Abstract
AIM: To validate the utility of magnetic resonance imaging (MRI) for the clinical management of acute ischemic colitis (IC).
METHODS: This is a magnetic resonance (MR) prospective evaluation of 7 patients who were proved to have acute IC on the basis of clinical, endoscopic and computed tomography (CT) findings and who were imaged in our institution between February 2011 and July 2012. The mean age of the patients was 72.28 years. Abdominal CTs were obtained using a 64-detector row configuration for all patients with un-enhanced and contrast-enhanced scans, in the late arterial phase (start delay 45-50 s) and in the portal venous phase (start delay 70-80 s). The MR examinations were performed using a 1.5T superconducting magnet, using Fast Imaging Employing Steady State Acquisition and T2-weighted fast-recovery fast-spin echo sequences in axial and coronal plane. CT and MRI examinations were analysed for the presence of colonic abnormalities and associated findings.
RESULTS: Segmental involvement was seen in 6 patients (85.71%), with a mean length of involvement of 412 mm (range 145.5-1000 mm). Wall thickness varied between 6 mm and 17.5 mm (mean 10.52 mm) upon CT examinations and from 5 to 15 mm (mean 8.8 mm) upon MR examinations. The MRI appearance of the colonic wall varied over the time: Type I appearance with a 3 layer sandwich sign was seen in 5 out of 12 examinations (41.66%), patients underwent MR within a mean of 36 h (ranging from 1 to 54 h) after the CT examination. Type II and III appearance with a 2 layer sign, was seen in 4 examinations (33.33%), patients underwent MR within a mean of 420.5 h (ranging from 121 to 720 h) after the CT examination. In the remaining three MRI examinations, performed within a mean of 410 h (ranging from 99.5 to 720 h) the colonic wall appeared normal.
CONCLUSION: MRI, only using precontrast images, may be used as a substitute for invasive procedures in diagnosis and follow-up of acute IC.
Keywords: Ischemic colitis, Magnetic resonance imaging, Medical management, Colon, Computed tomography
INTRODUCTION
Ischemic colitis (IC) is a relatively common disease[1] and it is considered the most frequent form of intestinal ischemia and the second most frequent cause of lower gastrointestinal bleeding[2]. It represents the consequence of an acute or, more commonly, chronic decrease or blockage in the colonic blood supply, which may be either occlusive or non- occlusive in origin[3]. The original insult causing the ischemic event can rarely be established, but frequently occurs in elderly patients with diffuse disease in small segmental vessels and various co-morbidities. Today, with the introduction of new therapies, pharmacological causes could also be considered[4,5]. The anatomic damage results in ischemic necrosis of variable severity that can range from superficial mucosal involvement to full-thickness transmural necrosis[6]. The treatment depends on the acuteness and severity of the presentation[3]. Most cases of IC are transient and resolve spontaneously and such patients do not require specific therapy, instead very mild cases can be managed on an outpatient basis with a liquid diet, close observation and antibiotics[7]. IC rarely presents itself in a gangrenous form (acute fulminant IC).
The incidence of IC is underestimated because it often has a mild transient nature, clinical presentation can be nonspecific and highly variable, therefore, the diagnosis largely depends on clinical suspicion. In this context the role of imaging techniques remains controversial[8]. Standard radiology yields non-specific and late findings, while computed tomography (CT), the main technique for the noninvasive diagnosis of mesenteric ischemia, is well suited to confirm the clinical suspicion of IC, to suggest IC when it is unsuspected and to diagnose complications, however it requires the use of radiation and an iodinates contrast agent, limiting the possibility to use this technique in a short term follow-up[9-14]. Recently Iacobellis et al[15] has proposed magnetic resonance imaging (MRI) as a substitute for invasive procedures in diagnosing and grading acute IC, allowing for the early identification of pathological findings and by defining the evolution of ischemic lesions with 7T magnetic resonance imaging (7T-MRI) on an animal model with acute IC. The purpose of this study was to validate the utility of MRI in the clinical management of acute IC. In particular to show our experience in daily practice, focusing the attention both on the diagnosis and follow-up of this pathological condition.
MATERIALS AND METHODS
Patients population
All human procedures were approved by our Institutional Care Committee.
This is a magnetic resonance (MR) prospective evaluation of 11 patients who were proved to have acute intestinal ischemia on the basis of clinical, endoscopic and CT findings and who were imaged in our institution between February 2011 and July 2012. Among the initial 11 patients, 7 were included in the study because they were identified as having IC. Two patients with extensive small intestinal infarction because of the occlusion of superior mesenteric artery and 2 patients with the occlusion of superior mesenteric vein were not included.
Of the 7 patients, 2 were men and 5 women, with a mean age of 72.28 years (range, 54-97 years). Clinical charts, CT and MR examinations were reviewed. Imaging evaluation was requested because of the following clinical findings: sudden-onset of abdominal pain in all the patients (n = 7) (100%), bloody diarrhoea or bright red rectal blood in 4 patients (57.14%), nausea and vomiting in 1 patient (14.29%), elevated white blood cells (WBC) in 5 patients (71.42%), elevated Lactate dehydrogenase (LDH) levels in 6 patients (85.71%).
Five (71.42%) of these patients had arteriosclerotic cardiovascular disease on the basis of the findings from a clinical evaluation or cardiac work-up. Their mean age was 77 years. One patient (14.29%, 54 years old), during treatment of Lenalidomide for a relapsed multiple myeloma and one patient (14.29%, 80 years old) had hypotensive episodes associated with a recent exacerbation of chronic pancreatitis.
The diagnosis of IC was suspected on the basis of the clinical presentation and CT findings and was confirmed in all the patients: in 5 patients, an endoscopic procedure alone (sigmoidoscopy or colonoscopy) was performed, alternatively an endoscopic procedure and biopsy was performed on 1 patient; and 1 patient, underwent endoscopy and surgery with histological specimen evaluation.
Multidetector-row computed tomography imaging protocol
Abdominal CTs were obtained using a 64-detector row configuration for all patients (Discovery CT 750HD, General Electric Healthcare, Milwaukee, WI, United States). In all patients the examination was performed with a spiral technique in a cranio-caudal direction (from the base of the lungs to the pelvic brim) and supine position. All patients underwent un-enhanced and contrast-enhanced CT, in the late arterial phase (start delay 45-50 s) and in the portal venous phase (start delay 70-80 s) with an intravenous injection of 2 mL/kg of non-ionic contrast material (Iopamiro 370; Bracco Diagnostics, Milan, Italy), followed by 40 mL of saline solution using a peristaltic semiautomated power injector (4 mL/s flow rate, SIAS 757, Bologna, Italy) with an 18-gauge needle in the antecubital vein. Oral medium contrast was not given to any of the patients; rectal air or rectal contrast material was not administered. The following technical parameters were used: effective slice thickness of 3.75 mm for plain acquisition, 1.25 mm in the late arterial phase and 2.5 mm in the portal venous phase; beam pitch of 0.938, reconstruction interval of 0.8 mm, tube voltage of 120-140 KVp and reference mAs of 250/700 mA. Automatic tube current modulation was used to minimise radiation exposure. Standard reconstruction algorithm was used. Patients were instructed not to breath during helical imaging to avoid motion artefacts.
MRI techniques
The MR examination was performed using a 1.5T superconducting magnet (SIGNA HD 1.5, General Electric Healthcare, Milwaukee, WI, United States), using the following sequences: Fast Imaging Employing Steady State Acquisition (FIESTA, TR/TE, 3.8/1.6; matrix size, 192 × 320; section thickness, 6 mm; intersection gap, 1 mm; field of view, 480 mm × 480 mm; NEX, 1; breath-hold) on coronal plane and T2-weighted fast-recovery fast-spin echo sequence (FRFSE: TR/TE, 6000/70, matrix size, 320 × 256; section thickness, 4mm; intersection gap, 0.4 mm; field of view, 480 mm × 480 mm; NEX, 4; respiratory triggering) in axial and coronal plane. An eight channel body phase-array surface coil was employed. Oral and intravenous medium contrast were not given to any of the patients.
Image analysis and comparison
CT scans and MR images were evaluated by two Radiologists (Mazzei MA and Mazzei FG) experienced in gastrointestinal imaging, reaching a consensus agreement. The following were assessed: the location and length of the colonic segment involved; the appearance and degree of wall thickening; the presence of a double-halo or target configuration (two or three concentric rings); pericolic streakiness, peritoneal fluid or blood, presence of intramural, mesenteric, or portal venous gas, and free intraperitoneal air or other relevant abdominal findings were also recorded.
The bowel wall was considered thickened if it measured more than 3 mm in diameter. Right side colonic involvement was defined as abnormalities affecting a segment or the entire ascending colon including the hepatic flexure. Left-side involvement was defined as abnormalities starting at or distal to the splenic flexure. Finally, the gross appearance of the affected colonic wall at CT was divided into three morphologic groups according to Balthazar et al[14]: (1) Type I CT (acute IC), wall thickening with heterogeneous enhancement and zones of low attenuation compatible with severe colonic edema; there was enhancement of the mucosa consistent with an acute process, a shaggy contour, a loss of colonic haustra, with varying degrees of pericolic streakiness; (2) Type II CT (subacute IC), the CT appearance showed concentric and symmetric mild mural thickening and homogeneous attenuation of the wall of the colon with a sharply defined contour and with or without minimal pericolic streakiness; and (3) Type III CT (gangrenous IC): there was circumferential intramural air consistent with pneumatosis coli.
A similar classification was realised by the analysis of MR images and a correlation between the gross appearance of the affected colonic wall on both CT and MRI and the time from the onset.
RESULTS
Patient demographic information (age and sex), pre-existing disease, number of CT and MR examinations during hospitalisation, diagnostic proof (S = surgery, B = biopsy and E = endoscopy) and status at the time of discharge (D = died; L = living) for the 7 patient are summarised in Table 1. The diagnostic CT examinations were performed within a mean of 45.5 h (range, 2-168 h) after the clinical onset of symptoms, while the first MR examinations were performed in 4 patients within 48 h (mean of 14.8 h; range, 1-40 h) after the date of the CT examination, in 5 patients between 48 h and 15 d (mean of 137.5 h, range 54-240 h), and in 3 patients after 15 d from the CT examinations (mean of 524 h, range 384-720 h). Three patients underwent only one MRI examination, 3 patients were studied twice and 1 patient was studied three times, for a total of 12 MRI examinations. The duration of the MRI follow-up of the 7 patients considered varied from 1 to 30 d from the date of the CT examination.
Table 1.
Patients demographic characteristic
| Patient | Sex | Age (yr) | Pre-existing disease | Number of CT/MR | Diagnostic proof | Status of discharge |
| 1 | F | 54 | Multiple myeloma relapsed treated with Lenalinomide | 1/2 | E | L |
| 2 | F | 89 | Ischemic heart disease, hypertension, rheumatoid arthritis | 1/1 | E | L |
| 3 | F | 80 | Hypertension, thrombotic disease | 1/2 | E + B | L |
| 4 | F | 97 | Diverticulitis, pancreatitis, hypertension in treatment | 1/1 | E | L |
| 5 | M | 67 | Crohn’s disease | 1/1 | B | D |
| 6 | F | 57 | Hypertension in treatment | 1/2 | E | L |
| 7 | M | 62 | Heart failure | 1/3 | E | L |
CT: Computed tomography; MR: Magnetic resonance; F: Female; M: Male; B: Biopsy; E: Endoscopy; D: Died; L: Living.
Among the 7 patients, 6 (85.71%) exhibited a segmental involvement of the colon and 1 patient (14.29%) had the entire colon involved. The length of involvement in the patients with a segmental distribution, obtained using 2D reformat reconstructions on CT images, ranged from 145.5 to 1000 mm, with a mean length of 412 mm. The thickness of the wall of the colon in the affected segments varied from 6 to 17.5 mm, with a mean bowel wall thickness of 10.52 mm on CT images, and from 5 to 15 mm, with a mean bowel wall thickness of 8.8 mm, on MR images. Two out of 6 patients (33.33%) with a segmental involvement of the colon exhibited a left-sided and splenic flexure colitis, 1 (16.66%) a left-sided colitis only, 1 (16.66%) a left-sided colitis and IC of the transverse colon and splenic flexure, 1 (16.66%) an IC of the transverse colon only, and 1 (16.66%) an IC of the sigmoid colon. In the 1 patient with ischemic pancolitis, the diagnosis was confirmed at colonoscopy and total colectomy.
The gross appearance of the affected colonic wall at CT, divided into three morphologic groups, according to Balthazar et al[14], showed that Type I CT was present in 5 out of 7 patients (71.4%) and Type II CT in 2 out of 7 patients (28.6%). The diagnostic CT examinations in 5 patients with Type I CT appearance was performed within a mean of 36 h (ranging from 2 and 48 h) after the clinical onset of symptoms, showing an acute form of IC.
As the morphologic CT groups, the gross appearance of the affected colonic wall upon MRI could be divided into 3 groups: (1) Type I MRI, in the first group, wall thickening with a 3 layer sandwich sign showing high signal intensity of the intermediate layer and the low signal intensity of the inner and outer layers, were present in 5 MR examinations (4 patients, 1 patient had 2 MR examinations with an interval of 48 h due to worsening of clinical symptoms). The MR examinations with Type I appearance were performed within a mean of 36 h (ranging from 1 to 54 h) after the CT examination and the same patients with Type I MRI showed the Type I appearance at CT, according to an acute form of IC (Figures 1 and 2); (2) Type II MRI, in the second group, which consisted of 2 patients, there was a wall thickening with a 2 layer sign (high signal intensity of the inner layer and low signal intensity of the outer layer. The MR examinations with Type II appearance were performed within a mean of 420.5 h (ranging from 121 to 720 h) after the CT examination, according to a subacute form of IC; and (3) Type III MRI, in the third group, which consisted of 2 patients, the MR appearance showed a wall thickening with a inversed 2 layer sign (high signal intensity of the outer layer and low signal intensity of the inner layer ). The MR examinations with Type III appearance were performed within a mean of 420.5 h (ranging from 384 to 457 h) after the CT examination, according to a subacute form of IC.
Figure 1.
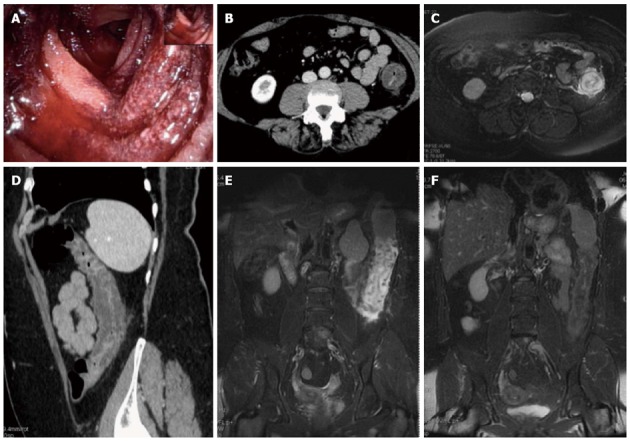
Magnetic resonance imaging follow-up of a patients with ischemic colitis resolved promptly. Ischemic colitis (IC) of left side colon in a 57 year old woman with a recent history of acute hypertensive crisis, who presented with left lower quadrant pain and massive rectal bleeding. A: Endoscopic procedure showed multiple necrotic area; B and C: Contrast-enhanced computed tomography (CT) and axial T2 fast-recovery fast-spin echo sequence (FRFSE) magnetic resonance imaging (MRI), after 32 h from CT, showed acute IC (Type I CT and MRI) with wall thickening, three layer sandwich sign and a mild amount of free fluid in the paracolic gutter; D and E: 2D coronal reformat CT and coronal T2 FRFSE MRI, at the same time, showed the entire involved tract; F: Ischemia risolved without complications with conservative therapy as shown in the follow-up MRI.
Figure 2.
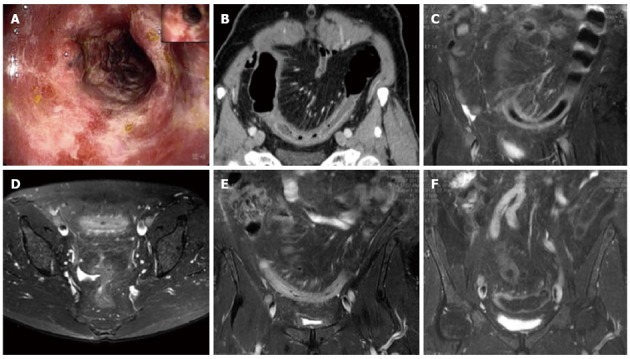
Magnetic resonance imaging follow-up of a patient with ischemic colitis and worsening of clinical symptoms. Ischemic colitis (IC) of sigmoid colon in a 62-year-old man with left lower quadrant pain and elevate lactate dehydrogenase levels, who presented with melena and a recent history of stenting procedures for ischemic cardiopathy. A: Endoscopic procedure showed multiple necrotic area; B: 2D (two dimensional) coronal reformat contrast-enhanced computed tomography (CT) showed acute IC (Type I CT); C-E: The patient had 2 magnetic resonance examinations (C and D-E) with an interval of 48 h due to worsening of clinical symptoms, with an increase of the length and thickness of the involved tract (D-E); F: The ischemic process resolved without complication after parenteral nutrition, as showed in the follow-up magnetic resonance imaging, performed after 384 h from the date of CT examination.
In the remaining 3 MRI examinations, performed within a mean of 410 h (ranging from 99.5 to 720 h) the colonic wall appeared normal.
No patients had circumferential intramural air consistent with pneumatosis coli.
Mild to moderate amounts of free abdomen fluid were present in all patients (100%) on the CT examination, and in 4 out of 4 of the first MR examinations (performed within 48 h from the date of CT examination) and in 1 out of 5 in the second MRI examinations (performed between 48 h and 15 d from the date of CT examination).
The free fluid was mainly located in the paracolic gutters and in the Douglas. No high-attenuation fluid consistent with blood was present. No reduction in caliber of mesenteric vessels was found.
On the basis of the result of the initial CT examination, endoscopic findings and clinical evaluation, no surgery was performed on any of the patients. Surgery was performed 3 wk after the initial episode because of the development of a sealed-off perforation of the transverse colon in one of the patients.
The site of the affected tract and it’s length (mm), gross appearance on CT (type according Balthazar et al[14], thickness of the affected wall and presence or absence of peritoneal fluid) and on MRI (type, thickness of the affected colonic wall and presence or absence of peritoneal fluid), in relation to the date of each examination were reported in Table 2.
Table 2.
Gross appearance on computed tomography and magnetic resonance imaging
| Patient | Involved tract/length (mm) | CT I range 2-168 h1 | MRI I within 48 h2 | MRI II > 48 h <15 d2 | MRI III > 15 d2 |
| 1 | Left side colon and splenic flexure/314 | Type I/12 mm; free fluid | Type I/10 mm; free fluid | Type III/6 mm; no free fluid | |
| 2 | Left side colon, splenic flexure and transverse colon/609 | Type I/12 mm; free fluid | Type II/7 mm; no free fluid | ||
| 3 | Left side colon and splenic flexure/380 | Type II/9.5 mm; free fluid | Type II/7 mm; no free fluid | normal appearance; no free fluid | |
| 4 | Transverse colon/219 | Type II/6 mm; free fluid | normal appearance; no free fluid | ||
| 5 | Entire colon/1000 | Type I/8.5 mm; free fluid | Type I/9.5 mm; free fluid | ||
| 6 | Left side colon/218 | Type I/17.5 mm; free fluid | Type I/15 mm; free fluid | Normal appearance; no free fluid | |
| 7 | Sigmoid colon/145.5 | Type I/8.2 mm; free fluid | Type I/7 mm; free fluid | Type I/8 mm; free fluid | Type III/5 mm; no free fluid |
After the clinical onset;
After the date of the CT examination. MRI: Magnetic resonance imaging; CT: Computed tomography.
DISCUSSION
The diagnosis of IC largely depends on clinical suspicion, especially since many other conditions (e.g., infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) are presented with abdominal pain, diarrhoea and hematochezia[3]. Endoscopy has become the diagnostic test of choice in establishing the diagnosis of IC, although, it can be limited because it could be performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics; in addition a minimal air insufflation should be used to prevent perforation[7,8].
From a radiological point of view, a CT examination is actually considered the main technique for the noninvasive diagnosis of mesenteric ischemia and also in cases of acute abdomen from different and various origins, because it can suggest IC when it is unsuspected, can diagnose complications and exclude other illnesses. However it requires the use of ionizing radiation and an iodinates contrast agent, limiting the possibility to use this technique in a short term follow-up[9-14].
Recently Iacobellis et al[15] has reported that MRI can play a relevant role in the diagnostic management of acute IC and may be substituted for other invasive endoscopic procedures in the diagnosis and grading of IC when an ischemic injury is suggested. Prior publications have described the feasibility of using MRI to evaluate a full range of colonic disease processes, including only one case of IC but without pathological confirmation and using precontrast and postcontrast imaging[16]. Our study is based on the assumption that a parallel between the experimental colonic ischemic damage in the animal model and humans is reasonable[15]. Then our aim has been to validate the utility of MRI in the clinical diagnosis and follow-up of IC, using combined Fast Imaging Employing Steady State Acquisition on a coronal plane and T2-weighted fast-recovery fast-spin echo sequences, both on an axial and coronal plane. About 71.4% of patients that underwent a CT examination within 48 h showed Type I of gross appearance of an involved colonic wall (wall thickening with heterogeneous enhancement and zones of low attenuation compatible with severe colonic edema and enhancement of the mucosa consistent with an acute process, a shaggy contour, a loss of colonic haustra, with varying degrees of pericolic streakiness). The same patients with Type I CT showed Type I appearance at MRI, according to an acute form of IC. The reason why the gross appearance of the involved colonic wall had a thickened and edematous appearance is related to the fact that usually IC is a form of non-occlusive ischemic disease and in most cases, however, there is no evidence of obstruction of a major artery or vein. Then a decrease in blood flow to 20% of the normal flow, associated with small-vessel disease (hypoxia), and reperfusion injury when the blood flow is reestablished are the responsible factors[17,18]. Consequently, any part of the colon can be involved, with no correlation established between the length and site of the involvement and distribution of the superior mesenteric or inferior mesenteric artery or vein[17,19,20]. Segments commonly affected by IC are the splenic flexure (Griffith point) because the marginal artery of Drummond (a system of arcades connecting the major arteries) is occasionally tenuous here and is absent in upto 5% of patients, and the anastomotic plexus between the inferior mesenteric artery distribution and the hypogastric vascular supply (point of Sudeck) at the rectosigmoid junction, because it is distal to the last collateral connection with proximal arteries[7,21]. In our case population a segmental distribution was apparent in 85.71% (6 patients).
The striking differences in the gross morphology of ischemic segment as detected at MRI is probably related to the timing of the examination and to the pathophysiology of the developing anoxic process. In the initial phases of anoxia, mucosal damage occurs first; with more severe and prolonged forms of anoxia, submucosa hemorrhage, edema, and pericolic congestive and edematous changes developing later due to the reperfusion event. Indeed, in the subacute phase (MRI examination performed between 3 and 30 d) the gross morphology has been changed (MRI Type II or III) with a reduction of the thickness of the involved wall (mean 7.2 mm, range 5-8 mm) and a double ring appearance (high signal intensity of the inner layer and low signal intensity of the outer layer for II type MRI and high signal intensity of the outer layer and low signal intensity of the inner layer for Type III MRI), probably for the reduction of edematous phenomena like the CT Type II according to Balthazar.
Although up to 85% of cases of IC managed conservatively improve within 1 or 2 d and resolve completely within 1 or 2 wk, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery. Surgical resection is required when an irreversible ischemic injury and chronic colitis develop as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhoea, and protein-loosing enteropathy[8]. The advantage of the use of MRI for clinical management of IC, is the possibility to perform a short term follow-up without the employment of ionizing radiation or intravenous contrast material. As demonstrated in our study MRI could be used, instead of CT to suggest the diagnosis of the IC in the proper clinical setting, particular when a segmental distribution is evident, in the depiction of other abnormal conditions that may be seen in patients suspected of having IC, and in a short term follow-up, when a clinical worsening occurs, to adequately manage the patient.
Some limitations of the present study should be outlined. Firstly, our patient selection process does not allow for an evaluation of the sensitivity or specificity of MRI for the diagnosis or detection of IC. The other major limitation of both CT and MR imaging in the diagnosis of colonic ischemia is the lack of specificity. The gross morphologic features overlap with those of inflammatory colitis, although the segmental distribution is more often seen in ischemia[22]. In spite of the limited number of subjects who were examined, we are convinced that MRI can provide a valid imaging for the identification of pathological findings of acute IC. Moreover MRI in combination with clinical suspicion, endoscopic and histological findings, can play a key role in the diagnosis and management of IC. In particular MRI can discriminate patients with urgent operative intervention from patients in which a follow-up can be proposed as an alternative to surgery. All these allow for an earlier detection and effective follow-up of IC with a possible earlier adequate treatment.
ACKNOWLEDGMENTS
We thank Miss Julia Hassall for reviewing the manuscript and Miss Francesca Seri and Mr. Duccio Guerrieri for performing the majority of MR examinations.
COMMENTS
Background
The incidence of ischemic colitis (IC) is underestimated because it often has a mild transient nature, clinical presentation can be nonspecific and highly variable, therefore, the diagnosis largely depends on clinical suspicion. In this context the role of imaging techniques remains controversial. Standard radiology yields non-specific and late findings, while computed tomography (CT), the main technique for the noninvasive diagnosis of mesenteric ischemia, is well suited to confirm the clinical suspicion of IC, to suggest IC when it is unsuspected and to diagnose complications, however it requires the use of radiation and an iodinates contrast agent, limiting the possibility to use this technique in a short term follow-up.
Research frontiers
The role of magnetic resonance imaging (MRI) in the diagnostic management of acute IC is still controversial, and nothing is known about the in vivo magnetic resonance findings of IC or about the relationship between MR findings and the onset of clinical symptoms.
Innovations and breakthroughs
To be known, this is the first study using MRI for the evaluation of IC and for the comparison between MRI and CT findings in this pathological condition.
Applications
MRI in combination with clinical suspicion, endoscopic and histological findings, can play a key role in the diagnosis and management of IC. In particular MRI can discriminate patients with urgent operative intervention from patients in which a follow-up can be proposed as an alternative to surgery. In particular the advantage of the use of MRI for clinical management of IC, is the possibility to perform a short term follow-up without the employment of ionizing radiation or intravenous contrast material.
Terminology
Fast imaging employing steady state acquisition sequence (FIESTA): provides images of fluid filled structures with very short acquisition times. The FIESTA sequence uses the T2 steady state contrast mechanism to provide high signal noise ratio images with strong signal from fluid tissues while suppressing background tissue for contrast and anatomic detail of small structures; IC: inflammation of the colon due to colonic ischemia resulting from alterations in systemic circulation or local vasculature.
Peer review
This paper is well written, is original and has good information. It merits to be admitted to publish without susbtantial changes.
Footnotes
P- Reviewer Rodrigo L S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet. 1963;116:53–60. [PubMed] [Google Scholar]
- 2.Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am. 2008;46:877–885, v. doi: 10.1016/j.rcl.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14:7302–7308. doi: 10.3748/wjg.14.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rha SE, Ha HK, Lee SH, Kim JH, Kim JK, Kim JH, Kim PN, Lee MG, Auh YH. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics. 2000;20:29–42. doi: 10.1148/radiographics.20.1.g00ja0629. [DOI] [PubMed] [Google Scholar]
- 5.Westgeest HM, Akol H, Schreuder TC. Pure naratriptan-induced ischemic colitis: a case report. Turk J Gastroenterol. 2010;21:42–44. doi: 10.4318/tjg.2010.0047. [DOI] [PubMed] [Google Scholar]
- 6.Stamatakos M, Douzinas E, Stefanaki C, Petropoulou C, Arampatzi H, Safioleas C, Giannopoulos G, Chatziconstantinou C, Xiromeritis C, Safioleas M. Ischemic colitis: surging waves of update. Tohoku J Exp Med. 2009;218:83–92. doi: 10.1620/tjem.218.83. [DOI] [PubMed] [Google Scholar]
- 7.Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med. 2003;70:920–91, 920-91, 920-91, passim. doi: 10.3949/ccjm.70.11.920. [DOI] [PubMed] [Google Scholar]
- 8.Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med. 2009;76:401–409. doi: 10.3949/ccjm.76a.08089. [DOI] [PubMed] [Google Scholar]
- 9.Angelelli G, Scardapane A, Memeo M, Stabile Ianora AA, Rotondo A. Acute bowel ischemia: CT findings. Eur J Radiol. 2004;50:37–47. doi: 10.1016/j.ejrad.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Romano S, Romano L, Grassi R. Multidetector row computed tomography findings from ischemia to infarction of the large bowel. Eur J Radiol. 2007;61:433–441. doi: 10.1016/j.ejrad.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Mazzei MA, Mazzei FG, Marrelli D, Imbriaco G, Guerrini S, Vindigni C, Civitelli S, Roviello F, Grassi R, Volterrani L. Computed tomographic evaluation of mesentery: diagnostic value in acute mesenteric ischemia. J Comput Assist Tomogr. 2012;36:1–7. doi: 10.1097/RCT.0b013e31823b4465. [DOI] [PubMed] [Google Scholar]
- 12.Mazzei MA, Guerrini S, Cioffi Squitieri N, Imbriaco G, Mazzei FG, Volterrani L. Non-obstructive Mesenteric Ischemia after Cardiovascular Surgery: not so uncommon. Ann Thorac Cardiovas. 2013:In press. doi: 10.5761/atcs.le.12.02154. [DOI] [PubMed] [Google Scholar]
- 13.Mazzei MA, Guerrini S, Cioffi Squitieri N, Genovese EA, Mazzei FG, Volterrani L. [Diagnosis of acute mesenteric ischemia/infarction in the era of multislice CT] Recenti Prog Med. 2012;103:435–437. doi: 10.1701/1166.12884. [DOI] [PubMed] [Google Scholar]
- 14.Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology. 1999;211:381–388. doi: 10.1148/radiology.211.2.r99ma28381. [DOI] [PubMed] [Google Scholar]
- 15.Iacobellis F, Berritto D, Somma F, Cavaliere C, Corona M, Cozzolino S, Fulciniti F, Cappabianca S, Rotondo A, Grassi R. Magnetic resonance imaging: a new tool for diagnosis of acute ischemic colitis? World J Gastroenterol. 2012;18:1496–1501. doi: 10.3748/wjg.v18.i13.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JJ, Semelka RC, Martin DR, Marcos HB. Colon diseases: MR evaluation using combined T2-weighted single-shot echo train spin-echo and gadolinium-enhanced spoiled gradient-echo sequences. J Magn Reson Imaging. 2000;12:297–305. doi: 10.1002/1522-2586(200008)12:2<297::aid-jmri12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Brandt LJ, Boley SJ. Ischemic and vascular lesions of the bowel. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal disease: pathophysiology, diagnosis, management. 5th ed. Philadelphia: Saunders; 1993. pp. 1940–1945. [Google Scholar]
- 18.Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65–83. doi: 10.1016/s0039-6109(16)45628-8. [DOI] [PubMed] [Google Scholar]
- 19.Wittenberg J, Athanasoulis CA, Williams LF, Paredes S, O'Sullivan P, Brown B. Ischemic colitis. Radiology and pathophysiology. Am J Roentgenol Radium Ther Nucl Med. 1975;123:287–300. doi: 10.2214/ajr.123.2.287. [DOI] [PubMed] [Google Scholar]
- 20.Bharucha AE, Tremaine WJ, Johnson CD, Batts KP. Ischemic proctosigmoiditis. Am J Gastroenterol. 1996;91:2305–2309. [PubMed] [Google Scholar]
- 21.Roger AI, David S. Intestinal blood flow and disease of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: Saunders; 1995. pp. 1212–1234. [Google Scholar]
- 22.Philpotts LE, Heiken JP, Westcott MA, Gore RM. Colitis: use of CT findings in differential diagnosis. Radiology. 1994;190:445–449. doi: 10.1148/radiology.190.2.8284397. [DOI] [PubMed] [Google Scholar]


