SUMMARY
A major roadblock in the biomedical treatment of human sensory disorders, including blindness, has been an incomplete understanding of the nervous system and its ability to adapt to changes in sensory modality. Likewise, fundamental insight into the evolvability of complex functional anatomies requires understanding brain plasticity and the interaction between the nervous system and body architecture. While advances have been made in the generation of artificial and biological replacement components, the brain's ability to interpret sensory information arising from ectopic locations is not well understood. We report the use of eye primordia grafts to create ectopic eyes along the body axis of Xenopus tadpoles. These eyes are morphologically identical to native eyes and can be induced at caudal locations. Cell labeling studies reveal that eyes created in the tail send projections to the stomach and trunk. To assess function we performed light-mediated learning assays using an automated machine vision and environmental control system. The results demonstrate that ectopic eyes in the tail of Xenopus tadpoles could confer vision to the host. Thus ectopic visual organs were functional even when present at posterior locations. These data and protocols demonstrate the ability of vertebrate brains to interpret sensory input from ectopic structures and incorporate them into adaptive behavioral programs. This tractable new model for understanding the robust plasticity of the central nervous system has significant implications for regenerative medicine and sensory augmentation technology.
KEY WORDS: vision, behavior, memory, transplantation, innervation, plasticity, frog
INTRODUCTION
One of the major challenges facing cognitive science and neurobiology is the integration of interdisciplinary approaches to understand the plasticity of the brain and nervous system. While developmental biology research has elucidated numerous molecular pathways that can be manipulated to induce targeted large-scale alterations in body plan and in organ positioning in several model species (Schneuwly et al., 1987; Crosby et al., 1992; Maden, 1993; Oviedo et al., 2010), very few of these studies have probed the functional connections of the brain to ectopic organs. Thus we still do not have an adequate picture of how the brain recognizes neural connections to specific organs in abnormal positions, or the degree of plasticity that might allow additional sensory or motor organs to be incorporated into adaptive cognitive programs. A mechanistic understanding of the relationship between brain and somatic organs is crucial for several disciplines.
Cognitive science and embodied artificial intelligence must incorporate lessons learned from highly successful biological models about how complex behavior can be generated from the interaction of computational modules and sensors/effectors. Indeed, a promising recent direction in this field is the development of cybernetic devices that are not pre-programmed with a description of their own structure but must discover their morphology dynamically (Bongard et al., 2006). Robust mechanisms of such self-modeling will have numerous applications for the development of fault-tolerant and resilient communications and control networks in many areas of engineering. In addition, the understanding of the biological flexibility that allows a behavioral program to adjust to modifications in body structure will shed light on evolutionary developmental biology. The ability of the central nervous system (CNS) and behavioral control programs to adjust to changes in body structure is an important contribution towards the evolvability of complex body plans, since anatomy can then be genetically altered without reducing the fitness of the animal (as would occur with a fixed behavioral/cognitive repertoire hard-wired to a particular body morphology) (Vandenberg et al., 2012).
Another key implication of plasticity research concerns the design of strategies in regenerative medicine. A primary goal of this research is to one day restore the function of damaged or missing sensory structures through the use of biological or artificial replacement components. Significant progress has been made in the engineering aspect of these studies; retinal prosthetics and cochlear implants have been successfully used in humans (Humayun et al., 2003; Mahadevappa et al., 2005; Yanai et al., 2007; Weiland et al., 2011) and functional eyes have been induced to form in vertebrate models (Viczian et al., 2009; Pai et al., 2012). However, it remains unclear how ectopic sensory organs connect to the central nervous system of the host, and whether the brain can adjust its function to make use of these ectopic organs. Implanted or regenerated structures may require specific connections to the CNS targets of native sensory organs in order to confer function. Alternatively, it is possible that CNS plasticity will allow ectopic organs to function even through novel pathways, with sensory information being transferred through the co-option of auditory, visual, mechanosensory, nociception, thermoreception, or other existing neural systems.
Sensory substitution data in humans and primate species suggest considerable CNS plasticity when processing sensory information. Brain computer interfaces are becoming increasingly sophisticated and primates have been trained to feed themselves with mechanical appendages wired into the motor cortex of the brain (Yang et al., 2005; Rothschild, 2010; Nicolas-Alonso and Gomez-Gil, 2012; Pasqualotto et al., 2012). In addition, a number of devices can transduce visual data into sound (echolocation), electrotactile stimulation of the skin, or electrical stimulation of the tongue. In the case of blind individuals, extended use of these devices stimulates neuronal activity within the visual cortex of the patient even though these ‘visual’ data are delivered through decidedly non-visual pathways (Bach-y-Rita et al., 1969; De Volder et al., 1999; Bach-y-Rita and Kercel, 2003; Ptito et al., 2005). However, the devices used in these studies make use of other existing sensory structures; how the brain responds to exogenous visual information delivered through additional novel structures and pathways remains unclear. Clearly, there is a need for the development of molecularly tractable models in which genetics, neurobiology and behavior can be investigated during the brain's adaptation to an altered body plan.
The African frog Xenopus laevis (Daudin 1802) is a developmental model system in which the molecular genetics (Beck and Slack, 2001; Slack et al., 2004) and biophysics (Adams et al., 2007; Vandenberg and Levin, 2010) of pattern formation can be readily manipulated at early stages, and behavior analyzed (Blackiston et al., 2010; Blackiston and Levin, 2012). In particular, Xenopus has been successfully used to analyze eye development, with well-documented pathways for eye induction, lens formation, retinal pathfinding and vascularization (Holt, 1984; Henry and Grainger, 1990; Chien et al., 1993; Hirsch and Harris, 1997; Kenyon et al., 1999; Lupo et al., 2005). In addition, studies have reported the generation of ectopic eyes in tadpoles through surgical techniques, and more recently through embryonic microinjection of a number of ‘eye control genes’ including Pax6, Rx1, Otx2 and Six3 (Koo and Graziadei, 1995; Chow et al., 1999; Ohuchi et al., 1999; Ashery-Padan and Gruss, 2001; Kenyon et al., 2001; Sedohara et al., 2003; Bailey et al., 2004), as well as through modulation of bioelectrical determinants of organ identity (Pai et al., 2012). Ectopic eyes induced in the cranial region of tadpoles appear to extend ‘cellular bridges’ and axons towards the optic tecta of the host and these structures are maintained across metamorphosis into adulthood (Harris, 1986; Koo and Graziadei, 1995; Sedohara et al., 2003). When ectopic eyes were transplanted just behind the head along the dorsal midline, axons from transplanted eyes appear to enter the spinal cord of the host, perhaps following the pathways of native Rohon–Beard neurons (Giorgi and Van der Loos, 1978; Katz and Lasek, 1978). However, it is still unknown whether ectopic eyes induced in either the cranial or caudal regions were functional (i.e. whether they conferred vision in the host).
One of the major hurdles to determining whether ectopic sensory organs are functional in frog species has been the difficulty in completing cognitive studies. Electrophysiology can reveal whether ectopic structures produce action potentials in response to a stimulus, but behavioral results are necessary to determine if such information is interpreted within the brain of the host. A definitive test of integration with the brain (one that rules out simple spinal reflexes) requires not merely a response to visual stimulus but the ability to incorporate these cues in learning paradigms. Unfortunately, Xenopus have been notoriously difficult to use in associative learning assays. Frogs have been reported to perish rather than avoid repeated electric shocks in aversive training procedures, and it has been suggested that frog species may be unable to learn in the laboratory setting (Mcgill, 1960; Boice, 1970; Thompson and Boice, 1975).
Our laboratory has recently developed protocols for visual conditioning in Xenopus (Blackiston and Levin, 2012) using a novel automated machine vision system capable of training tadpoles to specific colors or intensities of light (Blackiston et al., 2010). Using this device we established a protocol for investigating the brain–body interface in a tractable model system to ask: how plastic is the Xenopus brain? Having evolved for the use of two eyes located in the head, can it recognize the presence of eyes elsewhere in the body and use the information provided by these organs? We quantitatively analyzed whether ectopic eyes in Xenopus tadpoles confer vision in the host. Making use of fluorescent lineage tracers to examine the innervation patterns of ectopic eyes in vivo, we investigated specific innervation patterns in those animals that were able to use ectopic eyes far from the head to learn in light aversion assays. These data shed light on evolutionary and biomedical implications of the interaction between brain and ectopic sensory organs, and will inform future sensory augmentation programs seeking to treat disorders through the implantation of biological or synthetic structures.
MATERIALS AND METHODS
Animal husbandry
Xenopus laevis embryos were maintained according to standard protocols (Sive et al., 2000) in 0.1× Marc's modified Ringer (MMR), pH 7.8, and were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). For animals used in behavior trials, individuals were raised under a 12h:12h light:dark cycle at a temperature of 16°C, at no more than 30 individuals per 100×25 mm Petri dish. After stage 46, tadpoles were fed twice per day on standard Sera micron powdered food until behavioral testing. All experimental procedures involving the use of animals for experimental purposes were approved by the Institutional Animal Care and Use Committees (IACUC) and Tufts University Department of Laboratory Animal Medicine (DLAM) under the protocol number M2011-70.
Microinjection
Capped, synthetic tdTomato mRNA (Waldner et al., 2009) was dissolved in water and injected into embryos in 3% Ficoll using standard methods (Sive et al., 2000). Injections were made into both cells of two cell embryos to promote ubiquitous expression. Three hours after injection, embryos were washed and cultured at 16°C 0.1× MMR. At stage 18, Tomato-positive tissue donors were screened for fluorescence using an Olympus BX-61 equipped with a Hamamatsu ORCA AG CCD camera.
Microsurgery
Prior to surgery the vitelline membranes of stage 23 Tomato-positive donors and wild-type recipients were removed with surgical forceps and individuals were anesthetized in a 0.02% tricaine solution in 0.1× MMR. Eye primordia were excised from fluorescent donors using forceps, with care taken to remove as little of the underlying neural tissue as possible. Recipients were prepared by placing a small slit along the body axis into which the transplanted tissue was positioned. Donor tissue was placed at the most posterior possible location and directly ventral to the neural tube. Following surgery, individuals were allowed to heal for 30 min, at which time they were washed twice with 0.1× MMR and placed in a 16°C incubator.
To generate no-eyed tadpoles and to remove the native eyes of transplant recipients, stage 46 tadpoles were anesthetized in 0.02% tricaine solution. The epidermis covering the eye was gently removed using surgical forceps and the eye was removed by severing the optic nerve at the base of the eye. Following surgery, tadpoles were kept anesthetized for 30 min before being washed twice with 0.1× MMR. All animals were allowed to recover for a minimum of 48 h before being analyzed for behavior.
Behavioral apparatus
All behavioral testing was conducted using an automated training apparatus that has been documented previously (Blackiston et al., 2010). The overall structure of the device includes a rectangular array of cells, each with a disposable Petri dish in the field of vision of a machine vision camera (Insight-Micro 1400, Cognex Corporation, Natick, MA, USA). Each Petri dish houses a single animal that can be subjected to a series of individual conditions, with external illumination of each dish provided by red and/or blue LEDs (Osram Semiconductors, Sunnyvale, CA, USA; blue LED, 470 nm, part no. LBW5SM; red LED, 635 nm, part no. LRG6SP) housed in an illumination control module (ICM). Illumination is delivered from above the experimental environment and can vary both by quadrant and intensity, ranging between maximum or off in fifteen even steps. Xenopus are known to possess at least three cone classes, with the spectral sensitivity of the red and blue cone overlapping with the LEDs used in testing (Röhlich and Szél, 2000). Within each cell is also a set of six iridium oxide-coated titanium electrodes, allowing the delivery of mild to strong electric shocks. All shocks delivered during light-mediated training experiments were 1.2 mA AC currents, pulsed for 100 ms, followed by 300 ms of no shock. Constant current elicited erratic swimming behavior in tadpoles on rare occasions, and pulsed current created no such phenomenon. Computer-controlled software executes an experiment autonomously, recording the animal's position and speed in the dish, as well as changing lighting conditions or delivering shock as a result of time and/or behavior criteria.
A number of variables were optimized for learning experiments including feeding schedule, shock intensity and duration, day/night cycle and rest duration (Blackiston and Levin, 2012). All training trials began with a 20 min initial color preference analysis in which half of the dish was illuminated with red light and half with blue, with the position of the lights rotating after 10 min. Rotation of light regimes ensured that individuals remaining motionless would not be scored as having an absolute preference for one color or the other. Following this period, an initial training session was initiated, which mirrored the initial color preference lighting regime. However, during training, tadpoles received a 1.2 mA pulsed electric shock, as described above, when occupying the red half of the dish. As with the initial color preference phase, the lighting regimes were inverted after 10 min. Following training, a 90 min rest period was executed in which the entire dish was illuminated with blue light and no punishments were delivered. Upon completion of the rest, tadpoles were exposed to the initial lighting condition (half of the dish illuminated with red light and half with blue) for 5 min without punishment, to evaluate color preference in the absence of shock. The sequence of training, rest and testing were repeated a total of six times, after which the trial was terminated and tadpoles were transferred to their original rearing incubators. In the case of individuals that were tested over two successive days, the locations of each tadpole in the behavior machine were randomized between the two days to avoid any chamber-specific effects on learning. Furthermore, for all trials involving animals with ectopic eyes, wild-type animals and operated animals were siblings from the same clutch of embryos. All positional data during each trial was written to a log file, which was parsed and analyzed in Excel over discrete time blocks.
Statistics
All statistical analyses were performed using Prism v. 5 (GraphPad Software, La Jolla, CA, USA). Movement rates in response to light were analyzed using a repeated-measures ANOVA followed by Tukey's post hoc comparisons. Learning rates were analyzed using repeated-measures ANOVAs followed by Bonferroni post hoc comparisons between innate values and all testing values. Comparisons of time punished during training were made with a one-way ANOVA. A chi-squared (χ2) test was employed to compare learning between animals with different innervation patterns. Data conformed to parametric requirements and no corrections were necessary for normality or variance.
RESULTS
Ectopic eyes are produced at the site of an eye primordium transplant
In order to induce ectopic eyes at specific locations, we utilized surgical transplant of eye primordia between donor and recipient Xenopus embryos. Donor tissue was harvested from stage 24 embryos, via the removal of the entire presumptive eye region. Care was taken to excise little surrounding tissue and underlying neural tissue, and all surgery was carried out on the left side of the embryo (Fig. 1A). Once the tissue was removed, a small incision was made on the left side of the recipient and the graft was situated tightly in the wound. In all cases, the orientation of the transplanted tissue matched the orientation of the host with regard to the mediolateral axis, but may have been perturbed relative to the dorsoventral and anteroposterior axis. Within 30 min of transplant, the wound and graft showed healing and no defects to normal development were observed (Fig. 1B). Following surgery, animals were raised according to standard protocols alongside non-operated siblings. Twenty-four hours post-surgery, wounds were completely healed and developing eyes were visible as a defined region of raised tissue along the trunk of the animal (Fig. 1C). After hatching, we noted no swimming or feeding differences between tadpoles that received grafts and those that did not; all animals appeared unimpaired.
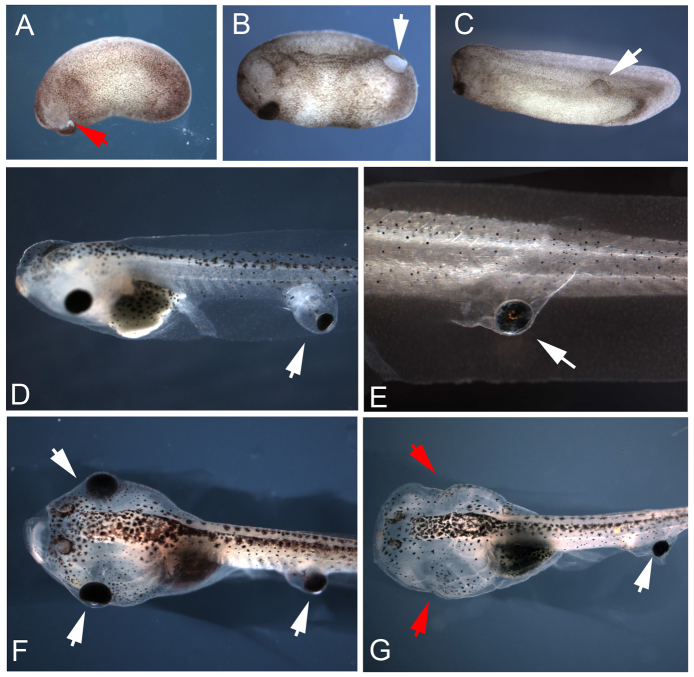
Fig. 1.
Eye primordia transplants produce ectopic eyes at the site of graft in Xenopus tadpoles. Donor tissue was excised from the eye field of stage 24 embryos (A, red arrow), grafted along the recipient's body, and healed within 30 min (B, white arrow). Ectopic eyes develop at the same rate as native eyes, and wounds heal completely within 24 h post-surgery (C). Eyes transplanted to caudal regions develop within pockets of tissue (D) or tightly along the trunk of the tail (E). Tadpoles receiving grafts could also have their native eyes removed through surgery at stage 46 (F, red arrows in G), leaving only ectopic visual structures.
Transplantation surgery was successful in generating ectopic eyes in 95% of cases (N=230), and eyes could be produced at any location along the body axis, with the exception of the posterior tip of the tail. In all cases, full ectopic eyes were generated that were generally of the same dimensions as those of the host, or occasionally slightly smaller than that of the host. By placing grafts at the most posterior location of stage 24 embryos, we were able to create eyes along the trunk of recipients (although no eyes formed at the posterior tip of the tail). Ectopic eyes induced in the tail revealed two distinct phenotypes. In the first, a small balloon of epidermis was formed, creating a pocket extending laterally from the trunk. The eye then developed in the pocket, but appeared to be anchored to the trunk through a bridge of tissue (Fig. 1D). In the second phenotype, the eye developed tightly along the trunk of the tadpole without an obvious bridge of tissue between the eye and trunk of the host (Fig. 1E). Ectopic eyes demonstrated the same mediolateral orientation as that of the host (in no cases did we observe eyes facing ‘inward’ towards the body – all were directed outward towards the external environment mirroring the orientation of the graft), and microscopy revealed that ectopic structures received blood supplies. In addition, the native eyes of tadpoles receiving grafts could be removed surgically at stage 46, leaving only ectopic structures intact (compare Fig. 1F,G). This procedure generated animals suited for future behavioral tests, as the only remaining visual structures were ectopic eyes arising from grafts.
Ectopic eyes innervate specific locations within the host
To characterize the functionality of ectopic eyes, we tracked the innervation from donor eyes into the host by making transplants between labeled donors and non-labeled recipients. Fluorescent donor eyes were created by injecting mRNA encoding the tdTomato fluorescent protein (Shaner et al., 2004) into two-cell Xenopus embryos. At stage 24, grafts from these individuals were transplanted to non-labeled recipients. Using this method, innervation arising from ectopic eyes could be visualized directly, in vivo, using fluorescence microscopy at swimming stages when tadpoles are optically transparent.
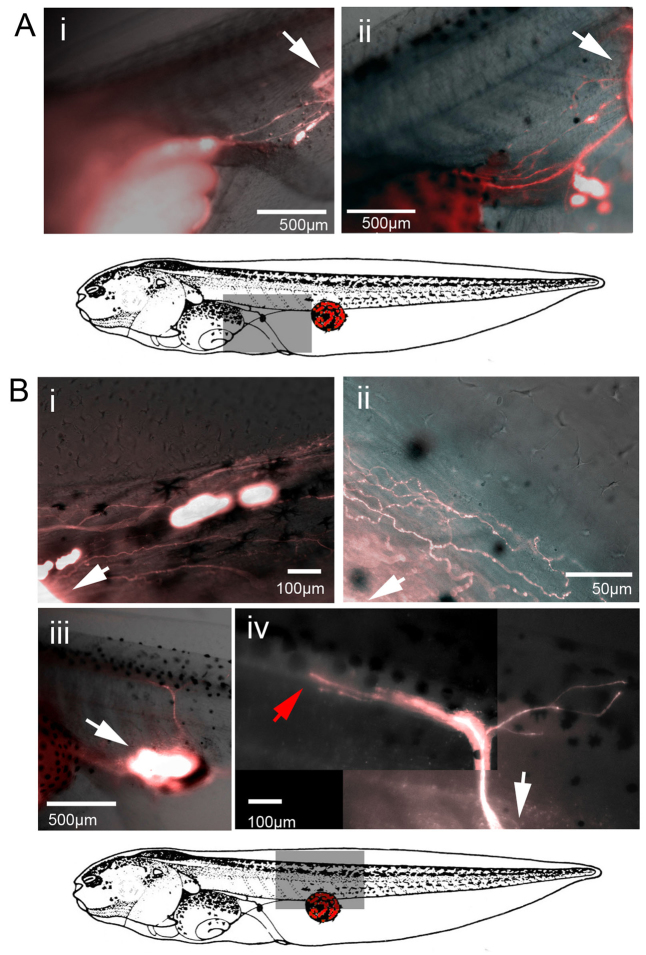
Visualization of tdTomato fluorescent protein when eyes were induced in the tail of the recipient revealed distinct innervation patterns. A total of 134 animals were imaged, of which 50% (N=67) showed no neuronal processes emanating from ectopic eyes. In contrast, in 26% (N=35) of the individuals, processes were observed proceeding anteriorly from the site of the graft to the stomach of the host (Fig. 2Ai,ii). In these cases, neuronal processes terminated at the point of contact with the gut, and no labeled tissue was observed at the anterior side of the stomach, closest to the head of the tadpole. In the remaining 24% (N=32) of animals imaged, neuronal processes were observed penetrating the host tissue dorsally, moving through the trunk of the host towards the spine. In the majority of these animals (N=26), the axons were organized in a loose network, extending both anteriorly and posteriorly from the point of entry within the trunk (Fig. 2Bi,ii). In more rare cases (N=6), bundles of neuronal processes penetrated the trunk of the host and terminated directly along the spinal cord (Fig. 2Biii,iv). In none of the animals did labeled neurons proceed into the cranial region of the host, and no signal was observed within the brain of any animal receiving caudal transplants. We conclude that the ectopic eye (donor) tissue sends out neuronal processes towards distinct anatomical locales within the host.
Fig. 2.
Ectopic eyes transplanted to caudal regions innervate host tissue. When donor eye primordia from a td-Tomato+ host was transferred to the trunk of a recipient, two distinct innervation patterns were observed. In the first, neuronal processes proceeded anteriorly though the fin and terminated along the lining of the stomach of the host (Ai,ii). In the second, labeled neuronal processes penetrated the trunk of the recipient and proceeded towards the spine of the host in either a highly arborized branching pattern (Bi,ii) or along a single path (Biii,iv). White arrows indicate the location of ectopic eyes. The red arrow in panel Biv indicates the termination point of ectopic neuronal processes in the trunk (not continuing to the brain).
Tadpoles with and without eyes respond to changes in light intensity
To determine whether ectopic eyes confer vision in the host, we made use of a custom machine vision system to track movement rates in response to light using a behavior apparatus described previously (Blackiston et al., 2010). Briefly, a circular arena rests in the field of vision of a motion-tracking camera. Automated software allows for red or blue LEDs to be turned on or off in individual quadrants of the arena, based on time criteria or animal behavior (Fig. 3A,B), and electric shocks can be delivered based on animal position within the arena. This occurs in real time, giving individual feedback to each animal based on its behavior, thus enabling studies of instrumental learning or classical conditioning. Twelve experimental arenas can be run simultaneously, with each having stimuli independently delivered, and quantitative, objective data recorded by automated software.
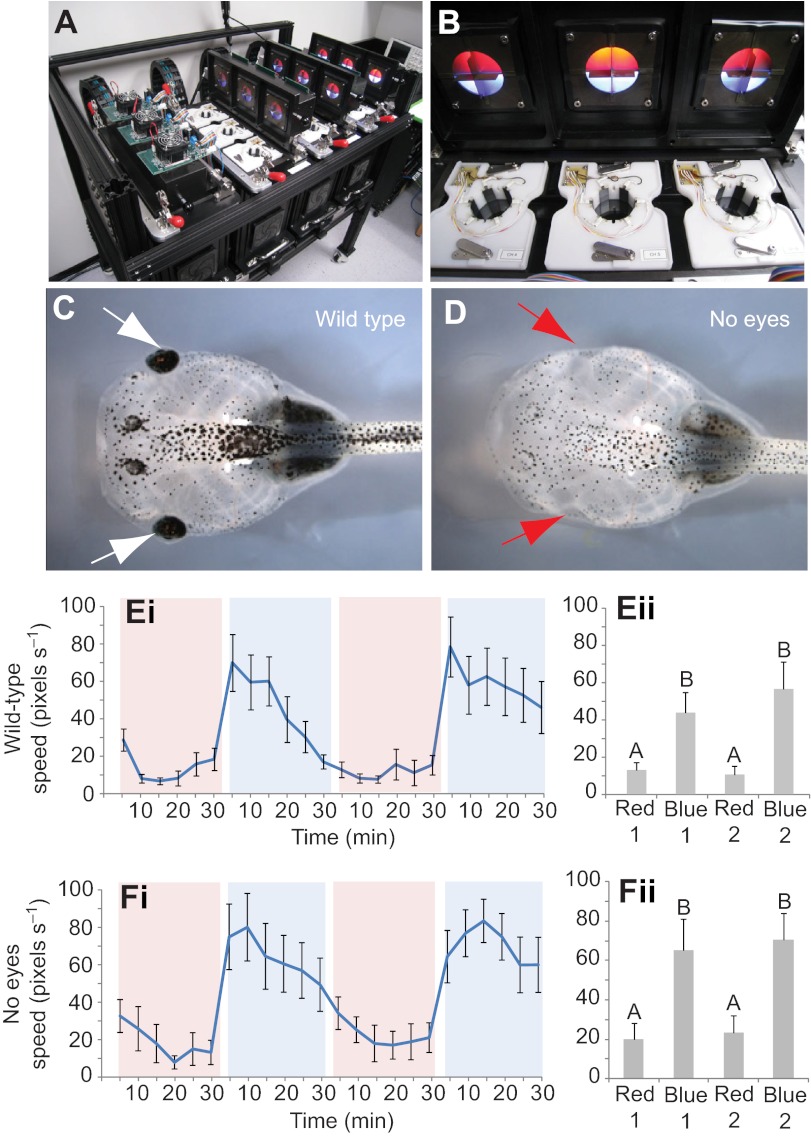
Fig. 3.
Eyeless tadpoles respond to changes in light intensity. Tadpoles were placed in a behavior analysis system (A) that contains a circular arena with overhead illumination delivered through red and blue LEDs (B). A machine vision camera system located below the dish tracked tadpole movement rates in response to changing light colors and intensities within the experimental arena. The movement rates of wild-type animals (C) and individuals with eyes removed through surgery (D) were compared. When presented with alternating lighting conditions between red and blue light, wild-type tadpoles showed an increase in movement rates when presented with blue light (Ei, and collapsed for each time period in Eii). In addition, eyeless tadpoles also showed a significant increase when presented with blue light, suggesting that they were able to sense blue wavelengths though a mechanism independent from visual sensory organs (Fi, and collapsed for each time period in Fii). Shaded red and blue areas indicate trial periods in which red or blue illumination was present. Error bars indicate ±1 s.e.m.; N=24 for both conditions; different letters represent significantly different columns by repeated-measures ANOVA.
First, a simple assay was developed to quantitatively test movement in response to changes in light levels. Tadpoles were adapted for 30 min under low intensity red light, after which low intensity blue lights were turned on in all quadrants of the arena for 30 min. Following this period, the red/blue regime was repeated a second time to demonstrate consistency of behavior in response to light changes. In addition to wild-type animals (Fig. 3C), no-eyed control animals were produced by surgically removing native eyes in stage 46 tadpoles (Fig. 3D). Animals with no eyes behaved as wild-type Xenopus tadpoles following surgery, feeding and circling the dish at rates comparable to un-operated siblings. Thus we could detect and characterize differences of light response between wild-type animals, those without eyes, and those with native eyes removed but ectopic eyes implanted, to determine whether the ectopic structures confer light sensitivity.
Over the course of the initial red-adapted phase, wild-type animals demonstrated minimal movement rates (Fig. 3E). However, in response to the onset of blue illumination, tadpoles responded with a rapid increase in movement rate (see shaded blue sections of Fig. 3E). Movement rate differences between each phase of the trial were significant, with rates being consistently higher during the blue illumination portions of the trial (ANOVA, P<0.001). Remarkably, animals with no eyes showed the same movement rate increases in response to light (Fig. 3F), with significant differences being detected between the red-adapted and blue-illuminated phases (ANOVA, P<0.001). These difference were not due to heat given off by the blue LEDs, as measurements revealed no changes in temperature to either the tadpole media or illumination head of the behavior apparatus over the course of the trial. We conclude that the Xenopus tadpole contains a light sensor other than the eyes that can rapidly trigger movement upon changes in light levels. Since eyeless tadpoles also exhibit a rapid response to changes of light, this simple protocol could not be used to test the vision properties of real ectopic eyes. Therefore, we designed a more complex associative learning task.
Intact tadpoles demonstrate associative learning in a light-mediated choice assay
We have recently developed a protocol (Blackiston and Levin, 2012) that results in significant aversive behavior in response to red light (Fig. 4A). Using the automated training device, tadpoles are first assayed for red or blue light preference in the absence of any training. Following this period, individuals then receive an electric shock whenever located in red portions of the arena. This is followed by a rest period, and then a test for light preference in the absence of punishment. By repeating this procedure multiple times, increasing aversion to red light in response to training can be assayed across the length of the trial.
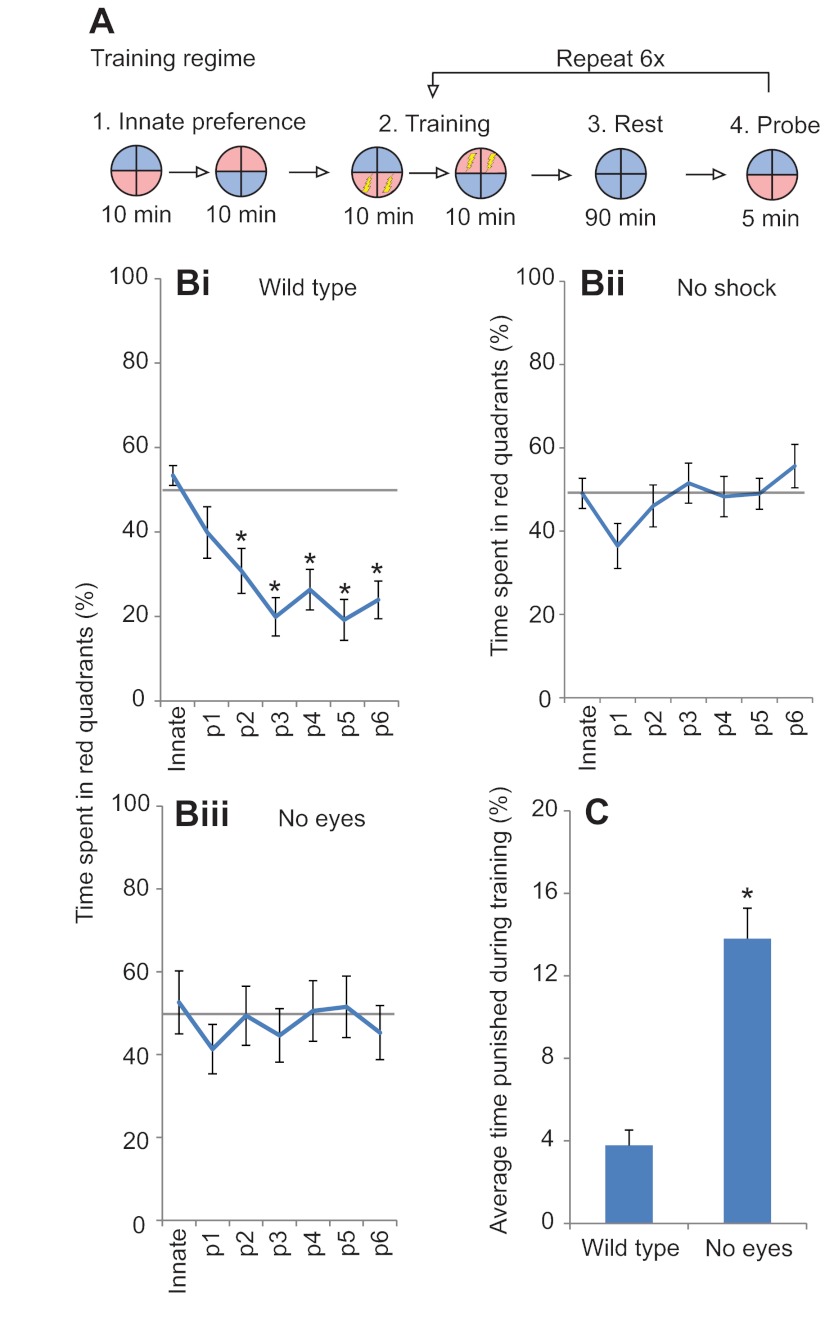
Fig. 4.
Tadpoles can learn associative stimulus avoidance in an automated assay. Tadpoles were placed individually into the behavior apparatus, and the automated software executed a training cycle. Animals were first tested for red or blue light preference in the absence of shock, followed by a training period where animals receive a shock when occupying the red half of the arena. Following training, all animals received a 90 min rest period in blue light before being tested for red and blue color preference in the absence of shock. Training, rest and testing sessions were repeated a total of six times across the trial (A). Prior to training, wild-type tadpoles demonstrated no preference for either red or blue halves of the dish. However, following two training sessions, a significant red light aversion was generated (Bi). In contrast, animals subjected to the same experiment in the absence of shock did not demonstrate red light aversion during testing (Bii). Furthermore, animals with eyes surgically removed at stage 45 were unable to learn red light avoidance in the presence of shock (Biii). During the training periods, when tadpoles are punished for occupying red halves of the arena, wild-type animals spent significantly less time in punishing areas compared with eyeless tadpoles (C). Error bars indicate ±1 s.e.m.; N=33, 36 and 36, for wild type, no shock and no eyes, respectively; *P<0.05, repeated-measures ANOVA (B), Student's t-test (C).
In this protocol, wild-type animals showed no innate preference for either red or blue light, spending half of their time under each color in the absence of punishment (Fig. 4B). Following each training phase, the preference for red light decreased, with significant differences from innate values appearing after two training periods upon reaching a 30% threshold. Red light aversion was maintained throughout the remainder of training, with values plateauing after the third testing session. To verify that red light aversion was due to associative training and not sensitization to blue light or red light, sibling animals were trained using the same protocol but without the delivery of electric shock during training phases. In these animals, no significant changes from innate values were observed (Fig. 4Bii). Such control tadpoles spent roughly half of their time under each of the two colored halves of the arena at the conclusion of the experiment. Taken together, these results represent the first report of Xenopus tadpoles learning in an aversive color training assay and suggest that they are amenable to training in a laboratory environment.
Eyeless tadpoles do not show associative learning to light-mediated cues
Eyeless tadpoles were also tested for associative color learning using the same protocol. In contrast to wild-type animals, those without eyes did not demonstrate any learning during the trial (Fig. 4Biii). Innate preferences for blue light were slightly elevated compared with those of wild-type animals, though not significantly different from those of controls. Following repeated training, eyeless animals continued to spend half of the time under each color light, with no significant color aversion at any time during the test (ANOVA, P=0.71). We conclude that although the tadpole contains some sensor of light outside of the eye that is able to trigger a movement very rapidly upon change in light levels, this sensor is not usable as input for true learning by the animal when interpreting wavelength-based cues. In addition to learning rates, we also analyzed each treatment to determine how successful individuals were at avoiding punishing halves of the arena during training periods. Over the course of all six training sessions, wild-type animals were punished only 3.9% of the time, indicating that they were proficient at avoiding red portions of the dish (Fig. 4C). In contrast, eyeless animals were punished 13.8% of the time, a fourfold increase above wild-type animals, signifying difficulty in distinguishing the areas in the dish that would result in a shock being delivered (ANOVA, P≤0.001). This combination, red light aversion during 70% or more of the test phase and rates of punishment below 10%, was never observed in eyeless animals and thus served as our criteria for light-mediated learning in subsequent trials.
Learning requires innervation of the central nervous system
Having identified a successful learning protocol in wild-type animals, we sought to determine if ectopic eyes could allow animals to learn in this protocol. Using in vivo imaging of ectopic eye innervation, animals were placed into separate groups based on innervation pattern, then tested for learning in the automated assay above. Animals were required to show both learning and shock avoidance to the same level as wild-type animals (spending <30% of their time in red quadrants during the final three testing periods, and being punished <10% of the training time), over two successive days of training, to avoid the possibility of randomly choosing quadrants that would appear as learning. Using this criterion, none of the animals from the no-shock or eyeless treatments qualify as ‘learners’. In addition, the position of the animal in the device was randomized between each day, using a random number generator to rule out any chamber-specific effects that may be associated with training.
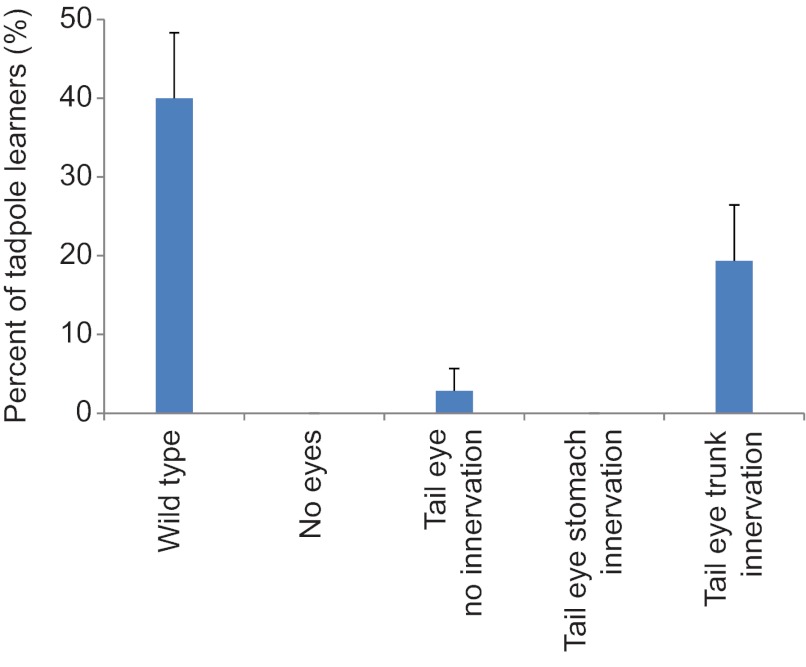
Using this procedure on new cohorts of tadpoles, 40% of wild-type animals demonstrated learning over two successive days of training (Fig. 5) while none of the eyeless animals tested met the criteria for learning (N=36 for both treatments). When examining animals with their native eyes removed but with an ectopic eye in the tail, we observed a single individual that learned in the absence of innervation. In animals with processes extending from ectopic eyes in the tail and terminating at the stomach, no learning was observed in any of the individuals tested (N=32). Surprisingly, we did observe measurable light-mediated learning in animals with posterior ectopic eyes that innervated the trunk and spine of the host, with six of 31 animals (19.2%) showing red avoidance on both days of testing. These results were significantly different from the null hypothesis expectation of equal distribution of results across treatments (χ2, P<0.001). We conclude that animals with ectopic eyes present in the tail are capable of providing data to the tadpole in light-mediated learning assays, and in the case of eyes present in the tail, these structures need not innervate the brain of the host for learning to occur.
Fig. 5.
Xenopus can learn a light-mediated task using ectopic eyes present in the tail of the tadpole. Tadpoles with their native eyes removed, but containing a single ectopic eye on the tail, were sorted based on the innervation pattern of the ectopic structure and tested for learning in the automated device. Each group was trained twice, with randomized placement between days of training, to avoid positional effects in the device and to reduce the possibility of false positives. Forty percent of wild-type tadpoles demonstrated learning on both days, while none of the eyeless tadpoles met the learning criteria. Tadpoles with eyes in the tail showed no innervation; those with eyes in the tail that innervated the stomach also showed no appreciable learning in the device. However, 20% of tested individuals with an ectopic eye in the tail that innervated the trunk and spine of the animal learned over both days of testing. Error bars indicate 95% confidence intervals.
DISCUSSION
Prior work showed that ectopic eyes induced in the cranial region of tadpoles extended cellular bridges and axons towards the optic tecta and were maintained through metamorphosis (Harris, 1986; Koo and Graziadei, 1995; Sedohara et al., 2003). When ectopic eyes were transplanted just behind the head along the dorsal midline, axons from transplanted eyes appeared to enter the spinal cord (Giorgi and Van der Loos, 1978; Katz and Lasek, 1978). Furthermore, eye transplantation studies done in Rana pipiens, in which donor tissue was transplanted to the ear position of recipients, generated animals with optic fibres that penetrated the medulla of the host and in some cases exited the brain and established tracts within the spinal cord (Constantine-Paton and Caprianica, 1975; Constantine-Paton and Capranica, 1976a). These eyes were physiologically active and could evoke basic behavioral responses (Constantine-Paton and Capranica, 1976b). However, the functional aspects of eyes far outside the head had not been explored, and it was unknown whether ectopic eyes could be functional (i.e. whether they conferred vision in the host).
Here, we demonstrate that embryonic eye primordia grafts induce ectopic eyes in Xenopus tadpoles, and that such tadpoles could be subjected to quantitative behavioral analysis. Eyes could be created at any location except the most posterior point of the tail and were morphologically normal. Ectopic eyes received blood supplies from the host, and persisted through at least 6 weeks of development post-transplant. Tadpoles with eyes along the tail appeared unimpaired with respect to motor function, as behavioral tests show no difference in movement rates or exploration behavior compared with wild-type individuals (data not shown).
To analyze whether ectopic eyes conferred vision, we created a robust, automated light-response assay, in which tadpoles were exposed to alternating conditions of dim red and bright blue light. Wild-type animals show characteristic elevations in movement rates when exposed to blue lights. Remarkably, eyeless individuals showed clearly increased movement rates in response to bright blue light. Thermoreception is an unlikely explanation, as the motor response was immediate upon light incidence; moreover, the LEDs used in the machine vision system create no detectable change in temperature as measured by an infrared digital thermometer. A plausible candidate may be the pineal organ (Jamieson and Roberts, 2000). Ecologically, this structure may underlie perception of location within the water column, as well as mediating color change in juvenile and adult animals (Korf et al., 1981). Alternatively, photoreceptors have been identified in the epidermis of amphibians, reptiles, avians and mammals, the skin of which exhibits electrical signals in response to light (Becker and Cone, 1966; Harth and Heaton, 1973; Tosini and Avery, 1996). Regardless of the receptor, eyeless vision (which may be a reflex not involving the brain at all) would be a confounding factor for attempts to study connection of the brain to ectopic sensory organs. Thus in light of these observations, we created a more sophisticated associative light-mediated learning assay to study true eye–brain communication.
Our quantitative, automated procedure trained tadpoles to occupy specific locations within a dish in response to light cues – to our knowledge, the first successful report of associative light-mediated learning in tadpoles. Consistent learning results required the optimization of a number of independent variables (Blackiston and Levin, 2012). Wild-type tadpoles demonstrated rapid, repeatable learning behavior using this training method, while eyeless individuals failed to learn at any point during the trial. Thus the above-described light sensitivity in eyeless animals is insufficient for true color aversion learning. Only animals with intact eyes could pair a specific light with a punishment, and learn to move away from that color. We cannot be certain that tadpoles are using wavelength information in learning per se (they may be cued by perceived intensity differences due to the sensitivity of their retinas to the two LED colors used during training). Regardless of the cue (wavelength or luminance), individuals are clearly able to use this signal to distinguish between different halves of the experimental arena and remember this information as part of a true learning assay.
Individuals containing a single ectopic eye (with their native eyes surgically removed) demonstrated repeatable light-mediated learning over two successive days of trials even when the eye was well outside of the head. Eyes induced in the tails of tadpoles never innervated the brain of the host, yet tadpoles were able to learn light-mediated tasks if innervation of the trunk and spine was observed (Fig. 2Civ). These results uncover the presence of a novel visual pathway capable of bestowing vision to the host: we show that the vertebrate brain is able to recognize a locus of ectopic tissue in a caudal body location and interpret its signals correctly as visual data, incorporating it into an adaptive behavioral program. Our results are consistent with classical studies in rats, in which retinal transplants placed on the surface of the brain could evoke pupillary responses and were functional in lever-pressing assays (Coffey et al., 1989; Lund et al., 1991; Dunnett, 1994; Lund and Coffey, 1994; Radel et al., 1995; Girman et al., 2003), but demonstrate that such functional connections can extend through long distances in the body.
While many perturbations have been used to alter the embryonic body plan, very few studies have probed the functional connections of the brain to ectopic organs. We successfully combined anatomical perturbation with a reliable learning assay, and the data raise a number of interesting questions for future studies, specifically involving pathfinding of neurons through existing tissue and the signal processing of data delivered to the spinal cord and brain by ectopic eyes. The discovery of molecular mechanisms guiding ectopic innervation paths, and a precise delineation of the limits of plasticity, will be essential to fully utilize the promise of bioengineered organ systems.
A mechanistic understanding of the relationship between brain and somatic organs is crucial for several disciplines. Learning to optimize neuronal connections is highly relevant for the development of functional regenerative therapies, as well as for the engineering of sensory/motor augmentation of human abilities (e.g. exploration of extreme environments such as for space travel). An algorithmic formulation of the steps carried out by living systems in monitoring their structure and adjusting information-processing programs accordingly may have important implications for robotics and the creation of robust communication and control networks that maintain performance levels despite reconfiguration or damage.
The ability of the host organism to accommodate changes in anatomical arrangement of sensory organs has important implications for our basic understanding of evolution and the function of the CNS. For random genetic change to be an effective driver of increases in morphological complexity, it is necessary that alterations in morphogenetic regulatory networks do not result in an organism whose hardwired behavior is unable to accommodate the resulting body plan (low fitness), even when the morphological change itself could have been highly advantageous. The tadpole brain is not merely hardwired for the use of two eyes in prespecified locations but contains the capacity to recognize visual organs wherever they may be located, incorporating them into adaptive behavioral programs. This sort of functional, modular plasticity is consistent with similar self-monitoring and dynamic flexible repair that we recently observed to operate in Xenopus craniofacial patterning (Vandenberg et al., 2012), as well as known data on remodeling in early embryos (Cooke, 1981).
Moreover, understanding the robust mechanisms of such plasticity will have numerous applications for the development of fault-tolerant and resilient communications and control networks in many areas of engineering. Indeed a promising recent direction is the development of cybernetic devices that are not preprogrammed with a description of their own structure but must discover their morphology dynamically (Bongard et al., 2006). The interplay between investigations of somatic self-modeling during embryogenesis and implementations of such programs in bioengineered or synthetic systems will enrich numerous fields.
One of the most fascinating areas for future investigation is the question of how the brain recognizes that electrical signals coming from a patch of tissue near the gut is to be interpreted as visual data. In computer engineering, this problem is usually solved by a ‘header’ – a piece of metadata attached to each packet of information that indicates its source and type. Whether electric signals from eyes impinging on the spinal cord carry such an identifier remains a hypothesis to be tested. Likewise, extension of this paradigm to encompass ectopic effector organs (e.g. additional limbs), or indeed sensory organs appropriate to different species, will be necessary to fully map the range of functional plasticity of vertebrate brains. Such information on the relationship between brain and body will extend our understanding of neural structure and function, with important implications for engineering, artificial life, cognitive science and regenerative biomedicine.
ACKNOWLEDGEMENTS
We thank Punita Koustubhan, Amber Currier and Claire Stevenson for Xenopus husbandry assistance, Gerhart Ryffel and Roger Tsien for the tdTomato clones, and Daniel Lobo and Laura Vandenberg for helpful comments on the manuscript.
FOOTNOTES
FUNDING
We are grateful for support of the National Institutes of Health (grants MH081842 to M.L., EY018168 to M.L., 5T32DE007327-09 to D.B.), the Leila Y. Mathers Charitable Foundation and US Army Medical Research and Materiel Command (USAMRMC, award W81XWH-10-2-0058 to M.L.). Deposited in PMC for release after 12 months.
REFERENCES
- Adams D. S., Masi A., Levin M. (2007). H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 134, 1323-1335 [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R., Gruss P. (2001). Pax6 lights-up the way for eye development. Curr. Opin. Cell Biol. 13, 706-714 [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P., Kercel S. W. (2003). Sensory substitution and the human-machine interface. Trends Cogn. Sci. 7, 541-546 [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P., Collins C. C., Saunders F. A., White B., Scadden L. (1969). Vision substitution by tactile image projection. Nature 221, 963-964 [DOI] [PubMed] [Google Scholar]
- Bailey T. J., El-Hodiri H., Zhang L., Shah R., Mathers P. H., Jamrich M. (2004). Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 48, 761-770 [DOI] [PubMed] [Google Scholar]
- Beck C. W., Slack J. M. (2001). An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol. 2, REVIEWS1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H. E., Cone R. A. (1966). Light-stimulated electrical responses from skin. Science 154, 1051-1053 [DOI] [PubMed] [Google Scholar]
- Blackiston D., Levin M. (2012). Aversive training methods in Xenopus laevis: general principles. Cold Spring Harb. Protoc. 2012, doi: 10.1101/pdb.top068338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D., Shomrat T., Nicolas C. L., Granata C., Levin M. (2010). A second-generation device for automated training and quantitative behavior analyses of molecularly tractable model organisms. PLoS ONE 5, e14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice R. (1970). Avoidance learning in active and passive frogs and toads. J. Comp. Physiol. Psychol. 70, 154-156 [Google Scholar]
- Bongard J., Zykov V., Lipson H. (2006). Resilient machines through continuous self-modeling. Science 314, 1118-1121 [DOI] [PubMed] [Google Scholar]
- Chien C. B., Rosenthal D. E., Harris W. A., Holt C. E. (1993). Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron 11, 237-251 [DOI] [PubMed] [Google Scholar]
- Chow R. L., Altmann C. R., Lang R. A., Hemmati-Brivanlou A. (1999). Pax6 induces ectopic eyes in a vertebrate. Development 126, 4213-4222 [DOI] [PubMed] [Google Scholar]
- Coffey P. J., Lund R. D., Rawlins J. N. (1989). Retinal transplant-mediated learning in a conditioned suppression task in rats. Proc. Natl. Acad. Sci. USA 86, 7248-7249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M., Caprianica R. R. (1975). Central projection of optic tract from translocated eyes in the leopard frog (Rana pipiens). Science 189, 480-482 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Capranica R. R. (1976a). Axonal guidance of developing optic nerves in the frog. I. Anatomy of the projection from transplanted eye primordia. J. Comp. Neurol. 170, 17-31 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Capranica R. R. (1976b). Axonal guidance of developing optic nerves in the frog. II. Electrophysiological studies of the projection from transplanted eye primordia. J. Comp. Neurol. 170, 33-51 [DOI] [PubMed] [Google Scholar]
- Cooke J. (1981). Scale of body pattern adjusts to available cell number in amphibian embryos. Nature 290, 775-778 [DOI] [PubMed] [Google Scholar]
- Crosby J. L., Varnum D. S., Washburn L. L., Nadeau J. H. (1992). Disorganization is a completely dominant gain-of-function mouse mutation causing sporadic developmental defects. Mech. Dev. 37, 121-126 [DOI] [PubMed] [Google Scholar]
- De Volder A. G., Catalan-Ahumada M., Robert A., Bol A., Labar D., Coppens A., Michel C., Veraart C. (1999). Changes in occipital cortex activity in early blind humans using a sensory substitution device. Brain Res. 826, 128-134 [DOI] [PubMed] [Google Scholar]
- Dunnett S. B. (1994). Behavioural consequences of neural transplantation. J. Neurol. 242 Suppl. 1, S43-S53 [DOI] [PubMed] [Google Scholar]
- Giorgi P. P., Van der Loos H. (1978). Axons from eyes grafted in Xenopus can grow into the spinal cord and reach the optic tectum. Nature 275, 746-748 [DOI] [PubMed] [Google Scholar]
- Girman S. V., Wang S., Lund R. D. (2003). Cortical visual functions can be preserved by subretinal RPE cell grafting in RCS rats. Vision Res. 43, 1817-1827 [DOI] [PubMed] [Google Scholar]
- Harris W. A. (1986). Homing behaviour of axons in the embryonic vertebrate brain. Nature 320, 266-269 [DOI] [PubMed] [Google Scholar]
- Harth M. S., Heaton M. B. (1973). Nonvisual photic responsiveness in newly hatched pigeons (Columba livia). Science 180, 753-755 [DOI] [PubMed] [Google Scholar]
- Henry J. J., Grainger R. M. (1990). Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev. Biol. 141, 149-163 [DOI] [PubMed] [Google Scholar]
- Hirsch N., Harris W. A. (1997). Xenopus Pax-6 and retinal development. J. Neurobiol. 32, 45-61 [PubMed] [Google Scholar]
- Holt C. E. (1984). Does timing of axon outgrowth influence initial retinotectal topography in Xenopus? J. Neurosci. 4, 1130-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun M. S., Weiland J. D., Fujii G. Y., Greenberg R., Williamson R., Little J., Mech B., Cimmarusti V., Van Boemel G., Dagnelie G., et al. (2003). Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 43, 2573-2581 [DOI] [PubMed] [Google Scholar]
- Jamieson D., Roberts A. (2000). Responses of young Xenopus laevis tadpoles to light dimming: possible roles for the pineal eye. J. Exp. Biol. 203, 1857-1867 [DOI] [PubMed] [Google Scholar]
- Katz M. J., Lasek R. J. (1978). Eyes transplanted to tadpole tails send axons rostrally in two spinal-cord tracts. Science 199, 202-204 [DOI] [PubMed] [Google Scholar]
- Kenyon K. L., Moody S. A., Jamrich M. (1999). A novel fork head gene mediates early steps during Xenopus lens formation. Development 126, 5107-5116 [DOI] [PubMed] [Google Scholar]
- Kenyon K. L., Zaghloul N., Moody S. A. (2001). Transcription factors of the anterior neural plate alter cell movements of epidermal progenitors to specify a retinal fate. Dev. Biol. 240, 77-91 [DOI] [PubMed] [Google Scholar]
- Koo H., Graziadei P. P. C. (1995). Eye primordium transplantation in Xenopus embryo. Anat. Embryol. 191, 155-170 [DOI] [PubMed] [Google Scholar]
- Korf H. W., Liesner R., Meissl H., Kirk A. (1981). Pineal complex of the clawed toad, Xenopus laevis Daud.: structure and function. Cell Tissue Res. 216, 113-130 [DOI] [PubMed] [Google Scholar]
- Lund R. D., Coffey P. J. (1994). Visual information processing by intracerebral retinal transplants in rats. Eye 8, 263-268 [DOI] [PubMed] [Google Scholar]
- Lund R. D., Radel J. D., Coffey P. J. (1991). The impact of intracerebral retinal transplants on types of behavior exhibited by host rats. Trends Neurosci. 14, 358-362 [DOI] [PubMed] [Google Scholar]
- Lupo G., Liu Y., Qiu R., Chandraratna R. A., Barsacchi G., He R. Q., Harris W. A. (2005). Dorsoventral patterning of the Xenopus eye: a collaboration of retinoid, hedgehog and FGF receptor signaling. Development 132, 1737-1748 [DOI] [PubMed] [Google Scholar]
- Mcgill T. E. (1960). Response of the leopard frog to electric shock in an escape-learning situation. J. Comp. Physiol. Psychol. 53, 443-445 [Google Scholar]
- Maden M. (1993). The homeotic transformation of tails into limbs in Rana temporaria by retinoids. Dev. Biol. 159, 379-391 [DOI] [PubMed] [Google Scholar]
- Mahadevappa M., Weiland J. D., Yanai D., Fine I., Greenberg R. J., Humayun M. S. (2005). Perceptual thresholds and electrode impedance in three retinal prosthesis subjects. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 201-206 [DOI] [PubMed] [Google Scholar]
- Nicolas-Alonso L. F., Gomez-Gil J. (2012). Brain computer interfaces, a review. Sensors 12, 1211-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1967). Normal table of Xenopus laevis (Daudin). Amsterdam: North-Holland Publishing Company; [Google Scholar]
- Ohuchi H., Tomonari S., Itoh H., Mikawa T., Noji S. (1999). Identification of chick rax/rx genes with overlapping patterns of expression during early eye and brain development. Mech. Dev. 85, 193-195 [DOI] [PubMed] [Google Scholar]
- Oviedo N. J., Morokuma J., Walentek P., Kema I. P., Gu M. B., Ahn J. M., Hwang J. S., Gojobori T., Levin M. (2010). Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 339, 188-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V. P., Aw S., Shomrat T., Lemire J. M., Levin M. (2012). Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 139, 313-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto E., Federici S., Belardinelli M. O. (2012). Toward functioning and usable brain-computer interfaces (BCIs): a literature review. Disabil. Rehabil. Assist. Technol. 7, 89-103 [DOI] [PubMed] [Google Scholar]
- Ptito M., Moesgaard S. M., Gjedde A., Kupers R. (2005). Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain 128, 606-614 [DOI] [PubMed] [Google Scholar]
- Radel J. D., Kustra D. J., Das S., Elton S., Lund R. D. (1995). The pupillary light response: assessment of function mediated by intracranial retinal transplants. Neuroscience 68, 909-924 [DOI] [PubMed] [Google Scholar]
- Röhlich P., Szél A. (2000). Photoreceptor cells in the Xenopus retina. Microsc. Res. Tech. 50, 327-337 [DOI] [PubMed] [Google Scholar]
- Rothschild R. M. (2010). Neuroengineering tools/applications for bidirectional interfaces, brain-computer interfaces, and neuroprosthetic implants – a review of recent progress. Front Neuroeng. 3, 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. (1987). Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. Nature 325, 816-818 [DOI] [PubMed] [Google Scholar]
- Sedohara A., Komazaki S., Asashima M. (2003). In vitro induction and transplantation of eye during early Xenopus development. Dev. Growth Differ. 45, 463-471 [DOI] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567-1572 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M. (2000). Early Development of Xenopus laevis. New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Slack J. M., Beck C. W., Gargioli C., Christen B. (2004). Cellular and molecular mechanisms of regeneration in Xenopus. Philos. Trans. R. Soc. Lond. B 359, 745-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. A., Boice R. (1975). Attempts to train frogs - review and experiments. J. Biol. Psychol. 17, 3-13 [Google Scholar]
- Tosini G., Avery R. A. (1996). Dermal photoreceptors regulate basking behavior in the lizard Podarcis muralis. Physiol. Behav. 59, 195-198 [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Levin M. (2010). Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development 137, 1095-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Adams D. S., Levin M. (2012). Normalized shape and location of perturbed craniofacial structures in the Xenopus tadpole reveal an innate ability to achieve correct morphology. Dev. Dyn. 241, 863-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczian A. S., Solessio E. C., Lyou Y., Zuber M. E. (2009). Generation of functional eyes from pluripotent cells. PLoS Biol. 7, e1000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner C., Roose M., Ryffel G. U. (2009). Red fluorescent Xenopus laevis: a new tool for grafting analysis. BMC Dev. Biol. 9, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. D., Cho A. K., Humayun M. S. (2011). Retinal prostheses: current clinical results and future needs. Ophthalmology 118, 2227-2237 [DOI] [PubMed] [Google Scholar]
- Yanai D., Weiland J. D., Mahadevappa M., Greenberg R. J., Fine I., Humayun M. S. (2007). Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am. J. Ophthalmol. 143, 820-827 [DOI] [PubMed] [Google Scholar]
- Yang B. H., Yan G. Z., Yan R. G. (2005). A review of brain-computer interfaces (BCIs). Zhongguo Yi Liao Qi Xie Za Zhi 29, 353-357 [PubMed] [Google Scholar]