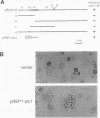
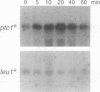
Abstract
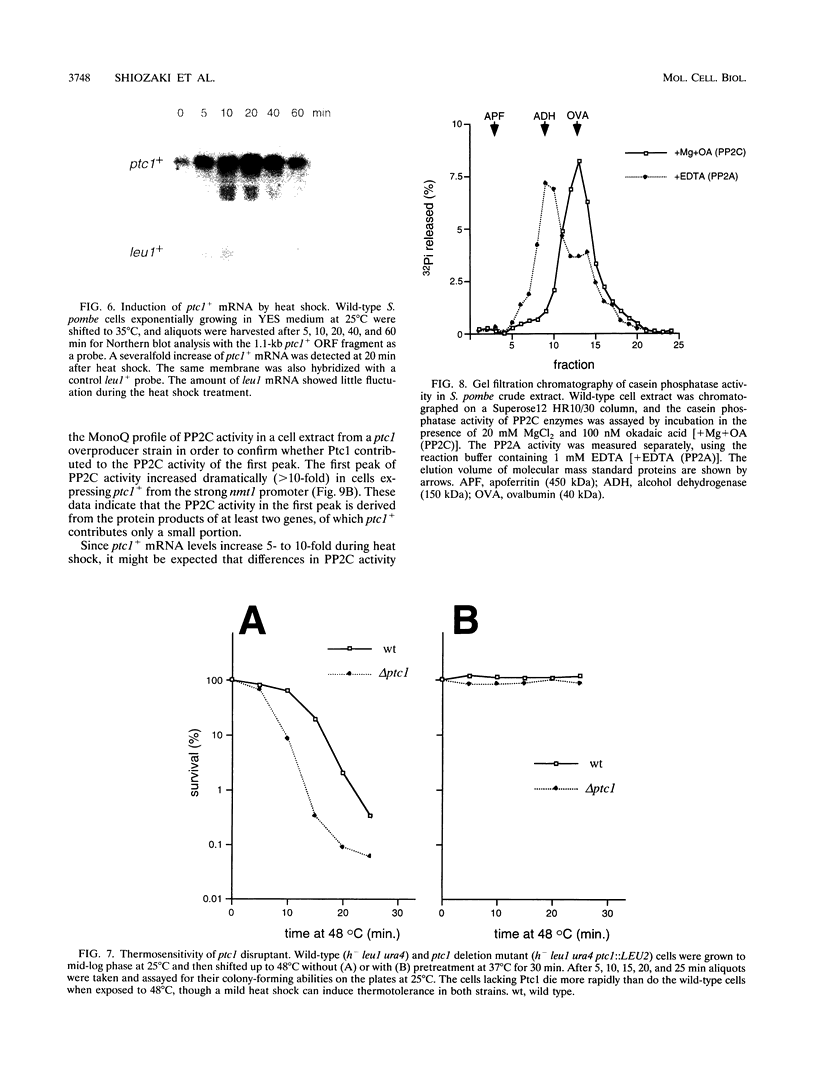
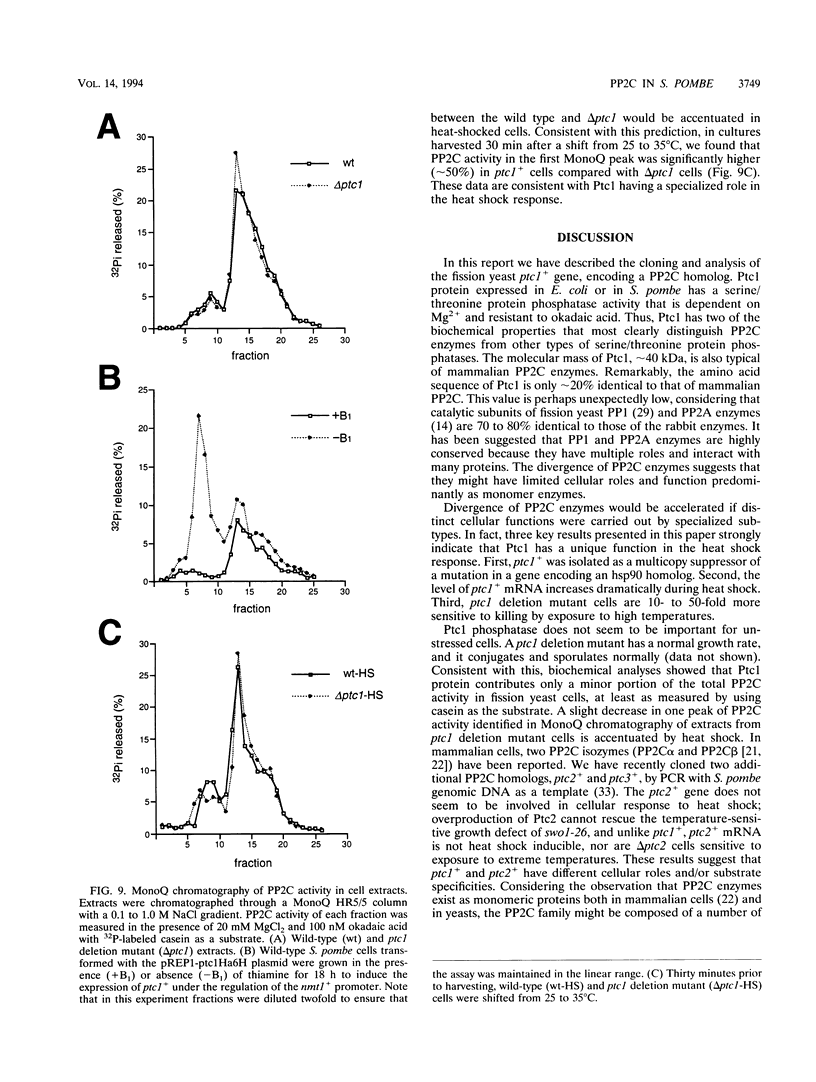
Protein phosphatase 2C (PP2C), an Mg(2+)-dependent enzyme that dephosphorylates serine and threonine residues, defines one of the three major families of structurally unrelated eukaryotic protein phosphatases. Members of the two other families of protein phosphatases are known to have important cellular roles, but very little is known about the biological functions of PP2C. In this report we describe a genetic investigation of a PP2C enzyme in the fission yeast Schizosaccharomyces pombe. We discovered ptc1+ (phosphatase two C) as a multicopy suppressor gene of swo1-26, a temperature-sensitive mutation of a gene encoding the heat shock protein hsp90. The ptc1+ gene product is a 40-kDa protein with approximately 24% identity to a rat PP2C protein. Purified Ptc1 has Mg(2+)-dependent casein phosphatase activity, confirming that it is a PP2C enzyme. A ptc1 deletion mutant is viable and has approximately normal levels of PP2C activity, observations consistent with the fact that ptc1+ is a member of a multigene family. Although a ptc1 deletion mutant is viable, it has a greatly reduced ability to survive brief exposure to elevated temperature. Moreover, ptc1+ mRNA levels increase 5- to 10-fold during heat shock. These data, demonstrating that Ptc1 activity is important for survival of heat shock, provide one of the first genetic clues as to the biological functions of PP2C.
Full text
PDF

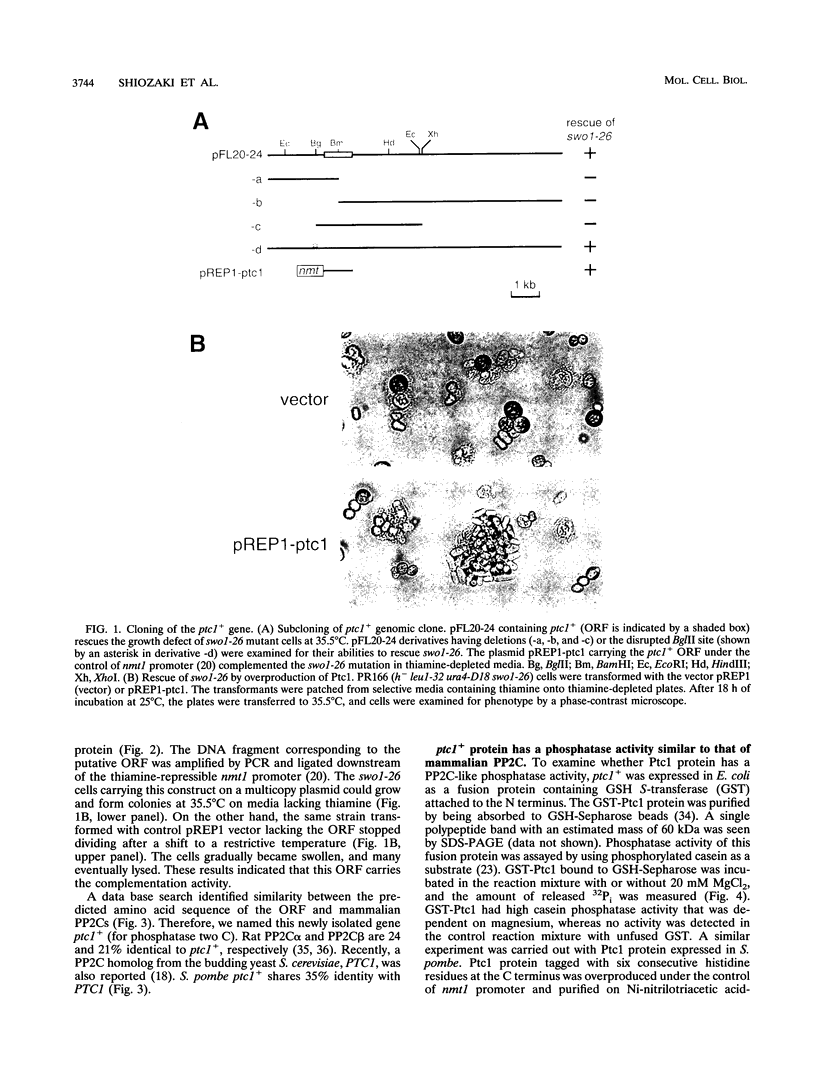


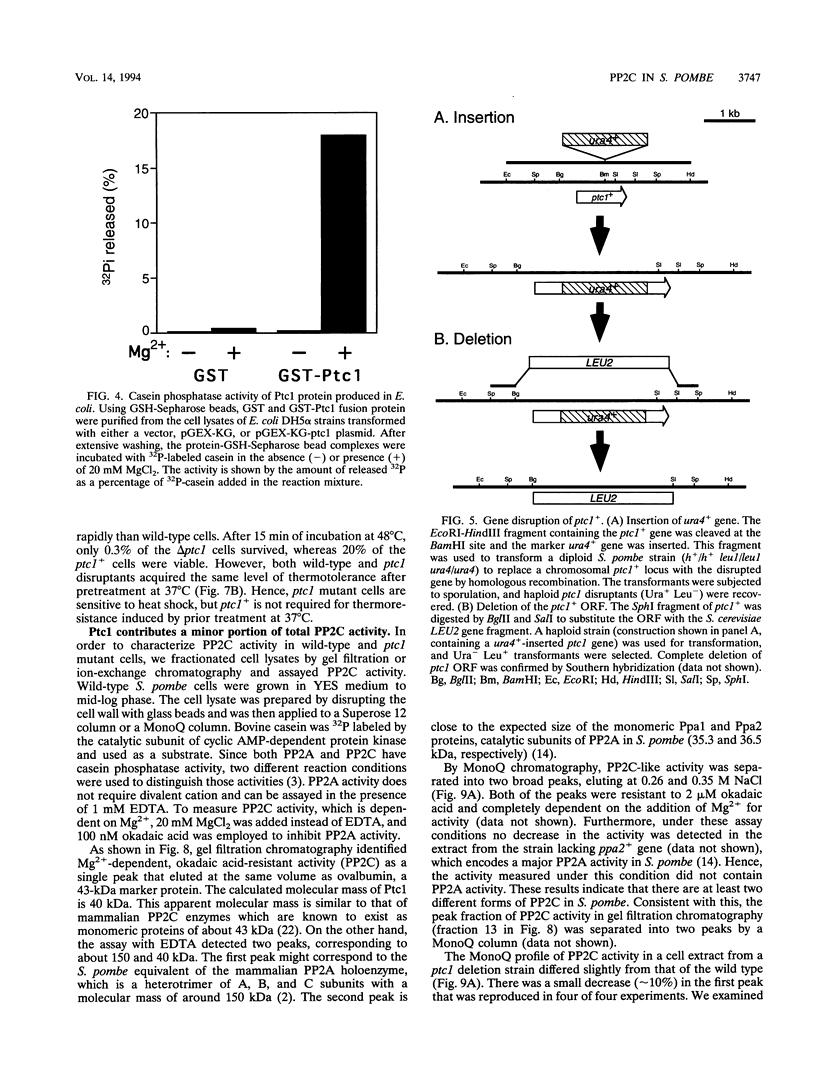


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Simmen U., Hottiger T., Boller T., Wiemken A. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation, and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990 Oct 29;273(1-2):107–110. doi: 10.1016/0014-5793(90)81062-s. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Rabideau D. A., Iannotti A. M., Sullivan W. P., Toft D. O. Identification of the 90 kDa substrate of rat liver type II casein kinase with the heat shock protein which binds steroid receptors. Biochim Biophys Acta. 1987 Jan 19;927(1):74–80. doi: 10.1016/0167-4889(87)90067-x. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Heyer W. D., Sipiczki M., Kohli J. Replicating plasmids in Schizosaccharomyces pombe: improvement of symmetric segregation by a new genetic element. Mol Cell Biol. 1986 Jan;6(1):80–89. doi: 10.1128/mcb.6.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochuli E., Döbeli H., Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J Chromatogr. 1987 Dec 18;411:177–184. doi: 10.1016/s0021-9673(00)93969-4. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Emslie E. A. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992 Oct 15;359(6396):644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Anderson C. W. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989 Feb 15;264(5):2431–2437. [PubMed] [Google Scholar]
- Legagneux V., Morange M., Bensaude O. Heat shock increases turnover of 90 kDa heat shock protein phosphate groups in HeLa cells. FEBS Lett. 1991 Oct 21;291(2):359–362. doi: 10.1016/0014-5793(91)81320-8. [DOI] [PubMed] [Google Scholar]
- Maeda T., Tsai A. Y., Saito H. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5408–5417. doi: 10.1128/mcb.13.9.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. J., Campbell D. G., McGowan C. H., Cohen P. T. Mammalian protein serine/threonine phosphatase 2C: cDNA cloning and comparative analysis of amino acid sequences. Biochim Biophys Acta. 1992 Feb 28;1130(1):100–104. doi: 10.1016/0167-4781(92)90471-b. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- McGowan C. H., Campbell D. G., Cohen P. Primary structure analysis proves that protein phosphatases 2C1 and 2C2 are isozymes. Biochim Biophys Acta. 1987 Sep 14;930(2):279–282. doi: 10.1016/0167-4889(87)90041-3. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Cohen P. Identification of two isoenzymes of protein phosphatase 2C in both rabbit skeletal muscle and liver. Eur J Biochem. 1987 Aug 3;166(3):713–721. doi: 10.1111/j.1432-1033.1987.tb13570.x. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Cohen P. Protein phosphatase-2C from rabbit skeletal muscle and liver: an Mg2+-dependent enzyme. Methods Enzymol. 1988;159:416–426. doi: 10.1016/0076-6879(88)59041-9. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Lenaers G., Russell P. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 1992 Dec;11(13):4933–4941. doi: 10.1002/j.1460-2075.1992.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992 Feb 7;68(3):407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Ota I. M., Varshavsky A. A gene encoding a putative tyrosine phosphatase suppresses lethality of an N-end rule-dependent mutant. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2355–2359. doi: 10.1073/pnas.89.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tamura S., Lynch K. R., Larner J., Fox J., Yasui A., Kikuchi K., Suzuki Y., Tsuiki S. Molecular cloning of rat type 2C (IA) protein phosphatase mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1796–1800. doi: 10.1073/pnas.86.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk J., Trompeter H. I., Pettrich K. G., Cohen P. T., Campbell D. G., Mieskes G. Molecular cloning and primary structure of a protein phosphatase 2C isoform. FEBS Lett. 1992 Feb 3;297(1-2):135–138. doi: 10.1016/0014-5793(92)80344-g. [DOI] [PubMed] [Google Scholar]
- Wu L., Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993 Jun 24;363(6431):738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]