Abstract
AIM: To investigate the effects of different concentrations of Schistosoma japonicum (S. japonicum) egg antigen on fibrogenesis and apoptosis in primary hepatic stellate cells (HSCs).
METHODS: A mouse model of schistosomiasis-associated liver fibrosis (SSLF) was established by infecting mice with schistosomal cercaria via the abdomen. HSCs were isolated from SSLF mice by discontinuous density gradient centrifugation, and their identity was confirmed by immunofluorescence double staining of α-smooth muscle actin (α-SMA) and desmin. The growth inhibitory effect and 50% inhibitory concentration (IC50) of S. japonicum egg antigen for primary HSCs (24 h) were determined using a cell counting kit-8 (CCK-8) assay. The expression levels of α-SMA, matrix metalloproteinase-9 (MMOL/LP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in HSCs in response to different concentrations of S. japonicum egg antigen were detected by Western blotting and real-time reverse transcription-polymerase chain reaction. The levels of phospho-P38 (P-P38), phospho-Jun N-terminal kinase (P-JNK) and phospho-Akt (P-AKT) in HSCs were detected by Western blotting.
RESULTS: An SSLF mouse model was established, and primary HSCs were successfully isolated and cultured. S. japonicum egg antigen inhibited HSC proliferation in a concentration-dependent manner. The IC50 of the S. japonicum egg antigen was 244.53 ± 35.26 μg/mL. S. japonicum egg antigen enhanced α-SMA expression at both the mRNA and protein levels and enhanced TIMP-1 expression at the mRNA level in HSCs (P < 0.05), whereas the expression of MMOL/LP-9 was attenuated at both the mRNA and protein levels in a concentration-dependent manner (P < 0.05). A high concentration of S. japonicum egg antigen enhanced P-P38, P-JNK and P-AKT activation (P < 0.05). The changes in α-SMA and MMOL/LP-9 expression induced by S. japonicum egg antigen were closely correlated with P-P38 and P-JNK activation (P < 0.05). The attenuation of MMOL/LP-9 was also correlated with P-AKT activation (P < 0.05), but the increase in α-SMA expression was not. TIMP-1 expression was not correlated with P-P38, P-JNK or P-AKT activation.
CONCLUSION: S. japonicum egg antigen promotes fibrogenesis, activates the P38/JNK mitogen-activated protein kinase and AKT/PI3K signaling pathways and inhibits proliferation in primary HSCs isolated from SSLF mice in a concentration-dependent manner.
Keywords: Schistosomiasis, Liver fibrosis, Hepatic stellate cells, Mitogen-activated protein kinase, Akt
INTRODUCTION
Schistosomiasis is a water-borne parasitic disease that plagues many tropical and subtropical regions. At least 200 million people in 76 countries are currently afflicted by this disease, and a further 500-600 million people are at risk of infection[1]. Schistosoma japonicum (S. japonicum), the Asian schistosome, causes schistosomiasis in China, Japan, the Philippines and Indonesia. A nationwide schistosomiasis survey carried out in 2003 indicated that there were still more than 800 000 people infected with S. japonicum in China[2].
Despite recent progress in anti-schistosomal strategies, clinical management remains a challenge because schistosomiasis-associated liver fibrosis (SSLF) is a complex, multi-step and often fatal disease. Cercaria that are transmitted through the skin can lay a large number of eggs, which then pass through the sinusoidal endothelial vascular system, settle in the liver, release immol/Lunocompetent products, interact with various liver cells and finally lead to liver fibrosis[3]. This liver fibrosis could develop into an irreversible advanced stage upon repeated exposure to the causative agents (i.e., S. japonicum eggs). Primary hepatic stellate cells (HSCs) are believed to be the crucial contributors to the fibrotic process by producing extracellular matrix and interrupting the balance of fiber generation vs degradation[4]. Some studies have investigated the mechanism underlying the pathogenesis of SSLF. S. mansoni eggs can stimulate hepatic endothelial cell proliferation[5] and migration, promote angiogenesis[6], induce fibroblast proliferation[7] and collagen synthesis[8] and down-regulate LX-2 activation and fibrogenesis[9]. However, little is known about the mechanisms that are active in HSCs during the development and progression of SSL[3,10].
In this study, primary HSCs isolated from SSLF mice were exposed to different concentrations of S. japonicum egg antigen and then analyzed to better understand the interaction between HSCs and SSLF.
MATERIALS AND METHODS
Animals
Healthy 4- to 6-wk-old male BALB/C mice were obtained from a schistosomiasis control station in Hubei province, China.
Reagents
S. japonicum egg antigen [0.01 g/mL in phosphate-buffered saline (PBS)] was obtained from the Hubei schistosomiasis control station and diluted to the working concentration in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% fetal bovine serum (FBS) before use.
Animal model development
The SSLF model was established by abdominal infection with schistosomal cercaria according to the method used in a previous study[11]. In brief, mice in the model group were percutaneously infected with S. japonicum by placing a glass slide carrying 20 ± 2 cercariae in non-chlorinated water on the abdomen of each mouse for 15 min. Mice in the control group were treated with non-chlorinated water containing no cercariae. All mice were raised for 6 wk under pathogen-free conditions with free access to food and water. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Chinese Council on Animal Care. Liver samples were taken from 10 infected and 10 normal mice and fixed in 4% (v/v) paraformaldehyde (PFA) in PBS.
Masson staining
Masson staining was performed using standard methods to observe collagen fiber deposition. About 5 middle-power fields were randomly selected from each sample for analysis, and the ratio of the area occupied by collagen fibers to the total area was quantified using Image Pro Plus 6.0 software (Media Cybernetics Inc., United States).
Hepatic stellate cell isolation and culture
HSCs were prepared by the discontinuous density gradient centrifugation technique previously described by Schafer et al[12], with minor modifications. Briefly, the liver was perfused with Solution I [137 mmol/L NaCl, 5.4 mmol/L KCl, 0.6 mmol/L NaH2PO4•2H2O, 0.8 mmol/L Na2HPO4•12H2O, 10 mmol/L HEPES, 0.5 mmol/L EGTA, 4.2 mmol/L NaHCO3, 5 mmol/L glucose, 100 U/mL penicillin and 100 U/mL streptomycin (Gibco, United States), pH = 7.4] and Solution II [137 mmol/L NaCl, 5.4 mmol/L KCl, 0.6 mmol/L NaH2PO4•2H2O, 0.8 mmol/L Na2HPO4•12H2O, 10 mmol/L HEPES, 3.8 mmol/L CaCl2•2H2O, 24.2 mmol/L NaHCO3, 5 mmol/L glucose, 600 mg/L collagenase IV (Gibco, United States), 400 mg/L pronase (Sigma, United States), 20 mg/L DNAse (Gibco, United States), pH = 7.4] through the hepatic portal vein. The livers were removed and forced through a 200 gauge mesh. Parenchymal cells were separated by centrifugation at 20 g for 5 min. The supernatant was transferred to a 50 mL centrifuge tube and centrifuged at 500 g for 7 min. The pellet was resuspended in 15% OptiPrep (Sigma, United States), and 11% OptiPrep and 5 mL GBSS (120 mmol/L NaCl, 5 mmol/L KCl, 0.84 mmol/L Na2HPO4•2H2O, 0.22 mmol/L KH2PO4, 1.9 mmol/L MgCl2•6H2O, 1.5 mmol/L CaCl2•2H2O, 27 mmol/L NaHCO3, 5 mmol/L glucose, pH = 7.4) were then layered on the top of the 15% OptiPrep, and the gradient was centrifuged at 1400 g for 17 min. The interface between the 11% OptiPrep and the GBSS was collected and washed twice with GBSS. The collected cells were cultured in DMEM containing 10% FBS (Gibco, United States). The cell viability, as measured by a Trypan Blue exclusion assay, was approximately 90%. Primary HSCs from passages 7-8 were used in this study.
Immunofluorescence staining
Immunofluorescence double staining of α-smooth muscle actin (α-SMA) and desmin was used to identify activated HSCs. Briefly, cell slides were fixed in 4% PFA and incubated with a blocking solution containing 0.1% Triton X-100 and 5% bovine serum albumin (BSA) in PBS, followed by incubation with anti-desmin (Abcam, United Kingdom) and anti-α-SMA (Boster, China) primary antibodies at 4 °C overnight. After three washes with PBS, the slides were incubated with secondary FITC-conjugated antibodies and Cy3-conjugated antibodies (Proteintech, United States) for 1 h at 37 °C and then with Hoechst 33258 for 5 min. The cells were observed and imaged using a TE-2000 Nikon Inverted fluorescence microscope, and the number of positive cells per 100 cells was analyzed.
Cell counting Kit-8
The cell growth inhibitory effect and 50% inhibitory concentration (IC50) of S. japonicum egg antigen for primary HSCs were determined using a cell counting kit-8 (CCK-8) assay. HSCs were treated as follows: cultured cells (1 × 104/well) in a 96-well plate were exposed to a range of concentrations of S. japonicum egg antigen (1, 5, 25, 125, 250, 625, 1250, 2500 and 3125 μg/mL) for 24 h, and 10 μL of water soluble tetrazolium-8 (WST-8) (Promoter, China) was then added to the medium containing the S. japonicum egg antigen. The cells were incubated then for 2 h. The absorbance of each well was read at 450 nm, and a blank well that contained only culture medium and was used for background correction. The percent inhibition was calculated according to the following formula: Inhibition (%) = [1 - (treated/control)] × 100[13,14]. The IC50 was determined using Statistical Product and Service Solutions (SPSS) 16.0 software (SPSS Inc., United States).
Real-time reverse transcription-polymerase chain reaction
Cultured cells were incubated with various concentrations of S. japonicum egg antigen (0, 5, 10, 15, 50, 250 μg/mL) for 24 h. Total RNA was isolated from HSCs using the TRIzol reagent (Invitrogen, United States) according to the protocol described by the manufacturer. RNA samples were quantified by measuring the absorbance at 260 nm and 280 nm using a spectrophotometer, and all samples had an A260/A280 ratio between 1.8 and 2.0, which indicated a high purity of the extracted RNA. The RNA concentrations were calculated based on the absorbance at 260 nm. Aliquots of total RNA (0.5 μg) from each sample were reverse transcribed into cDNA according to the instructions provided with the first-strand cDNA synthesis kit (TaKaRa, Japan). Equal amounts of the reverse transcription products were subjected to polymerase chain reaction amplification using SYBR Green as a fluorescent indicator on an AB iCycler system (AB, United States). The levels of α-SMA, matrix metalloproteinase-9 (MMOL/LP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) mRNAs were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The fold change in the expression of target genes between the experimental and control samples was calculated using the 2-ΔΔCT method, as previously described[15]. The primers used in this study were synthesized by Invitrogen Ltd. (Invitrogen, United States): α-SMA sense CGGGAGAAAATGACCCAGATT, α-SMA antisense GGACAGCACAGCCTGAATAGC, MMOL/LP-9 sense ACAGCCAACTATGACCAGGAT, MMOL/LP-9 antisense CAGGAAGACGAAGGGGAAGAC, TIMP-1 sense CTTGGTTCCCTGGCGTACTC, TIMP-1 antisense ACCTGATCCGTCCACAAACAG, GAPDH sense GGTTGTCTCCTGCGACTTCA and GAPDH antisense GGGTGGTCCAGGGTTTCTTA.
Western blotting
Western blot analysis was performed as described previously[16]. In brief, proteins from HSCs were extracted using RIPA lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, pH = 7.4) containing protease inhibitors. After the samples were boiled for 5 min at 95 °C in 5 × loading buffer, equal amounts (50 μg) of cell homogenates were separated by 12% SDS-PAGE. The proteins were then electrophoretically transferred at 250 mAH onto polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk or BSA in Tris-buffered saline-Tween 20 (TBST) and probed at 4 °C overnight with primary antibodies against α-SMA (1:200, Boster, China), MMOL/LP-9 (1: 200, Boster, China), TIMP-1and β-actin (1:500, Santa Cruz, United States), phospho-P38 (P-P38) and total P38 (T-P38) (1:1000, Cell Signaling Technology, United States), phospho-JNK (P-JNK) and total-JNK (T-JNK) (1:1000, Cell Signaling Technology, United States), phospho-AKT (P-AKT) (1: 1000, Cell Signaling Technology, United States) and total-AKT (T-AKT) (1:400, Santa Cruz, United States). The membranes were then washed and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies diluted 1:5000 or 1:2000. The membranes were washed, and all blots were visualized using an ECL detection system (Biouniquer, China). The bands were quantitated in grayscale using Image J software (NIH, United States).
Statistical analysis
The results of multiple observations are presented as the means ± SD of at least 3 independent experiments. Student′s t test was used to evaluate the difference in the ratio of the collagen fiber-deposited area to the total area between infected and normal mouse livers. ANOVA was used to evaluate the differences between the S. japonicum egg antigen-treated group and the control group. Person linear correlation analysis was used to analyze the correlations between the α-SMA, MMOL/LP-9 and TIMP-1 expression levels and the P-P38, P-JNK and P-AKT levels. All P values were two sided, and P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 16.0 software.
RESULTS
Establishment of the mouse model of SSLF
Masson staining showed that collagen fibers were deposited at the periphery of the eosinophilic granuloma and that eggs were deposited in the venae of mouse liver tissues at 6 wk post-infection; in contrast, there were no collagen fibers deposited in the normal mouse livers except in the vessels (Figure 1). In addition, the ratio of the collagen fiber-deposited area to the total area was significantly greater in the SSLF livers than in the normal livers (Table 1). The above results suggest that the SSLF mouse model was established successfully.
Figure 1.
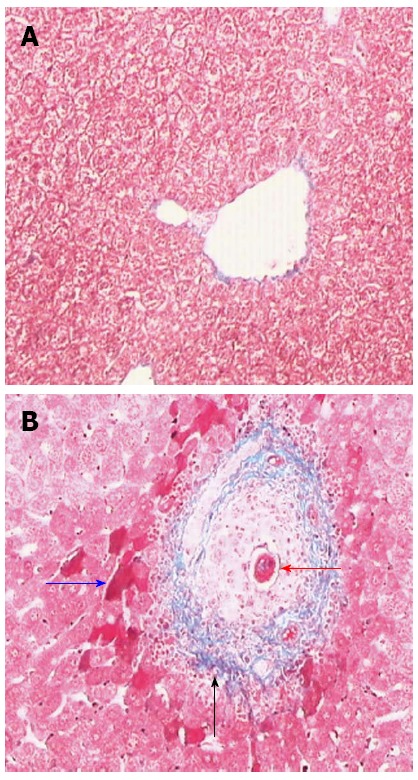
Masson staining of 6 wk post-infect mice livers (× 200). A: Normal mice; B: Infected mice, the red arrow indicated the eggs deposited in the vein, the blue represented acidophilic necrosis, and the black was the collagen fiber deposited around the vein.
Table 1.
Ratio of collagen fiber deposited area to the totalin the normal and infected mice livers (mean ± SD)
| Group | n | Ratio of deposited collagen fiber area against the total |
| Normal | 10 | 5.18% ± 1.88% |
| Infected | 10 | 14.53% ± 2.90%a |
aP < 0.05 vs Normal.
Identification of isolated HSCs
Primary HSCs isolated from SSLF mice displayed a quiescent phenotype. When cultured in plastic culture dishes, these cells began to exhibit an activated phenotype. The expression levels of α-SMA and desmin increased, and this feature was used to identify activated HSCs. Immunofluorescence double staining showed that α-SMA and desmin were expressed in the cytoplasm of HSCs (Figure 2). Approximately 95% of the cells expressed both α-SMA and desmin, suggesting that primary HSCs were isolated successfully.
Figure 2.
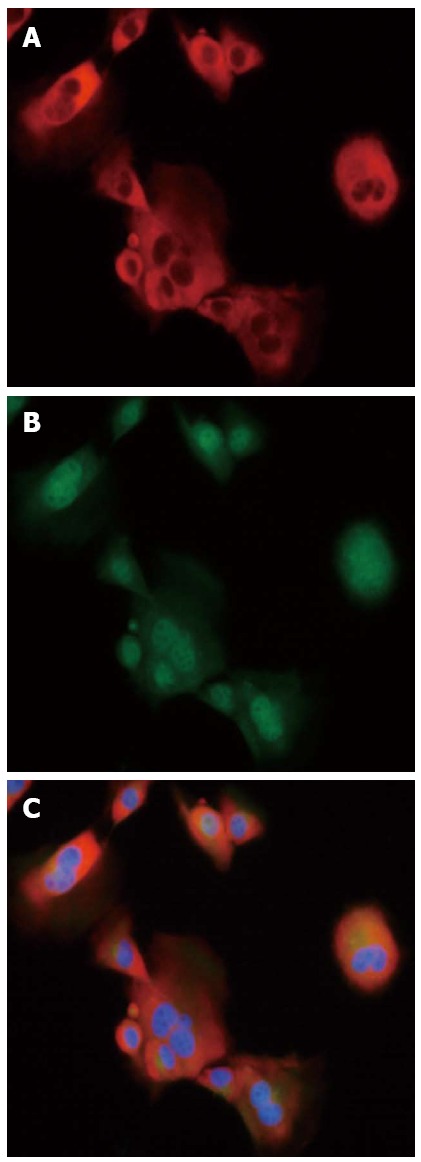
Immunofluorescence staining in primary primary hepatic stellate cells (× 400). A: α-Smooth muscle actin (red hue); B: Desmin (green hue); C: Overlay.
Inhibitory effect and IC50 of S. japonicum egg antigen for primary HSCs
Figure 3 shows that S. japonicum egg antigen inhibited the proliferation of primary HSC in a concentration-dependent manner. The percent inhibition values for S. japonicum egg antigen were 7.06% ± 2.26% for 1 μg/mL, 11.13% ± 1.90% for 5 μg/mL, 17.25% ± 1.95% for 25 μg/mL, 26.16% ± 1.28% for 125 μg/mL, 68.96% ± 4.73% for 250 μg/mL, 75.77% ± 3.87% for 625 μg/mL, 75.95% ± 4.40% for 1250 μg/mL, 75.84% ± 5.33% for 2500 μg/mL and 75.30% ± 4.84% for 3125 μg/mL. The IC50, defined as the concentration of a substance that reduces cell survival by 50%, is a useful parameter to quantify the effect of a substance on cell survival. In this study, the IC50 of S. japonicum egg antigen was calculated to be 244.53 ± 35.26 μg/mL.
Figure 3.
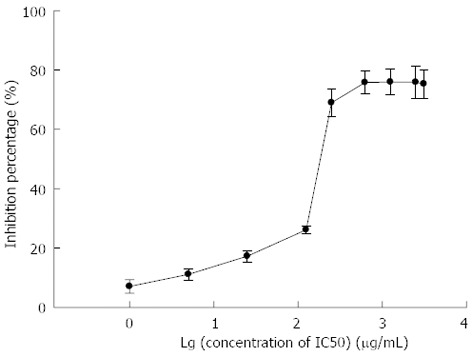
Inhibitory proliferation of Schistosoma japonicum egg antigen in primary hepatic stellate cells. The black dots were quantification of 3 independent experiments.
Expression of α-SMA, MMP-9 and TIMP-1 in response to S. japonicum egg antigen in vitro
As shown in Figure 4, S. japonicum egg antigen at 50 or 250 μg/mL enhanced α-SMA expression in HSCs at both the mRNA and protein levels. At the mRNA level, the fold change in α-SMA expression was 1.97 ± 0.18 in response to 50 μg/mL and 2.18 ± 0.22 in response to 250 μg/mL relative to the control (1.00 ± 0.10, P < 0.05) (Figure 4C). At the protein level, the α-SMA/β-actin ratio was 0.94 ± 0.03 at 50 μg/mL and 1.06 ± 0.04 at 250 μg/mL, compared with 0.63 ± 0.05 for the control (P < 0.05, Figure 4A and B).
Figure 4.
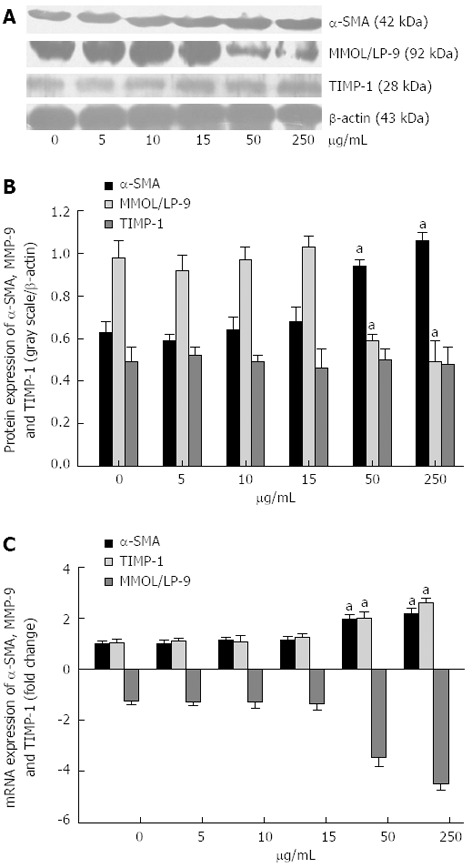
Western blotting (A, B) and real-time polymerase chain reaction (C) of α-smooth muscle actin, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 induced by Schistosoma japonicum egg antigen in primary hepatic stellate cells. A: Western blotting of α-smooth muscle actin (α-SMA), matrix metalloproteinase-9 (MMOL/LP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1), and representatives of 3 independent experiments; B: The histograms reported as ratio of respective target gray scale to that of β-actin. C: The histograms represented the fold change of target genes normalized to glyceraldehyde-3-phosphate dehydrogenase levels and quantified of 3 independent experiments. aP < 0.05 vs 0 μg/mL.
However, at the mRNA level, 3.45 ± 0.36-fold reduction in response to 50 μg/mL and 4.49 ± 0.28-fold reduction in response to 250 μg/mL in MMOL/LP-9 expression were observed after 24h with egg antigen treatment, compared with 1.05 ± 0.12 for the control (P < 0.05, Figure 4C). At the protein level, the MMOL/LP-9/β-actin ratios were 0.59 ± 0.03 at 50 μg/mL and 0.49 ± 0.10 at 250 μg/mL, compared with 0.98 ± 0.08 for the control (P < 0.05, Figure 4A and B). In addition, S. japonicum egg antigen also enhanced TIMP-1 expression at the mRNA level. The fold change in TIMP-1 mRNA expression was 2.00 ± 0.27 in response to 50 μg/mL and 2.62 ± 0.18 in response to 250 μg/mL compared with 1.05 ± 0.13 for the control (P < 0.05, Figure 4C). However, as shown in Figure 4A and B, S. japonicum egg antigen had no effect on TIMP-1 expression at the protein level.
Collectively, the above observations indicate that S. japonicum egg antigen promoted fibrogenesis in HSCs and participated in the extracellular matrix remodeling observed in SSLF.
P38/JNK MAPK and AKT signaling pathway activation by S. japonicum egg antigen in vitro
As shown in Figure 5, the P-P38, P-JNK and P-AKT activation levels were enhanced by stimulation with the S. japonicum egg antigen. The P-P38/T-P38 ratio was 1.03 ± 0.08 at 50 μg/mL and 1.05 ± 0.01 at 250 μg/mL vs 0.18 ± 0.02 for the control (P < 0.05). The P-JNK/T-JNK ratio was 0.58 ± 0.04 at 15 μg/mL, 0.88 ± 0.05 at 50 μg/mL and 0.67 ± 0.03 at 250 μg/mL vs 0.20 ± 0.01 for the control (P < 0.05). The P-AKT/T-AKT ratio was 0.57 ± 0.11 at 250 μg/mL v 0.29 ± 0.03 for the control (P < 0.05). The above results indicate that S. japonicum egg antigen activated the P38/JNK mitogen-activated protein kinase (MAPK) and AKT signaling pathways in a concentration-dependent manner.
Figure 5.
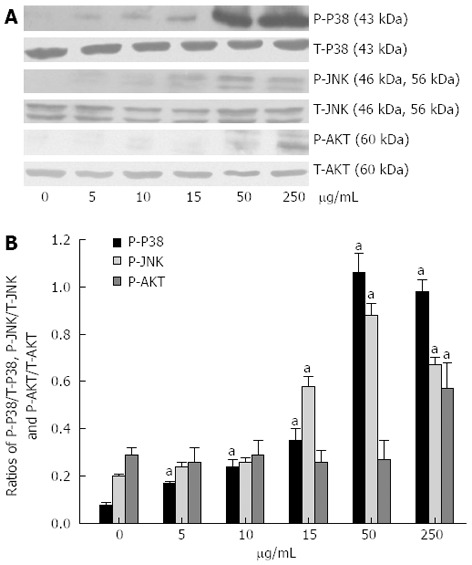
Western blotting of phospho-P38, phospho-Jun N-terminal kinase and phospho-Akt activation induced by Schistosoma japonicum egg antigen in primary hepatic stellate cells. A: Western blotting of phospho-P38 (P-P38), phospho-Jun N-terminal kinase (P-JNK) and phospho-Akt (P-AKT), and representatives of 3 independent experiments; B: The histograms respectively reported as ratio of P-P38/T-P38, P-JNK/T-JNK, and P-AKT/T-AKT. aP < 0.05 vs 0 μg/mL.
Relationships among the levels of the α-SMA, MMP-9 and TIMP-1 and P-P38, P-JNK and P-AKT in HSCs
α-SMA protein expression was positively correlated with the P-P38 (r = 0.669, P = 0.002) and P-JNK levels (r = 0.686, P = 0.002). MMOL/LP-9 expression was negatively correlated with the P-P38 (r = -0.976, P = 0.000), P-JNK (r = -0.86, P = 0.000) and P-AKT levels (r = -0.529, P = 0.024). However, TIMP-1 expression was not correlated with the P-P38, P-JNK or P-AKT level. α-SMA expression was not correlated with the P-AKT level.
DISCUSSION
HSC activation is considered the key step in liver fibrogenesis, representing a transition from the quiescent state into a proliferative, fibrogenic and contractile state. HSCs reside in the space of disse and are recruited to areas of hepatic injury by chemokines, where they become activated[17]. During this process, HSCs are transformed from the quiescent to activated phenotype, a process that is accompanied by an increase in the expression levels of α-SMA and desmin[18,19], which are considered to be markers of HSC activation. The activated HSCs adopt a myofibroblast-like phenotype and secrete collagen-rich matrix into the extracellular space, leading to liver fibrosis[20]. HSCs have been implicated as the effector cells of hepatic fibrosis and cirrhosis associated with hepatitis B virus, hepatitis C virus, genetic hemochromatosis, biliary atresia, cystic fibrosis and alcoholic liver disease in humans[20-23]. HSCs also play a role in eosinophilic granulomatous inflammol/Lation and SSLF[24-26]. Bartley et al[25] showed that activated HSCs could be detected at the edges of eosinophilic granulomas in S. japonicum-infected mice livers at 6 wk post-infection, and the localization of these cells was coincident with areas of collagen deposition. In this study, collagen fiber deposition was observed at the periphery of the eosinophilic granuloma, consistent with the previous reports[25]. In addition, we also found that S. japonicum egg antigen inhibited HSC proliferation in a concentration-dependent manner.
Activated HSC-secreted MMOL/LPs (e.g., MMOL/LP-9) and their inhibitors (e.g., TIMP-1) remodel the tissue matrix[22], and an imbalance in MMOL/LP/TIMP expression has been associated with cumulative fibrosis[27]. The expression of α-SMA is increased in the livers of SSLF patients and infected mice and is closely correlated with the degree of liver fibrosis[25,27]. In our experiment, the S. japonicum egg antigen attenuated MMOL/LP-9 expression and enhanced α-SMA expression at both the mRNA and protein levels, and it also enhanced TIMP-1 expression at the mRNA level. However, the TIMP-1 protein level was not affected. The mRNA and protein expression levels are not always proportional due to the activities of various regulatory mechanisms, e.g., post-transcriptional modulation by miRNAs. The above results imply that the S. japonicum egg antigen is involved in MMOL/LP-9-mediated matrix remodeling and thus collagen deposition, leading to liver fibrosis. The results of our study partially contradict to the previous report that S. mansoni eggs can down-regulate the activation of and fibrogenesis in the human hepatic stellate cell line LX-2[9]. One potential reason for this discrepancy is the different cell models and schistosomal egg antigens used in the two studies. In vitro exposure to schistosome eggs induces only limited activation of human dendritic cells (DCs) but strong activation of murine DCs[28]. Similarly, in this study, the same concentrations of egg antigen (5 μg/mL, 10 μg/mL, and 15 μg/mL) that attenuated α-SMA expression in human LX-2 cells had no effect on primary mouse HSCs. In addition, S. japonicum and S. mansoni eggs release different antigens[29], which may play a role in the discrepancy.
MAPKs are pivotal transmitters of extracellular signals such as hormones, cytokines, growth factors, and various environmental stress signals[30]. The MAPK family has three major subfamilies: ERK, P38, and JNK, and the P38 and JNK pathways are involved in HSC transformation[31] and in the regulation of the complex life cycle and host-parasite interactions of S. japonicum[32]. Many studies have shown that the regulation of MMOL/LP-9[33-36] and α-SMA[37-39] in various cell models is dependent on the activation of the P38 and (or) JNK signaling pathways. In addition, AKT/PI3K activation contributes to liver[40], hear[41] and pulmonar[42-44] fibrosis. To gain insight into the potential signaling mechanisms mediating the HSC responses induced by the S. japonicum egg antigen, we investigated the roles of the P38/JNK MAPK and AKT signaling pathways by assessing their phosphorylation status in primary HSCs after stimulation by the S. japonicum egg antigen. In this study, the P-P38 and P-JNK levels were significantly enhanced after the treatment. The increase in α-SMA expression that was stimulated by the S. japonicum egg antigen was positively correlated with the P-P38 and P-JNK levels but not the P-AKT level. Attenuated MMOL/LP-9 expression was negatively correlated with the P-P38, P-JNK and P-AKT levels. TIMP-1 expression was not correlated with the P-P38, P-JNK or P-AKT level. We also noted that only S. japonicum egg antigen concentrations of 250 μg/mL or greater were associated with AKT activation, suggesting that AKT signaling may have a less important role than P38/JNK MAPK signaling in the induction of fibrogenesis by the S. japonicum egg antigen.
In conclusion, the results of this study suggest that the S. japonicum egg antigen promotes fibrogenesis and inhibits the proliferation of primary HSCs in a concentration-dependent manner. These effects of the S. japonicum egg antigen may be attributed to the activation of the P38/JNK signaling pathways in HSCs. Thus, inhibitors of the P38/JNK signaling pathways may serve as potential therapeutic treatments for SSLF.
ACKNOWLEDGMENTS
The authors thank Doctor Ying Chang for her revision of the manuscript. The authors would also like to acknowledge Doctor Zhi-Jun Wang for providing technical assistance during preliminary experiments.
COMMENTS
Background
Under the action of stimulus, hepatic stellate cells (HSCs) are activated and secreted extracellular matrix (ECM) into extracellular space, which leads to liver fibrosis. HSCs have been attributed as the effector cells for schistosomiasis-associated liver fibrosis (SSLF). Continuous schistosome eggs deposition in liver tissue was the chief factors leading to SSLF. So the study on the interaction between schistosome eggs antigen and HSCs is very important.
Research frontiers
As the effector cells of liver fibrosis, HSC is usually used as the cell model to study the liver fibrosis in vitro. The research hotspot on SSLF is the effects induced by schistosome eggs in HSCs and the involved mechanisms, and finding out the targets of treatment on SSLF for future.
Innovations and breakthroughs
The authors isolated primary HSCs which maintained the original features of HSC genes, and tried to explore the involved signaling pathways. The results of this study showed that Schistosoma japonicum (S. japonicum) eggs promoted the fibrogenesis and inhibited the proliferation of primary HSCs in vitro, P38/JNK MAPK signaling pathways might play an important role in the fibrogenesis induced by S. japonicum eggs, however AKT/PI3K signaling pathway might be not involved.
Applications
The results of this study suggest that S. japonicum egg antigen induced-fibrogenesis is related with P38/JNK MAPK signaling pathway activation, and the inhibitors of the P38/JNK signaling pathways may serve as potential therapeutic treatments for SSLF.
Terminology
Schistosomiasis is a water-borne parasitic disease that plagues many tropical and subtropical regions in world. There are 6 types schistosome which can infect humans: Schistosoma haematobium, Schistosoma mansoni, S. japonicum, Schistosoma mekongi, Schistosoma intercalatum, Schistosoma malayensis. S. japonicum, the Asian schistosome, causes schistosomiasis in China, Japan, the Philippines and Indonesia. Cercariae of schistosome parasitize in oncomelania that live in water and infect humans through bare skins, develop into imaginess in human bodies, and produce a large number of eggs which can deposit in liver tissues. With the eggs continuous deposition, eosinophilic granuloma and liver fibrosis, which are the pathological characteristics of schistosomiasis, are finally induced.
Peer review
This is a good study in which authors describe the fibrogenesis and inhibitory proliferation effects induced by S. japonicum in primary HSCs, and analyzed the signaling pathways (e.g., P38/JNK MAPK) which may be involved in the above effects. The results are interesting and suggest that the inhibitors of the P38/JNK signaling pathways may serve as potential therapeutic treatments for SSLF in future.
Footnotes
Supported by Natural Science Foundation of China, No. 81071381
P- Reviewer Venugopal SK S- Editor Zhai HH L- Editor A E- Editor Zhang DN
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Xiao DL, Yu Q, Dang H, Guo JG, Zhou XN, Wang LY. Schistosomiasis situation in People’s Republic of China in 2003. Zhonghua Xue Xi Chong Bing Fangzhi Zazhi. 2004;16:401–405. [Google Scholar]
- 3.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth M, Mwatha JK, Joseph S, Jones FM, Kadzo H, Ireri E, Kazibwe F, Kemijumbi J, Kariuki C, Kimani G, et al. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172:1295–1303. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DO, Ottesen EA. Eggs of Schistosoma mansoni stimulate endothelial cell proliferation in vitro. J Infect Dis. 1988;158:556–562. doi: 10.1093/infdis/158.3.556. [DOI] [PubMed] [Google Scholar]
- 6.Kanse SM, Liang O, Schubert U, Haas H, Preissner KT, Doenhoff MJ, Dennis RD. Characterisation and partial purification of Schistosoma mansoni egg-derived pro-angiogenic factor. Mol Biochem Parasitol. 2005;144:76–85. doi: 10.1016/j.molbiopara.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Wyler DJ, Tracy JW. Direct and indirect effects of soluble extracts of Schistosoma mansoni eggs on fibroblast proliferation in vitro. Infect Immun. 1982;38:103–108. doi: 10.1128/iai.38.1.103-108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boros DL, Lande MA. Induction of collagen synthesis in cultured human fibroblasts by live Schistosoma mansoni eggs and soluble egg antigens (SEA) Am J Trop Med Hyg. 1983;32:78–82. doi: 10.4269/ajtmh.1983.32.78. [DOI] [PubMed] [Google Scholar]
- 9.Anthony B, Mathieson W, de Castro-Borges W, Allen J. Schistosoma mansoni: egg-induced downregulation of hepatic stellate cell activation and fibrogenesis. Exp Parasitol. 2010;124:409–420. doi: 10.1016/j.exppara.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Anthony B, Allen JT, Li YS, McManus DP. Hepatic stellate cells and parasite-induced liver fibrosis. Parasit Vectors. 2010;3:60. doi: 10.1186/1756-3305-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen BL, Zhang GY, Yuan WJ, Wang SP, Shen YM, Yan L, Gu H, Li J. Osteopontin expression is associated with hepatopathologic changes in Schistosoma japonicum infected mice. World J Gastroenterol. 2011;17:5075–5082. doi: 10.3748/wjg.v17.i46.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäfer S, Zerbe O, Gressner AM. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987;7:680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- 13.Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- 14.Li HN, Nie FF, Liu W, Dai QS, Lu N, Qi Q, Li ZY, You QD, Guo QL. Apoptosis induction of oroxylin A in human cervical cancer HeLa cell line in vitro and in vivo. Toxicology. 2009;257:80–85. doi: 10.1016/j.tox.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–H612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 17.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 18.Chang D, Ramalho LN, Ramalho FS, Martinelli AL, Zucoloto S. Hepatic stellate cells in human schistosomiasis mansoni: a comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006;97:318–323. doi: 10.1016/j.actatropica.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells) Hepatology. 1984;4:709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- 20.Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143–147. doi: 10.1136/bmj.327.7407.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW. Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997;26:584–592. doi: 10.1016/s0168-8278(97)80424-2. [DOI] [PubMed] [Google Scholar]
- 22.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527–535. doi: 10.1016/S0002-9440(10)65595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewindon PJ, Pereira TN, Hoskins AC, Bridle KR, Williamson RM, Shepherd RW, Ramm GA. The role of hepatic stellate cells and transforming growth factor-beta(1) in cystic fibrosis liver disease. Am J Pathol. 2002;160:1705–1715. doi: 10.1016/s0002-9440(10)61117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbosa Júnior Ade A, Pfeifer U, Andrade ZA. Role of fat-storing cells in schistosomal hepatic fibrosis of mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:91–96. doi: 10.1007/BF02915100. [DOI] [PubMed] [Google Scholar]
- 25.Bartley PB, Ramm GA, Jones MK, Ruddell RG, Li Y, McManus DP. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Maksimov VI, Molodova GA, Bespalova TI, Denisov VM. [Effect of different pH values on the activity and quaternary structure of asparaginase in Escherichia coli extracts] Prikl Biokhim Mikrobiol. 1975;11:539–545. [PubMed] [Google Scholar]
- 27.Singh KP, Gerard HC, Hudson AP, Boros DL. Expression of matrix metalloproteinases and their inhibitors during the resorption of schistosome egg-induced fibrosis in praziquantel-treated mice. Immunology. 2004;111:343–352. doi: 10.1111/j.0019-2805.2004.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perona-Wright G, Jenkins SJ, MacDonald AS. Dendritic cell activation and function in response to Schistosoma mansoni. Int J Parasitol. 2006;36:711–721. doi: 10.1016/j.ijpara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Mathieson W, Wilson RA. A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: the miracidium, hatch fluid and secretions. Int J Parasitol. 2010;40:617–628. doi: 10.1016/j.ijpara.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 31.Reeves HL, Dack CL, Peak M, Burt AD, Day CP. Stress-activated protein kinases in the activation of rat hepatic stellate cells in culture. J Hepatol. 2000;32:465–472. doi: 10.1016/s0168-8278(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Yang Z, Li Y, Yu F, Brindley PJ, McManus DP, Wei D, Han Z, Feng Z, Li Y, et al. Reconstruction and in silico analysis of the MAPK signaling pathways in the human blood fluke, Schistosoma japonicum. FEBS Lett. 2006;580:3677–3686. doi: 10.1016/j.febslet.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 33.Liu WH, Chang LS. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell Physiol. 2010;224:775–785. doi: 10.1002/jcp.22180. [DOI] [PubMed] [Google Scholar]
- 34.Cho HJ, Jeong YJ, Park KK, Park YY, Chung IK, Lee KG, Yeo JH, Han SM, Bae YS, Chang YC. Bee venom suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and NF-kappaB-dependent mechanisms. J Ethnopharmacol. 2010;127:662–668. doi: 10.1016/j.jep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Hsu HH, Liu CJ, Shen CY, Chen YJ, Chen LM, Kuo WH, Lin YM, Chen RJ, Tsai CH, Tsai FJ, et al. p38α MAPK mediates 17β-estradiol inhibition of MMP-2 and -9 expression and cell migration in human lovo colon cancer cells. J Cell Physiol. 2012;227:3648–3660. doi: 10.1002/jcp.24072. [DOI] [PubMed] [Google Scholar]
- 36.Cheng CY, Hsieh HL, Hsiao LD, Yang CM. PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth in human limbal epithelial cells on intact amniotic membrane. Stem Cell Res. 2012;9:9–23. doi: 10.1016/j.scr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Peng J, Feng D, Chu L, Li X, Jin Z, Lin Z, Zeng Q. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung. 2006;184:33–42. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 38.Nomiyama Y, Tashiro M, Yamaguchi T, Watanabe S, Taguchi M, Asaumi H, Nakamura H, Otsuki M. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas. 2007;34:364–372. doi: 10.1097/MPA.0b013e31802f0531. [DOI] [PubMed] [Google Scholar]
- 39.Akamatsu N, Nakajima H, Ono M, Miura Y. Increase in acetyl CoA synthetase activity after phenobarbital treatment. Biochem Pharmacol. 1975;24:1725–1727. doi: 10.1016/0006-2952(75)90013-1. [DOI] [PubMed] [Google Scholar]
- 40.Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512–1523. doi: 10.1002/hep.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lian H, Ma Y, Feng J, Dong W, Yang Q, Lu D, Zhang L. Heparin-binding EGF-like growth factor induces heart interstitial fibrosis via an Akt/mTor/p70s6k pathway. PLoS One. 2012;7:e44946. doi: 10.1371/journal.pone.0044946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Z, Gao Y, Deng Y, Li W, Chen Y, Xing S, Zhao X, Ding J, Wang X. Lipopolysaccharide induces lung fibroblast proliferation through Toll-like receptor 4 signaling and the phosphoinositide3-kinase-Akt pathway. PLoS One. 2012;7:e35926. doi: 10.1371/journal.pone.0035926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wygrecka M, Zakrzewicz D, Taborski B, Didiasova M, Kwapiszewska G, Preissner KT, Markart P. TGF-β1 induces tissue factor expression in human lung fibroblasts in a PI3K/JNK/Akt-dependent and AP-1-dependent manner. Am J Respir Cell Mol Biol. 2012;47:614–627. doi: 10.1165/rcmb.2012-0097OC. [DOI] [PubMed] [Google Scholar]
- 44.Conte E, Fruciano M, Fagone E, Gili E, Caraci F, Iemmolo M, Crimi N, Vancheri C. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One. 2011;6:e24663. doi: 10.1371/journal.pone.0024663. [DOI] [PMC free article] [PubMed] [Google Scholar]


