Abstract
Circadian rhythms control multiple physiological and pathological processes, including embryonic development in mammals and development of various human diseases. We have recently, in a developing zebrafish embryonic model, discovered that the circadian oscillation controls developmental angiogenesis. Disruption of crucial circadian regulatory genes, including Bmal1 and Period2, results in marked impairment or enhancement of vascular development in zebrafish. At the molecular level, we show that the circadian regulator Bmal1 directly targets the promoter region of the vegf gene in zebrafish, leading to an elevated expression of VEGF. These findings can reasonably be extended to developmental angiogenesis in mammals and even pathological angiogenesis in humans. Thus, our findings, for the first time, shed new light on mechanisms that underlie circadian clock-regulated angiogenesis.
Keywords: zebrafish, circadian, angiogenesis, Bmal1, Period2, VEGF, Clock, development, vasculogenesis, vasculature
Introduction
The light and darkness shift in our solar system has generated a circadian system in most invertebrate and vertebrate organisms. The circadian system plays a central role in regulation of almost all physiological processes, particularly cellular activity in various tissues and organs, leading to circadian clock-dependent physiological activity.1 Disruption of the circadian clock system would not only affect the physiological activity of an organism, but also often leads to the onset, development and progression of various diseases.2 Many human disorders have been associated with circadian clock disruption, including cancer,2 myocardial infarction,3 stroke,4 obesity5 and diabetes.6
In mammals, the retina receives light/dark signals and transmits them to a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus, which, in turn, synchronizes individual cellular clocks both in the brain and in the periphery.7 Synchronization and coordination between the central nervous system and peripheral tissues ensure that all organs and tissues in the body act in concert within the 24-h rhythmic cycle.8 The cellular clock in SCN neurons and other neuronal cells consists of a transcriptional feedback loop in which the central clock transcription factor, brain and muscle ARNT-like-1 (Bmal1), heterodimerizes with circadian locomotor output cycles kaput (Clock) or neuronal PAS domain containing protein 2 (NPAS2), to drive expression via, in most cases, E-box elements of target genes, including members of the Period and Cryptochrome families.9-11 These genes could act as transcriptional repressors, inhibiting both their own transcription and that of other E-box-containing genes.10 This core circadian transcription/translation feedback loop is strengthened by auxiliary loops acting via retinoic acid receptor-related orphan receptor elements (ROREs) and D-boxes to establish circadian rhythmicity of the clock-controlled output systems.12
Angiogenesis, the growth of new blood vessels from the existing vasculature, is essentially required for all tissue growth in mammals,13 and deregulated angiogenesis contributes to the development of a number of human disorders, including cancer,14-16 ophthalmological disorders,14 cardiovascular diseases17 and metabolic disorders, such as obesity18 and diabetes.19 During embryonic development, the vasculature is formed via two main processes, angiogenesis—i.e., sprouting of new capillaries from existing vessels—and vasculogenesis—i.e., de novo formation of the initial vascular system.20 Both angiogenesis and vasculogenesis are regulated by multiple angiogenic factors and cytokines that display overlapping and yet distinct functions on various cell types in the vessel wall and luminal endothelial cells.21,22 Although the process of angiogenesis is a relatively well-studied process, the role of the circadian clock in controlling vascular development has not been studied. In one of our recent publications,23 we, for the first time, show that key circadian clock genes including Bmal1 and Period2 are involved in regulation of vascular development in developing zebrafish embryos. The underlying mechanism of the circadian-regulated developmental angiogenesis involves modulation of VEGF expression in this fish model.23 Since these key clock genes also exist in mammals, including humans, our findings can be reasonably expanded to mammalian embryonic development and even to pathological angiogenesis in human diseases.
Impairment of Developmental Angiogenesis by Disruption of the Circadian Rhythm
To study the role of the circadian clock in regulation of developmental angiogenesis, we took the advantage of using transgenic zebrafish in our studies. We were particularly interested in developing an in vivo system, in which angiogenesis could be kinetically studied without mechanical disruption. To achieve this goal, we chose zebrafish embryos as a model system for the following reasons: (1) it has been long known that zebrafish have a well-developed circadian clock system; (2) the genetic information of zebrafish is completely known; (3) genetic interference of a given gene function can be achieved by the well-established morpholino technology; (4) a transgenic strain of zebrafish expressing enhanced green fluorescent protein (EGFP) specifically in vascular endothelial cells, Fli1:EGFP, is available; (5) vascular development in zebrafish embryos occurs fast, and the experimental turnover rate is relatively short; (6) it is a relatively inexpensive to use the zebrafish model; (7) zebrafish has diurnal physical activity that recapitulates human diurnal activity and thus is clinically relevant; (8) genes involved in the regulation of the circadian clock are highly homologous to those in humans; (9) zebrafish embryos are transparent in nature and vascular development can be easily monitored in living embryos without scarification; and (10) it is known that most cells in zebrafish embryos respond to light-dark cycles. Based on these advantages, we studied regulation of zebrafish developmental angiogenesis by the circadian clock. In the initial experiments, we exposed Fli1:EGFP zebrafish embryos to 12 h light/12 h dark (LD), constant light (LL) or constant dark (DD) environments. Surprisingly, exposure of zebrafish embryos to LL resulted in retarded angiogenesis of intersegmental vessels (ISVs) (Fig. 1) compared with those exposed in LD and DD. In contrast to LL, DD exposure did not significantly alter ISV growth.23 These findings suggested to us that disruption of the circadian rhythm might modulate developmental angiogenesis in zebrafish embryos.
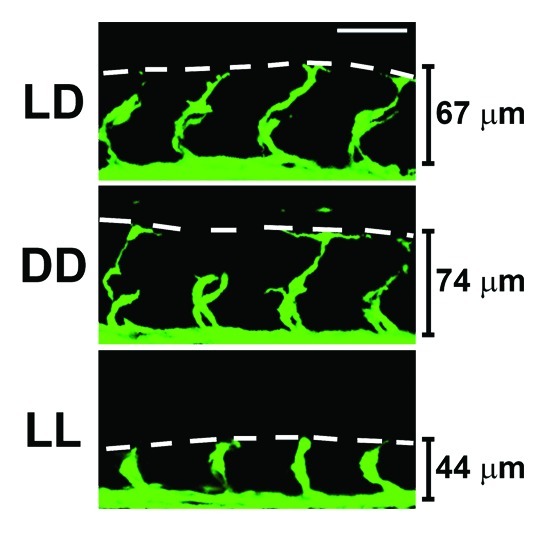
Figure 1. Constant light exposure leads to impaired developmental angiogenesis. Transgenic fli1:EGFP zebrafish embryos expressing GFP in endothelial cells were exposed to either 12 h light/dark cycles (LD), constant dark (DD) or constant light (LL) from 1 h post fertilization (hpf). Images of the vasculature were obtained by confocal microscopy at 24-hpf, and representative images of the intersegmental vessels (ISVs) are shown. It is clear that ISV development is impaired in LL compared with LD and DD. The lines indicate the height of the ISV vessels. The white scale bar is 50 μm.
Opposing Effects of Bmal1 and Period2 in Regulation of Angiogenesis
Since DD and LL exposure could lead to differential expression of various circadian clock genes, we then investigated mRNA expression levels of a couple of key circadian regulators, including Bmal1 and Period2. Markedly, Period2 expression was substantially elevated during LL exposure, whereas Bmal1 appeared to be downregulated.23 To delineate the functions of these two clock gene functions, we used the morpholino technique to specifically interfere with Bmal1 or Period2 functions in developing zebrafish embryos. Interestingly, inhibition of Bmal1 function by its specific morpholino resulted in marked suppression of ISV growth in zebrafish embryos (Fig. 2). Surprisingly, injection of Period2 morpholino into developing zebrafish embryos led to accelerated rather than decreased ISV development (Fig. 2). These findings demonstrate, for the first time, that the circadian clock plays a major role in regulation of vascular development in zebrafish embryos, and that Bmal1 and Period2 might display opposing functions in modulation of angiogenesis.
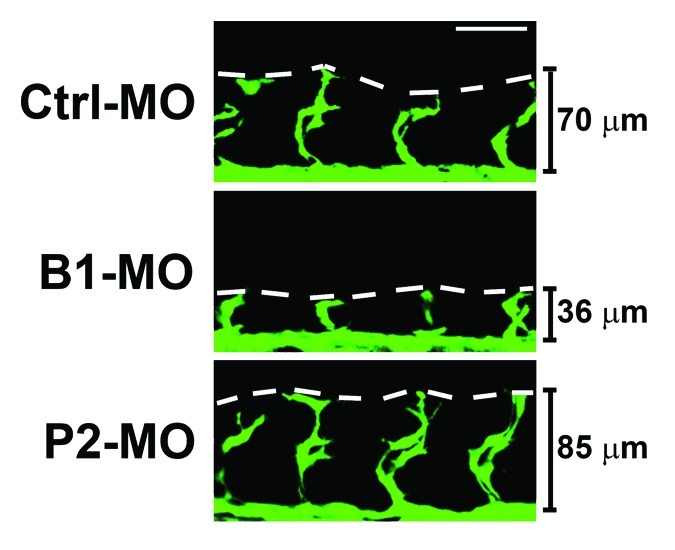
Figure 2. Bmal1 or Period2 deficiency led to opposing effects on developmental angiogenesis. Transgenic fli1:EGFP zebrafish embryos expressing GFP in endothelial cells were injected with 0.2 picomole of scrambled control or specific Bmal1 or Period2 morpholino at 15 min post-fertilization. Images of the vasculature were obtained by confocal microscopy at 24-hpf, and representative images of the ISVs are shown. It is clear that Bmal1 morpholino injection lead to impaired ISV development, whereas Period2 morpholino injection lead to enhanced ISV development compared with scrambled control morpholino injected embryos. The lines indicate the height of the ISV vessels. The white scale bar is 50 μm.
Bmal1 Modulates VEGF-Dependent Angiogenesis
Since Bmal1 is critically involved in the circadian clock-regulated angiogenesis, we next studied the signaling system that mediates Bmal1-regulated angiogenesis. VEGF is known as one of the key factors in modulation of developmental angiogenesis in zebrafish.20,24 Indeed, VEGF expression levels in zebrafish embryos also exhibited a circadian rhythm, which was well coordinated with Bmal1 expression levels. Similar to the Bmal1 rhythmic pattern, LL exposure nearly completely impaired the rhythmic expression pattern of zebrafish VEGF. We analyzed the promoter region of the zebrafish Vegf gene and revealed the existence of several potential Bmal1-binding E-boxes. We showed that Bmal1 directly binds to the promoter region of the Vegf gene and controls the promoter activity. Interestingly, deletion of these E-boxes in the promoter region of the zebrafish Vegf gene results in inactivation of the promoter activity.23 These findings demonstrate that Bmal1 transcriptionally targets VEGF to regulate its expression levels that exhibit the circadian rhythmic pattern.
Circadian Clock Modulates Notch Signaling-Mediated Angiogenesis
It is known that the Notch signaling system reciprocally and intimately interacts with the VEGF signaling pathway for vascular development in embryos.25,26 Similar to VEGF-defective mice, deletion of only one allele of the Dll4 (a Notch ligand) gene resulted in a haploinsufficient phenotype, where the embryos die during development owing to vascular defects.27,28 The Notch signaling is required for prevention of undirected vascular sprouting in angiogenic vessels.29-32 We used three independent approaches to study the role of the notch signaling system in relation to the circadian clock in regulation of developmental angiogenesis: (1) administration of DAPT, a γ-secretase inhibitor, to zebrafish embryos results in the formation of high-density vascular networks and excessive vascular sprouts; (2) delivery of a morpholino specifically targeting recombining binding protein suppressor of hairless (rbpsuh) leads to excessive angiogenesis; (3) genetic deletion of mind-bomb, a ubiquitin ligase, required for mediating the Notch signaling also results in an enhanced angiogenic phenotype. However, delivery of the Bmal1 morpholino to zebrafish embryos significantly reduced the Notch inhibition-induced angiogenesis.23 It is likely that inhibition of Bmal1 would significantly reduce the expression level of VEGF as one of its target genes.
Conclusion Remarks and Perspectives
The circadian clock system has been known to regulate multiple physiological and pathological processes in mammals. Our present study presents one of the first examples that the circadian clock plays a crucial role in regulation of developmental angiogenesis, which is essential for development of multiple tissues and organs in embryos. One of the most intriguing findings in our study is that expression levels of VEGF, as one of the key angiogenic factors, also exhibit a circadian rhythm. Indeed, VEGF is a direct transcriptional target of Bmal1 and is essentially involved in modulating the circadian clock-regulated developmental angiogenesis. Despite the existence of obvious vascular phenotypes in clock-disrupted zebrafish embryos, the development of zebrafish does not seem to be affected by defective angiogenesis. One possible explanation is that developing embryos have a robust compensatory mechanism against defective angiogenesis.33,34 Indeed, vasculogenesis is another major process of neovascularization during embryogenesis and the role of the circadian clock in regulation of vasculogenesis remains uncharacterized. Another possibility is that early embryos have an extremely high tolerance to hypoxia.35,36 Reduction of blood vessels by disruption of the circadian clock might not lead to major developmental defects.
Although our findings have been obtained from zebrafish, they can reasonably be extended to mammals, especially humans, as the circadian clock genes are conserved and play crucial roles in regulation of multiple physiological processes in humans. It is also likely that the circadian clock controls pathological angiogenesis, such as tumor angiogenesis. Since VEGF is involved in the onset, development and progression of various human disorders such as cancer,37,38 ophthalmological disorders,25 cardiovascular disease39 and obesity,18,19 it is highly plausible that circadian clock-regulated VEGF expression levels are involved in pathological angiogenesis in these common and lethal human diseases. Thus, targeting the circadian clock-regulated angiogenic pathway would, in principle, provide a therapeutic option for treatment of these common human diseases.
Acknowledgments
We thank Dr. Jeannette Söderberg for critical reading of the manuscript. Y.C.’s laboratory is supported by research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute Distinguished Professor Award, the Torsten Söderbergs Foundation, Söderbergs stiftelse, the Tianjin Natural Science Foundation (CMM-Tianjin, No. 09ZCZDSF04400) for international collaboration between Tianjin Medical University and Karolinska Institutet, ImClone Systems Inc./EliLilly, the European Union Integrated Project of Metoxia (Project no. 222741), and the European Research Council (ERC) advanced grant ANGIOFAT (Project no 250021). L.D.J. is supported by grants from Svenska Sällskapet för Medicinsk Forskning, Lions Forskningsfond mot Folksjukdomar, Karolinska Institutets Stiftelser, LiU-Cancer and Åke-Wibergs Forskningsfond.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23596
References
- 1.Bonny O, Firsov D. Circadian clock and the concept of homeostasis. Cell Cycle. 2009;8:4015–6. doi: 10.4161/cc.8.24.10224. [DOI] [PubMed] [Google Scholar]
- 2.Sahar S, Sassone-Corsi P. Circadian clock and breast cancer: a molecular link. Cell Cycle. 2007;6:1329–31. doi: 10.4161/cc.6.11.4295. [DOI] [PubMed] [Google Scholar]
- 3.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–22. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 4.Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, D’Agostino RB. Temporal patterns of stroke onset. The Framingham Study. Stroke. 1995;26:1343–7. doi: 10.1161/01.STR.26.8.1343. [DOI] [PubMed] [Google Scholar]
- 5.Shea SA. Obesity and pharmacologic control of the body clock. N Engl J Med. 2012;367:175–8. doi: 10.1056/NEJMcibr1204644. [DOI] [PubMed] [Google Scholar]
- 6.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol. 2007;72:551–6. doi: 10.1101/sqb.2007.72.033. [DOI] [PubMed] [Google Scholar]
- 8.McLoughlin S, Fitzgerald GA. Between a ROCK and a Hard Place: How to Align our Circadian Rhythms? Circulation. 2013;127:19–20. doi: 10.1161/CIRCULATIONAHA.112.150417. [DOI] [PubMed] [Google Scholar]
- 9.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama J, Miyamura N, Uchida Y, Asaoka Y, Honda R, Sawanobori K, et al. Common light signaling pathways controlling DNA repair and circadian clock entrainment in zebrafish. Cell Cycle. 2009;8:2794–801. doi: 10.4161/cc.8.17.9447. [DOI] [PubMed] [Google Scholar]
- 11.Ripperger JA, Schmutz I, Albrecht U. PERsuading nuclear receptors to dance the circadian rhythm. Cell Cycle. 2010;9:2515–21. doi: 10.4161/cc.9.13.12075. [DOI] [PubMed] [Google Scholar]
- 12.Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–98. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao R, Cao Y. Cancer-associated retinopathy: a new mechanistic insight on vascular remodeling. Cell Cycle. 2010;9:1882–5. doi: 10.4161/cc.9.10.11521. [DOI] [PubMed] [Google Scholar]
- 15.Rouhi P, Lee SL, Cao Z, Hedlund EM, Jensen LD, Cao Y. Pathological angiogenesis facilitates tumor cell dissemination and metastasis. Cell Cycle. 2010;9:913–7. doi: 10.4161/cc.9.5.10853. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y. Direct role of PDGF-BB in lymphangiogenesis and lymphatic metastasis. Cell Cycle. 2005;4:228–30. doi: 10.4161/cc.4.2.1421. [DOI] [PubMed] [Google Scholar]
- 17.Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–88. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 20.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–8. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen LD, Cao R, Cao Y. In vivo angiogenesis and lymphangiogenesis models. Curr Mol Med. 2009;9:982–91. doi: 10.2174/156652409789712738. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Langer R. Optimizing the delivery of cancer drugs that block angiogenesis. Sci Transl Med. 2010;2:ps3. doi: 10.1126/scitranslmed.3000399. [DOI] [PubMed] [Google Scholar]
- 23.Jensen LD, Cao Z, Nakamura M, Yang Y, Bräutigam L, Andersson P, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2012;2:231–41. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao R, Jensen LD, Söll I, Hauptmann G, Cao Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE. 2008;3:e2748. doi: 10.1371/journal.pone.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–29. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101:15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 29.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–4. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 30.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 31.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 32.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–80. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1:1143–7. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 34.Jensen LD, Rouhi P, Cao Z, Länne T, Wahlberg E, Cao Y. Zebrafish models to study hypoxia-induced pathological angiogenesis in malignant and nonmalignant diseases. Birth Defects Res C Embryo Today. 2011;93:182–93. doi: 10.1002/bdrc.20203. [DOI] [PubMed] [Google Scholar]
- 35.Rouhi P, Jensen LD, Cao Z, Hosaka K, Länne T, Wahlberg E, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc. 2010;5:1911–8. doi: 10.1038/nprot.2010.150. [DOI] [PubMed] [Google Scholar]
- 36.Cao Z, Jensen LD, Rouhi P, Hosaka K, Länne T, Steffensen JF, et al. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc. 2010;5:1903–10. doi: 10.1038/nprot.2010.149. [DOI] [PubMed] [Google Scholar]
- 37.Xue Y, Religa P, Cao R, Hansen AJ, Lucchini F, Jones B, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA. 2008;105:18513–8. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SL, Rouhi P, Dahl Jensen L, Zhang D, Ji H, Hauptmann G, et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA. 2009;106:19485–90. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lähteenvuo JE, Lähteenvuo MT, Kivelä A, Rosenlew C, Falkevall A, Klar J, et al. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–56. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]


