Abstract
AIM: To investigate lipopolysaccharide (LPS) related signal transduction in interstitial cells of Cajal (ICCs) from mouse small intestine.
METHODS: For this study, primary culture of ICCs was prepared from the small intestine of the mouse. LPS was treated to the cells prior to measurement of the membrane currents by using whole-cell patch clamp technique. Immunocytochemistry was used to examine the expression of the proteins in ICCs.
RESULTS: LPS suppressed the pacemaker currents of ICCs and this could be blocked by AH6809, a prostaglandin E2-EP2 receptor antagonist or NG-Nitro-L-arginine Methyl Ester, an inhibitor of nitric oxide (NO) synthase. Toll-like receptor 4, inducible NO synthase or cyclooxygenase-2 immunoreactivity by specific antibodies was detected on ICCs. Catalase (antioxidant agent) had no action on LPS-induced action in ICCs. LPS actions were blocked by nuclear factor κB (NF-κB) inhibitor, actinomycin D (a gene transcription inhibitor), PD 98059 (a p42/44 mitogen-activated protein kinases inhibitor) or SB 203580 [a p38 mitogen-activated protein kinases (MAPK) inhibitor]. SB 203580 also blocked the prostaglandin E2-induced action on pacemaker currents in ICCs but not NO.
CONCLUSION: LPS inhibit the pacemaker currents in ICCs via prostaglandin E2- and NO-dependent mechanism through toll-like receptor 4 and suggest that MAPK and NF-κB are implicated in these actions.
Keywords: Interstitial cells of Cajal, Lipopolysaccharide, Mitogen-activated protein kinases, Nuclear factor κB, Small intestine
INTRODUCTION
Many morphological or functional studies have disclosed the roles of interstitial cells of Cajal (ICCs) in many organs including gastrointestinal (GI) tract. An accumulation of evidence from these studies has revealed ICCs has the distinct role in pacemaker activity[1-3] and as mediators of enteric motor neurotransmission in GI tract[4-6]. For development and maintenance, the ICCs express the receptor tyrosine kinase Kit[1,3,7]. And, it is well known that c-Kit or ANO1 can be a selective molecular marker for researching the distribution and function of ICCs[8].
GI inflammation can be cause by inflammatory cytokines such as interleukins, chemical inflammatory mediators etc and lipopolysaccharide (LPS) from gram-negative bacteria is a major causative factor of GI inflammation[9]. Various vivo and vitro experiments showed LPS could play a major role in GI motility disorders[10-13]. Also, there are several reports that the morphological and functional changes of ICCs are involved in inflammation-induced GI motility[14-16]. These indicate ICCs may be important target for inflammation-induced motility disorders. Recently we reported that LPS inhibited the pacemaker currents in cultured ICCs from mouse small intestine. LPS-action was blocked by cyclooxygenase (COX)-2 inhibitor or nitric oxide (NO) synthase inhibitor, suggesting prostaglandins (PGs) and NO are involve in these actions[17], can verify this.
Generally, the stimulation of LPS rouses regulatory pathways such as the nuclear factor κB (NF-κB) pathway, as well as reactive oxygen species (ROS) signaling cascades via a toll like receptor-4-mediated signaling pathway[18]. Furthermore, the stimulation of LPS results in the activation of kinases including extracellular signal-regulated kinases, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPKs) critical for growth and cytokine production that induces inflammatory-related substances, such as interleukin, PGs, inducible NO synthase (iNOS) etc[19-21]. Our previous report suggested production of inflammatory-related substances, such as prostaglandin E2 (PGE2) or NO are involved in LPS-induced action on ICCs[17]. However, the details of the PGs or iNOS induction in ICCs by LPS stimulation remain unclear.
In the present study, we aimed to clarify the involvement of NF-κB, ROS and MAPKs that contribute to PGs or iNOS induction in ICCs and changing of pacemaker activity in LPS-treated ICCs.
MATERIALS AND METHODS
Solutions and drugs
The cells were bathed in a solution containing 5 mmol/L KCl, 135 mmol/L NaCl, 2 mmol/L CaCl2, 10 mmol/L glucose, 1.2 mmol/L MgCl2, and 10 mmol/L HEPES, adjusted to pH 7.2 with Tris. NaCl was replaced with equimolar N-methyl-D-glucamine for making Na+-free solution, and CaCl2 was omitted in the bath solution for Ca2+-free solution. The pipette solution contained 20 mmol/L K-aspartate, 120 mmol/L KCl, 5 mmol/L MgCl2, 2.7 mmol/L K2ATP, 0.1 mmol/L Na2GTP, 2.5 mmol/L creatine phosphate disodium, 5 mmol/L 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), and 0.1 mmol/L EGTA, adjusted to pH 7.2 with Tris.
The drugs used were LPS (salmonella enteric serotype typhimurium), JNK inhibitor II, SN-50 (NF-κB inhibitor) and catalase from Calbiochem, (San Diego, CA, United States) and the other compounds were purchased from Sigma.
Preparation of cells and tissues
Balb/C mice (3-8 d old) of either sex were anesthetized with ether and were sacrificed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the cecum were removed and opened along the mesenteric border. The luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa was removed by sharp dissection. Small strips of intestinal muscle were equilibrated in Ca2+-free Hank’s solution containing 5.36 mmol/L KCl, 125 mmol/L NaCl, 0.34 mmol/L NaOH, 0.44 mmol/L Na2HCO3, 10 mmol/L glucose, 2.9 mmol/L sucrose, and 11 mmol/L HEPES for 30 min, and the cells were dispersed with an enzyme solution containing 1.3 mg/mL collagenase (Worthington Biochemical Co, Lakewood, NJ, United States), 2 mg/mL bovine serum albumin (Sigma Chemical Co., St. Louis, MO, United States), 2 mg/mL trypsin inhibitor (Sigma) and 0.27 mg/mL ATP. Cells were plated onto sterile glass coverslips coated with poly L-lysine (2.5 μg/mL, Sigma) in 35 mm culture dishes. The cells were then cultured at 37 °C in a 5% CO2 incubator in SMGM (smooth muscle growth medium) (Clonetics Co., Walkersville, MD, United States) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, United States) and murine stem cell factor (SCF, 5 ng/mL, Sigma). ICCs were identified immunologically with the use of a monoclonal antibody for kit protein (ACK2) labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR, United States).
Patch clamp experiments
The whole-cell configuration of the patch clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from cultured ICC after 2-3 d in culture. We recorded from small clusters of ICC, as spontaneous inward currents from small groups of cells are more robust and regular than from single cells. Currents or potentials were amplified by Axopatch 200B (Axon Instruments, Foster, CA, United States). A command pulse was applied using an IBM compatible personal computer and pClamp software (version 9.2; Axon Instruments). The data were filtered at 5 kHz, and the recorded data were displayed on a computer monitor saved for data analysis and future references. Results were analyzed using the Clampfit program (Axon Instruments) and Graph Pad Prism (version 2.01) software. All experiments were performed at 30 °C.
Immunocytochemistry
Cultured cells were fixed in acetone (20 °C/5 min). Following fixation, preparations were washed for 60 min in phosphate buffered saline (PBS; 0.01 mol, pH 7.4). Cultured cells were then incubated in 10% goat serum containing 1% bovine serum albumin for 1 h at RT to reduce nonspecific antibody binding. For double immunostaining, cells were incubated overnight at 4 °C in a mixture of two primary antibodies raised in different species (a rat monoclonal c-Kit antibody, 1:200, Gibco-BRL, Gaithersburg, MD, United States; a goat polyclonal TLR4 antibody, 1:100, Santa Cruz Biotechnology, CA, United States; a goat polyclonal iNOS antibody, 1:100, Santa Cruz Biotechnology; a goat polyclonal COX-2 antibody, 1:100, Santa Cruz Biotechnology). The secondary antibodies were fluorescein isothiocyanate (FITC) for c-Kit and Texas Red for anti-TLR4, iNOS or COX-2, diluted 1:100 (all reagents from Vector Laboratories, Burlingame, CA). Immunoreactivity was detected using FITC-conjugated secondary antibody (FITC-anti-rat; Vector Laboratories, 1:100 in PBS, 1 h, room temperature). Control cultured ICCs were prepared in a similar manner, but, omitting ACK2 from the incubation medium. After washing with PBS, the mixture of labeled secondary antibodies was treated with FITC-conjugated donkey anti-rabbit IgG (1:100, Chemicon, Temecula, CA, United States) and Texas red-conjugated donkey anti-goat IgG (1:100, Jackson Immunoresearch Laboratories, Baltimore, PA, United States). The incubation of labeled secondary antibodies was performed for 1 h at room temperature. Cells were examined with a FV300 confocal microscope (Olympus, Tokyo, Japan) with an excitation wavelength appropriate for FITC (488 nm) and Texas Red (568 nm). Final images were constructed with Flow View Software Program (Olympus, Tokyo, Japan).
Ethics
All experiments were performed according to the Guiding Principles for the Care and Use of Animals approved by the Ethics Committee of Chosun University and the National Institute of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize both the number of animals used and the suffering of the animals.
Statistical analysis
Data are expressed as the mean ± SE. Differences in the data were evaluated by use of the Student’s t test. P values less than 0.05 are considered a statistically significant difference. The n values reported in the text refer to the number of cells used in the patch clamp experiments.
RESULTS
Confirmation of prostaglandin or NO production involvement on LPS-induced action in ICCs
First, we verified inhibitory action of LPS on ICCs and the involvement of PG or NO production on LPS-induced action. Recordings were made from cells within networks that had morphologies similar to the cells that were immunopositive for c-Kit. Under voltage clamp mode at a holding potential of -70 mV, ICCs showed spontaneous inward pacemaker currents (Figure 1A) and this inhibited by 200 μg/mL LPS incubation for 12 h (Figure 1B). And inhibitory action of LPS on pacemaker activity in ICCs was blocked by 10 μmol/L AH6809 (a PGE2-EP2 receptor antagonist) or 10 μmol/L NG-Nitro-L-arginine Methyl Ester (L-NAME) (an inhibitor of NO synthase) (n = 5, Figure 1C and D, bar graph not shown). These could make sure the involvement of PG or NO production on LPS-induced action in ICCs.
Figure 1.
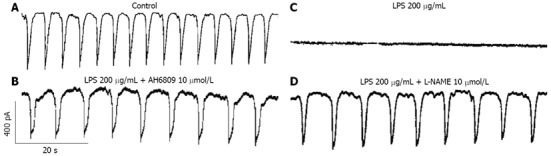
Effects of AH6809 or NG-Nitro-L-arginine Methyl Ester on lipopolysaccharide-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) at a holding potential of -70 mV in control condition; B: Pacemaker currents of ICCs incubated at 37 °C with 200 μg/mL of lipopolysaccharide (LPS) for 12 h; C: Pacemaker currents in ICCs pretreated with AH6809 (10 μmol/L) for 2 h prior to LPS incubation; D: Pacemaker currents in ICCs pretreated with NG-Nitro-L-arginine Methyl Ester (10 μmol/L) for 2 h prior to LPS incubation. LPS: Lipopolysaccharide; L-NAME: NG-Nitro-L-arginine Methyl Ester.
Localization of toll like receptor-4, inducible NO synthase and COX-2 in ICCs
For searching the ability that can interact with TLR4 and produce NO or PGs in ICCs, we tried immunocytochemistry with specific antibody for TLR4, iNOS and COX-2 proteins. Double staining with anti-c-Kit and anti-TLR4, anti-iNOS or anti-COX-2 antibodies revealed TLR4, iNOS and COX-2 immunoreactivity in c-Kit immune-positive ICCs (Figure 2A-C).
Figure 2.
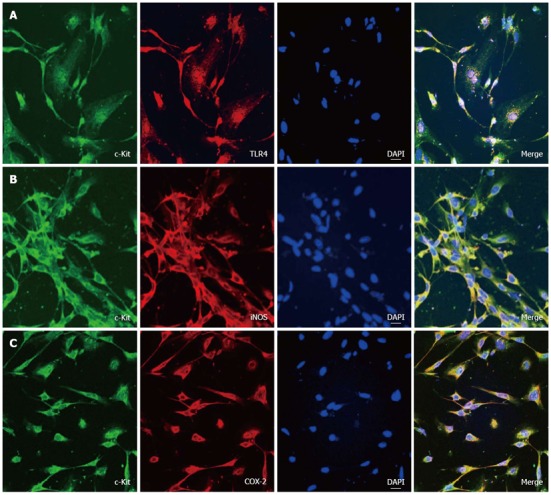
Expression of TLR4, iNOS and COX-2 in c-Kit positive cells. A: Double labeling of TLR4- and c-Kit-like immunoreactivity in cultured interstitial cells of Cajal (ICCs). Green (c-Kit) and red (TLR4) result in the mixed color yellow, indicating the colocalization of both peptides; B: Double labeling of iNOS and c-Kit-like immunoreactivity in ICCs. Green (c-Kit) and red (iNOS) result in the mixed color yellow, indicating the colocalization of both peptides; C: Double labeling of COX-2 and c-Kit-like immunoreactivity in ICCs. Green (c-Kit) and red (COX-2) result in the mixed color yellow, indicating the colocalization of both peptides (bar = 20 μm).
Effects of catalase on LPS-induced action in ICCs
For finding the involvement of ROS production on LPS-induced action, we used the antioxidant reagent, catalase. To investigate the effect of LPS, ICCs were incubated with 200 μg/mL LPS for 12 h and we could reconfirm the disappearance of pacemaker currents (Figure 3A). Pretreatment with catalase (3000 unit/mL) for 2 h prior to 200 μg/mL LPS incubation could not show any difference compared with LPS alone (Figure 3B). The frequency and amplitude values of pacemaker currents in catalase treatment with LPS were not significantly different compared with LPS alone (n = 4, Figure 3C and D).
Figure 3.
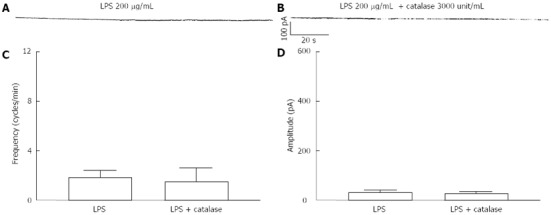
Effects of catalase on lipopolysaccharide-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) incubated at 37 °C with 200 μg/mL of lipopolysaccharide (LPS) for 12 h; B: Pacemaker currents in ICCs pretreated with catalase (3000 unit/mL) for 2 h prior to LPS incubation. The effects of catalase on the LPS-induced action in ICCs are summarized in C and D. Bars represent mean ± SE values (n = 4 per group).
Effects of SN-50 or actinomycin D on lipopolysacchardie-induced action in ICCs
For detecting the role for NF-κB or involvement of gene transcriptions on LPS-induced action, we used SN-50, the NF-κB inhibitor and actinomycin D, the gene transcription inhibitor. The incubation with LPS 200 μg/mL alone, the pacemaker currents were completely inhibited (Figure 4A). But when SN-50 (10 μmol/L) was treated for 2 h prior to LPS incubation, LPS-induced action on pacemaker currents in ICCs was blocked (Figure 4B). Also, we could find that actinomycin D (10 μmol/L) blocked the LPS-induced pacemaker currents suppression (Figure 4C). The values of frequency and amplitude induced by LPS in the presence of SN-50 or actinomycin D were significantly different from those obtained in the absence of NF-κB inhibitor (n = 5, Figure 4D and E).
Figure 4.
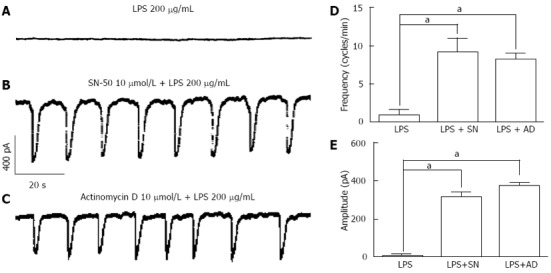
Effects of SN-50 or actinomycin D on lipopolysaccharide-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) incubated at 37 °C with 200 μg/mL of lipopolysaccharide (LPS) for 12 h at a holding potential of -70 mV; B: Pacemaker currents in ICCs pretreated with SN-50 (10 μmol/L) for 2 h prior to LPS incubation; C: Pacemaker currents in ICCs pretreated with actinomycin D (10 μmol/L) for 2 h prior to LPS incubation. The effects of SN-50 or actinomycin D on the LPS-induced action in ICCs are summarized in D and E. Bars represent mean ± SE values (n = 5 per group). aP < 0.05 vs LPS alone treatment. SN: SN-50, AD: Actinomycin D.
Effects of MAPK inhibitors on LPS-induced action in ICCs
For understanding the involvement of MAPKs on LPS-induced action, specific MAPKs inhibitors were used. When the pretreatments of ICCs with PD 98059 (a selective p42/44 inhibitor), SB 203580 (a selective p38 inhibitor) or JNK inhibitor II for 2 h prior to 200 μg/mL LPS incubation, the LPS-induced inhibitory action on pacemaker currents were blocked by PD 98059 (10 μmol/L), SB 203580 (10 μmol/L) but not by JNK inhibitor II (10 μmol/L) (Figure 5B-D). The values of frequency, amplitude and resting currents induced by LPS in the presence of PD 98059 or SB 203580 were significantly different from those obtained in the absence of these (n = 7, Figure 5E and F).
Figure 5.
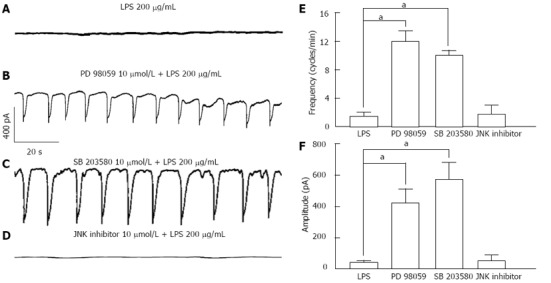
Effects of Mitogen-activated protein kinases inhibitors on lipopolysaccharide-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) incubated at 37 °C with 200 μg/mL of lipopolysaccharide (LPS) for 12 h at a holding potential of -70 mV; B: Pacemaker currents after pretreatment with PD 98059 (10 μmol/L) for 2 h prior to LPS incubation; C: Pacemaker currents after pretreatment with SB 203580 (10 μmol/L) for 2 h prior to LPS incubation; D: Pacemaker currents after pretreatment with c-Jun N-terminal kinase (JNK) inhibitor II (10 μmol/L) for 2 h prior to LPS incubation. The effects of mitogen-activated protein kinases inhibitors on the LPS-induced action are summarized in E and F. Bars represent mean ± SE values (n = 7 per group). aP < 0.05 vs LPS.
Effects of MAPK inhibitors on Prostaglandin E2-induced action in ICCs
For examining the involvement of MAPKs on PGE2 production by LPS stimulation in ICCs, we used p42/44 inhibitor and p38 inhibitor with PGE2. Because JNK inhibitor II did not show any influence on LPS-induced action in ICCs, we did not test with JNK inhibitor II in here. At first, we could see PGE2 (5 μmol/L) itself has the inhibitory effect on pacemaker currents in ICCs (Figure 6A). Then, we examined the effects of PGE2 on pacemaker currents in presence of PD 98059 or SB203580. The pretreatments of ICCs with PD 98059 (10 μmol/L) blocked the 5 μmol/L PGE2-induced inhibitory action on pacemaker currents but not SB 203580 (10 μmol/L) (Figure 6B and C). The values of frequency and amplitude currents induced by PGE2 in the presence of PD 98059 were significantly different from those obtained in the absence of PD 98059 (n = 4, Figure 6D and E).
Figure 6.
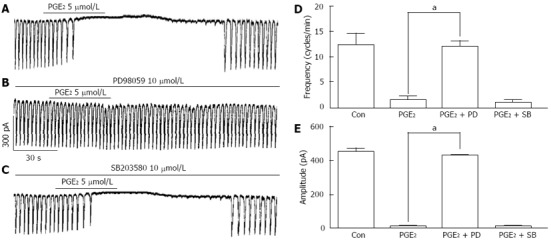
Effects of mitogen-activated protein kinases inhibitors on prostaglandin E2-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) treated with 5 μmol/L prostaglandin E2 (PGE2) at a holding potential of -70 mV; B: Pacemaker currents after pretreatment with PD 98059 (10 μmol/L) prior to PGE2 treatment; C: Pacemaker currents after pretreatment with SB 203580 (10 μmol/L) prior to PGE2 treatment. The effects of mitogen-activated protein kinases inhibitors on the PGE2-induced action are summarized in D and E. Bars represent mean ± SE values (n = 4 per group). aP < 0.05 vs PGE2. Con: Control, PD: PD 98059, SB: SB203580.
Effects of MAPK inhibitors on NO-induced action in ICCs
For examing the role of MAPKs on NO production by LPS stimulation in ICCs, we also used p42/44 inhibitor and p38 inhibitor with (±)-S-nitroso-N-acetylpenicillamine (SNAP), a NO donor. In our previous report[22], we already showed SNAP has inhibitory action on pacemaker currents in ICCs like as PGE2. In here, we also could see the inhibitory action of 100 μmol/L SNAP on pacemaker currents (Figure 7A). We examined the effects of SNAP on pacemaker currents in presence of PD 98059 or SB 203580. The pretreatments of ICCs with PD 98059 (10 μmol/L) or SB 203580 (10 μmol/L) did not show any influence on 5 μmol/L SNAP-induced inhibitory action on pacemaker currents (Figure 7B and C). The values of frequency and amplitude induced by SNAP in the presence of PD 98059 or SB 203580 were not significantly different from those obtained in the absence of PD 98059 or SB 203580 (n = 5, Figure 7E and F). Furthermore, we checked the reciprocal relation between PG and NO with PGE2 and L-NAME. In presence of 10 μmol/L L-NAME, 5 μmol/L PGE2 still inhibited the pacemaker current (n = 4, Figure 7D, bar graph not shown).
Figure 7.
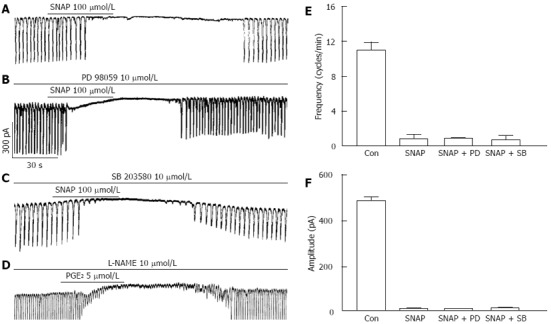
Effects of mitogen-activated protein kinases inhibitors on (±)-S-nitroso-N-acetylpenicillamine-induced action in interstitial cells of Cajal. A: Pacemaker currents of interstitial cells of Cajal (ICCs) treated with 100 μmol/L SNAP at a holding potential of -70 mV; B: Pacemaker currents after pretreatment with PD 98059 (10 μmol/L) prior to SNAP treatment; C: Pacemaker currents after pretreatment with SB 203580 (10 μmol/L) prior to SNAP treatment; D: Pacemaker currents after pretreatment with L-NAME (10 μmol/L) prior to prostaglandin E2 (PGE2) (5 μmol/L) treatment. The effects of mitogen-activated protein kinases inhibitors on the (±)-S-nitroso-N-acetylpenicillamine-induced action are summarized in E and F. Bars represent mean ± SE values (n = 5 per group). Con: Control, PD: PD 98059, SB: SB203580; SNAP: S-nitroso-N-acetylpenicillamine; L-NAME: NG-Nitro-L-arginine Methyl Ester.
DISCUSSION
PGE2 and NO are inhibitory mediators on intestinal motility and produced by COX (COX-1 or 2) and NOS (nNOS/eNOS/iNOS), respectively. We have shown that LPS inhibited pacemaker currents of intestinal ICCs by activating ATP-sensitive K+ channels through the release of PGE2 and NO[17]. In this experiment, we further found that MAPKs are involving in LPS-induced inhibition of pacemaker currents through NF-κB-dependent mechanisms in ICCs.
The binding of TLR4 with LPS activates NF-κB through myeloid differentiation primary response gene-88 (Myd88)-dependent pathway. LPS has been shown to induce NF-κB nuclear translocation in intestinal smooth muscle and myenteric plexus cells, but not in TLR4 knockout mice[23]. The activated NF-κB then induces various transcriptional genes including COX-2 and iNOS in GI tracts. In this study, LPS-induced inhibition of pacemaker currents was blocked by COX-2 inhibitor or iNOS inhibitor. And a specific NF-κB blocker or actinomycin D, a gene transcriptional blocker, suppressed LPS-induced inhibition of pacemaker currents. Furthermore, double staining with anti-c-Kit and anti-COX-2 antibodies or anti-iNOS antibodies revealed COX-2 or iNOS immunoreactivity in c-Kit immunopositive ICCs in cultured ICCs. These results suggest that NF-κB activation is essential to induce expression of COX-2 and iNOS exposed to LPS in ICCs.
MAPKs play an important role in the mediation of cellular responses, including transcriptional responses. Three types of MAPKs (p42/44 MAPK, p38 MAPK, and JNK) are involved in LPS-induced NF-κB or JNK activator protein-1 activation in various cells[24-27]. Recently, it was reported that three types of MAPKs were involved in the mechanism of LPS actions in rabbit ileum and the blockade of p38, JNK or p42/44 pathways suppressed the effects of LPS on the acetylcholine-induced contractions[28]. In our study, the LPS-induced effects in ICCs were blocked by PD98059 or SB20356, but not by JNK inhibitor II, indicating that activation of p42/44 and p38 MAPKs are involved in LPS-induced activation of NF-κB in ICCs. These results are consistent with those of others showing that p42/44 and p38 MAPKs are involved in COX-2 and iNOS induction by LPS in tracheal smooth muscle cells[26]. However, this study showed PGE2-induced inhibition of pacemaker currents is blocked by p42/44 MAPK inhibitor, but not by p38 MAPK inhibitor in intestinal ICCs and NO-induced inhibition of pacemaker currents was not blocked by both p42/44 MAPK inhibitor and p38 MAPK inhibitor. These results suggest that p42/44 MAPK and p38 MAPK play differential role in regulation of LPS-induced actions in ICCs. Thus, it seems that p38 MAPK may be involved in LPS-induced NF-κB activation to express COX-2 and iNOS, whereas p42/44 MAPK as a downstream pathway of NF-κB may be involved in PGE2-induced effects in ICCs not NO signaling. Our previous study could explains the possibility that NO-induced action on pacemaker currents is via activating ATP-sensitive K+ channels through cGMP-dependent mechanism in ICCs but not involvement of MAPKs[22].
LPS action was also mediated by ROS production through a nicotinamide adenine dinucleotide phosphate oxidase pathway. In rat aortic smooth muscles, LPS could induce the hydrogen peroxide signaling[29]. Also, we found that H2O2 inhibited pacemaker currents by activating ATP-sensitive K+ channels in ICCs, which was mediated through COX-2-dependent PGE2 production[30]. Therefore, in order to investigate whether H2O2 is involved in LPS-induced PGE2 production, we treated catalase, a hydrogen peroxide scavenger. However, catalase did not block the LPS-induced inhibition of pacemaker currents. This result suggests that hydrogen peroxide do not involve in LPS-induced actions in intestinal ICCs.
Many reports are arguing the relation between the production of COX and NOS. NO production by NOS enzyme could stimulate or inhibit the COX enzyme induction[31,32]. And some reports suggested products by COX enzyme stimulate the NOS pathway[33,34]. In our opinion, the cross talk between NOS and COX may depend on the cell type or enzyme type. In here, we need to check whether inhibitory effect of PGE2 on pacemaker activity is through induction of NO synthesis in ICCs. However, the proofs that PGE2 alone could inhibit the pacemaker activity in ICCs in our previous study and L-NAME could not show any influence on PGE2-induced action in here could establish no-interaction between the products of COX and NOS by LPS stimulation in ICCs.
In summary, the current study provides evidence that LPS inhibited pacemaker currents in cultured ICCs via PGE2 and NO-dependent pathways. NF-κB and MAP kinases are involved in this inhibitory action.
COMMENTS
Background
Interstitial cells of Cajal (ICCs) are the pacemaker cells present in the gastrointestinal tract (GI) that generate the pacemaker currents that regulate the GI motility. Lipopolysaccharides (LPS) are endotoxins and pro-inflammatory agents present in the cell wall of the Gram-negative bacteria responsible for various alterations of gastrointestinal functions including intestinal dysmotility.
Innovations and breakthroughs
LPS altered GI motility through release of prostaglandins or nitric oxide (NO) by nuclear factor κB (NF-κB) dependent gene transcription. This study showed LPS inhibited pacemaker currents of ICC. Prostaglandin E2 and NO contributed to the inhibitory effects of LPS together with mitogen-activated protein kinases and NF-κB.
Applications
LPS-induced inhibitory actions on pacemaker activities of ICC may be one possible mechanism to explain LPS-induced GI dysmotility.
Terminology
ICCs refer to the several types of cells present on the musculature of the GI tract. ICCs act as pacemaker cells in gastrointestinal tracts, regulating rhythmicity by activating calcium activated chloride channel or nonselective cation channels.
Peer review
The functional regulation of ICCs is still not fully understood and this study provides novel information on the TLR 4-mediated signaling pathways on LPS–suppressed pacemaker currents of ICCs.
Footnotes
Supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, No. 2012-0001335
P- Reviewers Alireza M, Chen XM S- Editor Jiang L L- Editor A E- Editor Lu YJ
References
- 1.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 2.Ordög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518(Pt 1):257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Park H, Calrk E, Cullen JJ, Conklin JL. Effect of endotoxin on opossum oesophageal motor function. Neurogastroenterol Motil. 2000;12:215–221. doi: 10.1046/j.1365-2982.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 11.van Miert AS, van Duin CT. Endotoxin-induced inhibition of gastric emptying rate in the rat. The effect of repeated administration and the influence of some antipyretic agents. Arch Int Pharmacodyn Ther. 1980;246:19–27. [PubMed] [Google Scholar]
- 12.Wechsung E. [The involvement of prostaglandins in the inhibiting effect of endotoxin on the myoelectric activity of the gastrointestinal system in pigs] Verh K Acad Geneeskd Belg. 1996;58:711–738. [PubMed] [Google Scholar]
- 13.Weisbrodt NW, Pressley TA, Li YF, Zembowicz MJ, Higham SC, Zembowicz A, Lodato RF, Moody FG. Decreased ileal muscle contractility and increased NOS II expression induced by lipopolysaccharide. Am J Physiol. 1996;271:G454–G460. doi: 10.1152/ajpgi.1996.271.3.G454. [DOI] [PubMed] [Google Scholar]
- 14.Der T, Bercik P, Donnelly G, Jackson T, Berezin I, Collins SM, Huizinga JD. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. 2000;119:1590–1599. doi: 10.1053/gast.2000.20221. [DOI] [PubMed] [Google Scholar]
- 15.Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997;273:G1233–G1245. doi: 10.1152/ajpgi.1997.273.6.G1233. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Berezin I, Mikkelsen HB, Der T, Bercik P, Collins SM, Huizinga JD. Pathology of interstitial cells of Cajal in relation to inflammation revealed by ultrastructure but not immunohistochemistry. Am J Pathol. 2002;160:1529–1540. doi: 10.1016/s0002-9440(10)62579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo DC, Choi S, Shahi PK, Kim MY, Park CG, Kim YD, Lee J, Chang IY, Lee HS, Yeom SC, et al. Action of lipopolysaccharide on interstitial cells of cajal from mouse small intestine. Pharmacology. 2012;90:151–159. doi: 10.1159/000340018. [DOI] [PubMed] [Google Scholar]
- 18.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 19.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987; quiz 988. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 22.Park CG, Kim YD, Kim MY, Kim JS, Choi S, Yeum CH, Parajuli SP, Park JS, Jeong HS, So I, et al. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:175–184. doi: 10.1007/s00210-007-0187-1. [DOI] [PubMed] [Google Scholar]
- 23.Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, Akira S, Uematsu S, Bignami P, Ceriani V, et al. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol. 2006;208:47–54. doi: 10.1002/jcp.20632. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–488. [PubMed] [Google Scholar]
- 25.Huang KT, Kuo L, Liao JC. Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase. Biochem Biophys Res Commun. 1998;245:33–37. doi: 10.1006/bbrc.1998.8384. [DOI] [PubMed] [Google Scholar]
- 26.Luo SF, Wang CC, Chien CS, Hsiao LD, Yang CM. Induction of cyclooxygenase-2 by lipopolysaccharide in canine tracheal smooth muscle cells: involvement of p42/p44 and p38 mitogen-activated protein kinases and nuclear factor-kappaB pathways. Cell Signal. 2003;15:497–509. doi: 10.1016/s0898-6568(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 27.Luo SF, Lin WN, Yang CM, Lee CW, Liao CH, Leu YL, Hsiao LD. Induction of cytosolic phospholipase A2 by lipopolysaccharide in canine tracheal smooth muscle cells: involvement of MAPKs and NF-kappaB pathways. Cell Signal. 2006;18:1201–1211. doi: 10.1016/j.cellsig.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalo S, Grasa L, Hernández LV, Arruebo MP, Plaza MÁ, Murillo MD. Mitogen activated protein kinases blockade improves lipopolysaccharide-induced ileal motor disturbances. Rev Esp Enferm Dig. 2012;104:305–309. doi: 10.4321/s1130-01082012000600004. [DOI] [PubMed] [Google Scholar]
- 29.Torrie LJ, MacKenzie CJ, Paul A, Plevin R. Hydrogen peroxide-mediated inhibition of lipopolysaccharide-stimulated inhibitory kappa B kinase activity in rat aortic smooth muscle cells. Br J Pharmacol. 2001;134:393–401. doi: 10.1038/sj.bjp.0704259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S, Yeum CH, Kim YD, Park CG, Kim MY, Park JS, Jeong HS, Kim BJ, So I, Kim KW. Receptor tyrosine and MAP kinase are involved in effects of H(2)O(2) on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010;14:257–266. doi: 10.1111/j.1582-4934.2008.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey MJ, Coles B, O'Donnell VB. Interactions of nitric oxide-derived reactive nitrogen species with peroxidases and lipoxygenases. Free Radic Res. 2001;35:447–464. doi: 10.1080/10715760100301471. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- 33.Galea E, Feinstein DL. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 1999;13:2125–2137. doi: 10.1096/fasebj.13.15.2125. [DOI] [PubMed] [Google Scholar]
- 34.Tetsuka T, Daphna-Iken D, Srivastava SK, Baier LD, DuMaine J, Morrison AR. Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc Natl Acad Sci USA. 1994;91:12168–12172. doi: 10.1073/pnas.91.25.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]


