Abstract
Microbial diversity was assessed in the soils of non-polluted rice fields of Central Rice Research Institute and Choudwar, and textile effluent contaminated (about 30 years) rice fields of Choudwar about 4 years after cessation of pollution. The soils contained 0.62–1.01 % organic C and 0.07–0.12 % total N, and measured 6.18–8.24 pH and 0.6–2.68 mS/cm Eh which were more in the polluted Choudwar soil. The microbial populations (×106 cfu/g soil) in the soils were: heterotrophs 1.21–10.9, spore formers 0.9–2.43, Gram (−)ve bacteria 4.11–8.0, nitrifiers 0.72–1.5, denitrifiers 0.72–2.43, phosphate solubilizers 0.14–0.9, asymbiotic nitrogen fixers 0.34–0.59, actinomycetes 0.07–0.11, fungi 0–0.5 and Bacillus thuringiensis (Bt) 0.4–0.61 which predominated in the polluted soil of Choudwar. The fungi were scarce in the polluted rice fields. The Bt isolates belonged to three motile and one non-motile group. Two motile Bt isolates were phenotyped as Bt subsp. sotto and israelensis, whereas, the non-motile isolate was Bt subsp. wahuensis. All Bt isolates produced extracellular protease, lipase and amylase enzymes. The microbial guilds had positive correlation among themselves, as well as, with soil physico-chemical characters but the fungi had negative relation and the nitrogen fixers were unrelated with the biotic and abiotic components.
Keywords: Microbial dynamics, Bacillus thuringiensis, Textile effluent, Rice, Soil
Introduction
Each soil habitat has unique and well-adapted microbial guilds whose functionalities maintain the nutritional status, degrade pollutants and control different pests and diseases [1, 2]. The adversities like salinity, sodicity, acidity, industrial effluent pollution etc. affect the microbiology, nutrition status and productivity of soil [3, 4]. The Bacillus thuringiensis (Bt) is a unique bacterium which possess polyvalent functions like control of pests and diseases by toxin, antibiotic, siderophore, HCN, bacteriocin etc. production and plant growth promotion (PGP) by auxin (IAA) and ammonia production, P solubilization etc. [2, 5]. The Bt is ubiquitous and constitutes up to 50 % spore forming soil bacteria which may infect more than 50 % insects of the rice fields [1]. So, terrestrial pollution with industrial wastes like textile effluents which contains high salt (40–100 g/l), N, P, K, Fe, Zn, Mn, Cu (0.05–7.42 mg/l), dyes, and a considerable amount of organic matters viz. carbohydrates, fats, phenolic compounds [6–8] change the physico-chemical properties and microbial functionalities of the soil and severely effect the ecology. Although, immediate impact of the textile effluents on aquatic and terrestrial microbiology have been studied [3, 4] but its persistent effects on microbiology in general and Bt diversity in particular, in the rice ecologies have not been explored. Therefore, to evaluate and understand post-pollution (residual) impact of textile effluent on soil functionality, microbial dynamics and diversity (especially Bt) were studied in Orissa Textile Mill (OTM) effluent polluted (about 30 years) rice fields of Choudwar, Cuttack, Orissa after 4 years of discontinuation of effluent discharge (Fig. 1) along with non-polluted rice fields of the Central Rice Research Institute (CRRI) and Choudwar, Cuttack, Orissa.
Fig. 1.
Location map of the sampling sites
Materials and Methods
Soil samples were collected from the wet (microaerobic, 20–30 % moisture content) non-polluted rice fields of CRRI (soil no. 52) and Choudwar (soil no. 53a), as well as, textile effluent polluted (about 30 years) rice fields of Choudwar (soil no. 53) but about 4 years after discontinuation of effluent discharge due to closure of the OTM (Fig. 1). The soil collection fields of CRRI were fertilized with farmyard manure (5 t/ha/year) and NPK (80:40:40 kg/ha/year) but the Choudwar fields received only (0.5 t/ha/year) farmyard manure only. To collect samples, about 1 cm top soil was removed, five samples (about 10 g each) were collected from five locations (at least about 2 m apart) of each field, mixed thoroughly and the composite soils were sealed in sterile polythene packets and brought to the laboratory for analysis.
The pH, Eh, organic carbon (C) and total nitrogen (N) were estimated from the composite soils. To determine soil pH, air-dried powdered soil (200 mesh sieved) sample was suspended in deionized double distilled water at 1:2.5 (w/v) ratio, shaken at 400 rpm for 1 h, centrifuged at 10,000×g and the pH was recorded through a pH meter from the supernatant [9]. To determine Eh, powdered soil sample was suspended in two volumes (w/v) of water, extracted like pH measurement and the Eh (mS/cm) of the supernatant was recorded through a conductivity meter against 0.1 and 1 M KCl standards [9].
To determine organic carbon (C) [10], 1 g air-dried soil (200 mesh sieved) was suspended in 10 ml 1 N potassium dichromate in a 500 ml conical flask and digested with 20 ml conc. H2SO4. The mixture was cooled to room temperature (25 ± 0.1 °C), and within 30 min 200 ml distilled water, 10 ml orthophosphoric acid (88 %, w/v), a pinch of NaF and 1 ml diphenylamine reagent (0.5 g dye dissolved in 20 ml water and made to 100 ml with conc. H2SO4) were added. The solution was titrated with 0.5 N ferrous ammonium sulfate until the colour changed from dark blue to dark green. Two control sets were monitored simultaneously. The organic carbon content (%) of the soil was calculated as: 3(B − T)/BW, where W = gram soil, B = volume of ferrous ammonium sulfate required for titration of the control and T = volume of ferrous ammonium sulfate required for titration of the digested soil.
To estimate total nitrogen (N) [11], 0.5 g air-dried soil (200 mesh sieved) sample was digested in a 30 ml Kjeldahl digestion flask with 1 ml conc. H2SO4 and a pinch of catalyst mixture (CuSO4·5H2O 1 g, selenium 1 g, K2SO4 20 g) until the final product turned colourless. The mixture was cooled to room temperature (25 ± 0.1 °C) and the volume was made up to 10 ml with distilled water. To 1 ml sample, 1 ml silicate mixture [equivolume mixture of 10 % (w/v) Na2SiO3 and 10 % (w/v) NaOH] and 5 ml Nessler’s reagent were added, absorbance was read at 540 nm and the total nitrogen was estimated as (NH4)2SO4 equivalents.
The media and ingredients were sterilized by autoclaving at 121 ± 0.1 °C for 15 min or passing through 0.22 μm membrane filter according to requirement. A portion of the composite soil was blotted to optimum dryness, 1 g soil was suspended in 9 ml sterile distilled water and diluted up to 10−3 level. Soil moisture content was estimated gravimetrically [11]. To enumerate different microbes (colony forming units i.e. cfu/g dr. soil), the soil suspensions (100 μl, 10−3 dilution) were mixed separately with 100 ml of the following media, plated in five plates and incubated at 30 ± 0.1 °C in a BOD incubator up to 7 days for fungi, actinomycetes and sulfur oxidizing bacteria; 25–30 days for nitrifying bacteria and 3 days for other organisms [1]. Heat-treated (60 ± 0.1 °C, 30 min) soil suspensions (100 μl, 10−3 dilution) were used to enumerate the spore forming, as well as, spore-crystal forming bacteria (tentative B. thuringiensis i.e. Bt). The heterotrophic, Gram (−)ve and spore forming bacteria were enumerated on nutrient agar (NA) (g/l: peptone 5, beef extract 3, NaCl 3, pH 7.0, agar 20) plates. Filter-sterilized crystal violet (0.001 g/l final conc.) was added with the medium before plating to visualize (violet colonies) and count the Gram (−)ve bacteria. The nitrifying and denitrifying bacteria were enumerated on Winogradsky’s medium (g/l: K2HPO4 1, NaCl 2, MgSO4·7H2O 0.5, FeSO4·7H2O trace, CaCl2·2H2O 0.02, pH 8.5) containing 1 g/l (NH4)2SO4 or KNO3, respectively. The plates were flooded with sulphanilic acid reagent (equivolume mixture of sulphanilic acid 8 g/l and α-naphthyl amine 5 g/l dissolved separately in 5 M acetic acid) and the pink colonies were counted. The asymbiotic nitrogen fixing bacteria were counted on the nitrogen-free medium (g/l: mannitol 10, K2HPO4 0.5, MgSO4·7H2O 0.2, NaCl 0.2, MnSO4·4H2O trace, FeCl3 trace, agar 18, pH 7.2). The Thiobacillus medium (g/l: Na2S2O3 0.5, (NH4)2SO4 0.4, KH2PO4 4, CaCl2·2H2O 0.25, MgSO4·7H2O 0.5, FeSO4·5H2O 0.01, agar 18), the mycological medium (g/l: peptic digest of soybean meal 10, dextrose 40, agar 18, pH 7) and Krainsky’s medium (g/l: glucose 10, asparagine 0.5, K2HPO4 0.5, agar 15, pH 7) were used to count the sulfur oxidizing bacteria (brown colonies), fungi and actinomycetes, respectively. The colonies which formed halo zones on the insoluble phosphate medium (g/l: glucose 10, Ca3(PO4)2 5, MgSO4·7H2O 0.25, MgCl2·2H2O 5, KCl 0.2, (NH4)2SO4 0.1, agar 18) were counted as the phosphate solubilizing microbes [12].
The bacterial colonies developed on NA plates from the heat-treated inocula were checked under a phasecontrast microscope (100×) and those produced crystal inclusions along with the spores were recorded, isolated, purified and maintained on NA slopes at 4 ± 0.1 °C. The organisms were phenotyped following the standard microbiological methods [13, 14]. Anaerobic growth of the bacteria was checked in 5 ml thioglycolate stab (g/l: peptone 15, glucose 5, yeast extract 5, l-cysteine 0.75, NaCl 2.5, agar 0.75, sodium thioglycolate 0.5, pH 7.2) inoculated with a loopful of bacteria at the bottom of the tubes (20 cm × 20 mm dia.) loosely fitted with screw caps with/without a paraffin overlay. Antibiotic sensitivity of the organisms was checked by standard disc assay technique. Effects of NaCl (3–15 %) and pH (3–13) were studied on NA plates and NB (NA without agar) media, respectively. The extracellular enzymes viz. amylase (starch hydrolysis), protease (gelatinase and caseinase), lipase (Tween 80, cholesterol and vegetable oil hydrolysis), lecithinase (egg yolk hydrolysis) and chitinase were assessed on NA plates, containing 1 % substrate [13].
To ascertain the phylogenetic relations of the representative strains, almost full-length (approximately 1.40–1.45 kbp) 16S rRNA genes were sequenced, matched with similar sequences through the BLAST algorithm and the best matching sequences were retrieved from the database. The 16S rRNA sequences of the isolates were edited for proper grouping, aligned and the similarity was analyzed using the CLUSTAL-X programme. Phylograms were constructed by neighbour-joining (NJ) through the MEGA5 programme. The resultant tree topologies were evaluated by bootstrap analysis based on 1,000 re-samplings.
Populations of the microbial guilds of different soils were analyzed numerically by UPGMA clustering to understand relative abundance of individual and different groups. Similarly, the phenotypic characters of the spore-crystal forming bacteria of each soil [non-polluted (n = 28) and polluted (n = 34) Choudwar soils and CRRI (n = 36) soil] were clustered by the UPGMA to consolidate them. The multivariate principal component analysis (PCA) was performed using SPSS13 SW for ordination of data matrices for physico-chemical properties of soil and their correlation with the population sizes of different groups of bacteria [15].
Results and Discussion
Moisture contents of the experimental soils were 20–30 %. The organic C, total nitrogen, pH and Eh of the soils were 0.78 %, 0.09 %, 6.18 mS/cm and 0.6 mS/cm, respectively in the CRRI soil (no. 52); 1.01 %, 0.12 %, 8.24 mS/cm and 2.68 mS/cm, respectively in the textile effluent polluted Choudwar soil (no. 53), and 0.62 %, 0.07 %, 6.91 mS/cm and 0.8 mS/cm, respectively for the non-polluted Choudwar soil (no. 53a). The results proved that the soil physico-chemical constituents of the effluent polluted soil (no. 53) were higher than the other two soils (nos. 52 and 53a) which, however, had comparable conditions. The polluted soil would be alkaline (pH 8.24) due to deposition of alkali salts of metals (CO32+, HCO3−, OH− etc.) of the effluent [16]. Release of more sodium chloride (40–100 g/l) in the effluent [7] would increase the salinity i.e. conductivity (Eh 2.68 mS/cm) of the polluted Choudwar soil which was not a severe, rather a moderate (0.7–3.0 mS/cm) osmotic stress condition [17]. However, the pH (6.18–8.24) and Eh (0.6–2.68 mS/cm) of the soils conformed to the permissible limit of Indian and international (APHA) standards of pH (8.33) and Eh (0.89 mS/cm) for textile effluents [16]. Nevertheless, substantially lower pH (6.8) of the polluted soil than that (pH 9.6–12.5) of the raw textile effluent [18] indicated reclamation of the soil within 4 years after closure of the OTM factory. Although toxic, the textile effluents would have enriched the Choudwar soil with organic carbon (starch, carboxymethyl cellulose, cotton fibre, gum, pectin etc.), oil, fat, wax, polyvinyl alcohol, surfactants, detergents, oxidizing and reducing agents, paste, dye, phenol, NaOCl, NaOH, sodium phosphate, nitrate, ammonium salts, sulphide, heavy metals (Cr, Al, Cu, Mn, Fe, Zn, Hg etc.), silicates etc. most of which have longer persistence in the soil [8, 19–22]. Thus, although fertilization (0.5 t/ha FYM) was lower than the CRRI fields, the C, N and Eh were higher in effluent polluted Choudwar soil, even 4 years after discontinuation of effluent discharge.
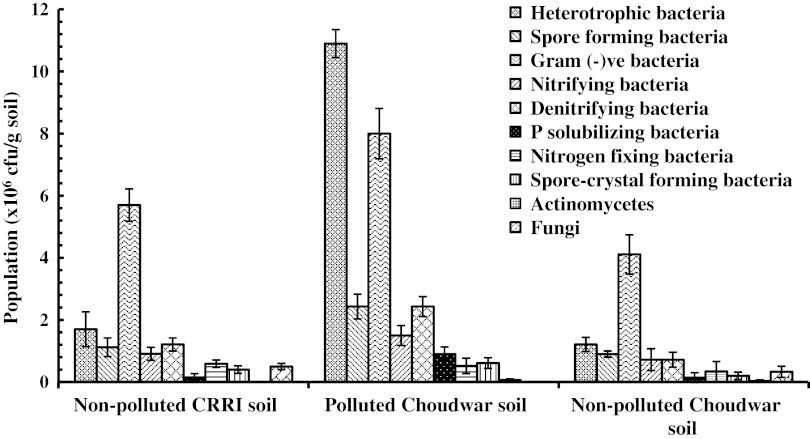
The population density (×106 cfu/g dr. soil) of heterotrophic (1.7, 10.9 and 1.21), spore forming (1.12, 2.43 and 0.9), Gram (−)ve (5.7, 8 and 4.11), nitrifying (0.91, 1.51 and 0.72), denitrifying (1.21, 2.43 and 0.72), phosphate solubilizing (0.15, 0.9 and 0.14) and the asymbiotic N2 fixing (0.59, 0.52 and 0.34) and spore-crystal forming (0.46, 0.54 and 0.23) microbes were comparable in the CRRI (soil no. 52) and non-polluted (no. 53a) Choudwar soils but they counted more in the polluted Choudwar soil (Fig. 2). Furthermore, unlike the non-polluted soils, degree of increase of heterotroph pool superseded the Gram (−)ve bacteria in the polluted soil (Fig. 2) which could be expected from higher population build up of the Gram (+)ve bacteria that predominate and establish more efficiently in soil [23]. Nevertheless, despite variations of the populations within the three soils, the microbial guilds grossly corroborated to those (106–108 cfu/g soil) of the other mesophilic and saline rice fields [1, 24] but were more than those (102–106 cfu/g) of the continuous or regular textile effluent polluted or irrigated rice fields [3]. As the Choudwar rice fields did not receive effluent for 4 years due to closure of the textile mill, natural reclamation of the soil might have reduced the residual toxic effects allowing re-establishment and build up of the resident microbes supported by residual organic C, N, K etc. of the textile effluent [19–22]. However, soil regeneration time for textile effluent pollution has been poorly studied in India, and therefore, optimum recovery period could not be ascertained from the present study. In the soils, the actinomycetes (0.07–0.11 cfu/g) and fungi (0–0.5 cfu/g) were negligible (Fig. 2) suggesting negative relation of the organisms with water content which affected their growth in the wet rice fields and reduced pool size [25, 26]. As the fungi and free-living nitrogen fixing bacteria favour acidic to near neutral pH, respectively [4, 26], they were minimum in the polluted (pH 8.24) soil (Fig. 2). Concomitant with descending order of carbon (C) and nitrogen (N) levels, the microbial guilds were more in the polluted soil of Choudwar (higher contents) followed by CRRI and non-polluted Choudwar soils (Fig. 2). Continuous rice cultivation with only about 0.5 t/ha FYM application in non-polluted Choudwar soil would limit C and N availability resulting in lower microbial population than the balanced fertilized (FYM: 5 t/ha and N:P:K::80:40:40 kg/ha) CRRI and nutrient enriched polluted Choudwar fields. Probably, the metal ions which precipitate in alkaline pH but solubilize in acidic pH [4] would have either reduced availability of the metal ions below the toxic limits (mg/l: Cr < 0.1; Al, Cu, Zn < 1; Mn 5, Fe 20 and Hg 0.05) in the alkaline (pH 8.24) polluted soil or was reclaimed naturally within 4 years of closer of OTM i.e. effluent discharge [19]. Soil microbes also metabolize Fe, Zn, Co, Cu, Pb, Ni, Mn, K, Hg, Se, As etc. which are useful for plant nutrition and mineralization [26]. The moderate salinity (2.68 mS/cm) level [17] of the polluted Choudwar soil would not be able to inhibit the microbes but would induce stress tolerance [27] and allow increase of population using higher C and N sources. Aerobic soil conditions would also counter the impact of pollution effected anoxic stress and support growth of the aerobic microbes [19]. Thus, increase in diverse microbial pool in the soil would recycle organic carbon, nitrogen, phosphorous etc. and remediate the toxic compounds at faster pace [19]. However, conflicting reports viz. enhancement of crop growth by irrigation of diluted effluent water due to supplementation of different nutrients [19, 28], as well as, its negative impact [3, 29] were observed by different workers.
Fig. 2.
Population dynamics of different microbes in the soil samples
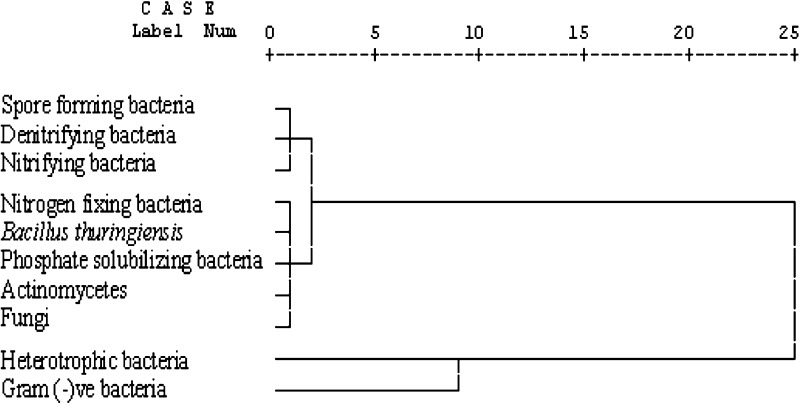
Correlation analysis of the soil physical characters and microbial populations revealed that all microbes (other than fungi) had positive relation with the former, as well as, among themselves but the free-living nitrogen fixing bacteria were independent and the fungi had negative relation with other microbes and soil properties (Table 1). As the fungi are primary decomposers, they would decline with concomitant increase of other microbial guilds [30]. Parallel trends of relations of the microbes (but fungi) with physical characters of the soils favoured that 4 years reclamation period would be helpful to sustain the ecology. The pool sizes of the spore forming, denitrifying and nitrifying bacteria in different soils clustered together revealing that the guilds were comparable (Fig. 3). Similarly, population sizes of the free-living N2 fixing, spore-crystal forming and P-solubilizing bacteria, actinomycetes and fungi were comparable in different soils (Fig. 3). The heterotroph and Gram (−)ve bacterial populations were more and formed a distinct cluster from the other guilds (Fig. 3) i.e. they predominated in the soils.
Table 1.
Correlation matrix among different groups of soil microbes with soil physical and chemical properties
| Components | Heterotrophic bacteria | Spore forming bacteria | Gram (−)ve bacteria | Nitrifying bacteria | Denitrifying bacteria | P solubilizing bacteria | N2 fixing bacteria | B. thuringiensisa | Actinomycetes | Fungi | Organic C | Total N | pH | Eh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterotrophic bacteria | 1 | |||||||||||||
| Spore forming bacteria | 0.996*** | 1 | ||||||||||||
| Gram (−)ve bacteria | 0.930*** | 0.959*** | 1 | |||||||||||
| Nitrifying bacteria | 0.981*** | 0.994*** | 0.983*** | 1 | ||||||||||
| Denitrifying bacteria | 0.972*** | 0.988*** | 0.990*** | 0.998*** | 1 | |||||||||
| P solubilizing bacteria | 0.999*** | 0.992*** | 0.918*** | 0.974*** | 0.963*** | 1 | ||||||||
| N2 fixing bacteria | 0.289ns | 0.372ns | 0.618* | 0.465ns | 0.506ns | 0.257ns | 1 | |||||||
| B. thuringiensisa | 0.893*** | 0.930*** | 0.995*** | 0.962*** | 0.974*** | 0.878*** | 0.687* | 1 | ||||||
| Actinomycetes | 0.795** | 0.738* | 0.519ns | 0.666* | 0.631* | 0.815** | −0.349ns | 0.439ns | 1 | |||||
| Fungi | −0.926*** | −0.889*** | −0.725* | −0.838** | −0.812** | −0.938*** | 0.092ns | −0.659* | −0.965*** | 1 | ||||
| Organic C | 0.930*** | 0.959*** | 0.999*** | 0.983*** | 0.990*** | 0.917*** | 0.620* | 0.996*** | 0.518ns | −0.723* | 1 | |||
| Total N | 0.934*** | 0.962*** | 0.999*** | 0.985*** | 0.991*** | 0.922*** | 0.611* | 0.996** | 0.528ns | −0.732* | 0.999*** | 1 | ||
| pH | 0.920*** | 0.882*** | 0.713* | 0.829** | 0.802** | 0.932*** | −0.108ns | 0.647* | 0.969*** | −0.999*** | 0.712* | 0.720** | 1 | |
| Eh | 0.991*** | 0.975*** | 0.874*** | 0.948*** | 0.932*** | 0.995*** | 0.160ns | 0.827** | 0.868*** | −0.968*** | 0.873*** | 0.879*** | 0.963*** | 1 |
ns not significant
Significant at * p < 0.05; ** p < 0.01; *** p < 0.001
aSpore-crystal forming bacteria
Fig. 3.
Cluster analysis of the microbial populations in different soils
The spore-crystal forming bacterial population in the soils varied between 4.01 and 6.12 × 105 cfu/g soil (Fig. 2) and was more in polluted Choudwar soil (Fig. 2). The spore-crystal forming bacterial index (ratio of spore-crystal forming and total spore forming bacteria) in CRRI, and non-polluted and polluted Choudwar soils were 0.36, 0.22 and 0.25, respectively which were either comparable or more than those of the other mesophilic and coastal saline soils [1]. From CRRI, as well as, polluted and non-polluted Choudwar fields, 36, 28 and 34 spore-crystal forming bacteria (n = 98), respectively, were isolated and they were divided into crystal morphotype groups. Among the 36 spore-crystal forming bacteria of CRRI, 20 (55.6 %) isolates produced heterogeneous, 8 (22.2 %) produced bipyramidal and 8 (22.2 %) produced spherical crystals. Similarly, out of 28 spore-crystal forming bacteria of the polluted Choudwar soil, 13 had heterogeneous (46.4 %), 11 had spherical (39.3 %) and 4 had bipyramidal (14.3 %) crystals. Out of the 34 spore-crystal forming bacteria, the non-polluted soil of Choudwar also harboured 15 heterogeneous (44.1 %), 10 bipyramidal (29.4 %) and 9 spherical (26.5 %) crystals. The results indicated that the three soils contained four diverse types of spore-crystal forming bacteria. More diversity (four types), pool size (0.61 × 106 cfu/g) and Bt index (0.36) in polluted Choudwar soil than the other two soils also supported natural detoxification of the soil in 4 years of with-holding the discharge.
The heterogeneous crystal forming bacteria predominated over the cumulative population of other groups which revealed that they were functionally dominant in the soils. Therefore, diversity of the heterogeneous crystal forming bacteria viz. TB81 and TB81a of the polluted and non-polluted Choudwar soils, respectively and TB19 and TB142 of CRRI soil were further characterized [14]. The spore-crystal forming strains produced circular, white, flat and undulate (TB19) or entire (TB81, TB81a and 142) colonies (Table 2). The sizes of vegetative cells, spores and crystals were 4.22–4.8 × 1.45–1.84, 1.58–1.85 × 0.93–1.1 and 1.4–2.2 × 0.98–1.35 μm, respectively (Table 2). The TB19 produced larger and TB142 produced smaller crystals (Table 2). The bacteria were Gram (+)ve, spore and crystal forming rods; other than TB19, they were motile, did not grow on mycological agar or anaerobically, tolerated 6–13 % NaCl and showed differences of some physiological and biochemical tests (Table 2). The phenotypic characters (Table 2) conclusively proved that the organisms belonged to Group I of the genus Bacillus and species thuringiensis [14]. Phenotyping tentatively identified the non-motile TB19 as Bt subsp. wahuensis; and TB81, TB81a as Bt subsp. sotto, and TB142 as Bt subsp. israelensis (Table 2) [19]. In spite of belonging to different subspecies, the Bt strains produced heteromorphic crystals (Table 2) which supported that serovar, crystal morphotype and Cry composition are not correlated [1]. On the basis of 16S rRNA gene sequences, a phylogenetic tree rooted with B. thuringiensis was constructed which showed that the strains were affiliated to B. cereus cluster (Fig. 4) and confirmed the identity of the organisms. All the spore-crystal forming bacterial isolates were sensitive to streptomycin, vancomycin, polymyxin B and norfloxacin but resistant to penicillin G and ampicillin/sulbactam (Table 2) which favoured that Bt are generally resistant to the penicillin group of antibiotics [1].
Table 2.
Phenotyping of the bacteria isolated from different soils
| Character | Bacteria (TB) | |||
|---|---|---|---|---|
| 19 | 81 | 81a | 142 | |
| Motility | Non-motile | Motile | Motile | Motile |
| Colony character1 | CWFUM | CWFEM | CWFEM | CWFEM |
| Bacterium character (l × w, μm) | Rod, 4.80 ± 0.1 × 1.45 ± 0.02 | Rod, 4.25 ± 0.12 × 1.80 ± 0.01 | Rod, 4.22 ± 0.08 × 1.84 ± 0.02 | Rod, 4.30 ± 0.09 × 1.80 ± 0.01 |
| Spore character (l × w, μm) | Elliptical, 1.58 ± 0.09 × 0.93 ± 0.01 | Elliptical, 1.83 ± 0.11 × 1.0 ± 0.01 | Elliptical, 1.85 ± 0.11 × 0.98 ± 0.04 | Elliptical, 1.85 ± 0.08 × 1.1 ± 0.02 |
| Crystal morphotype | Bipyramidal, rhomboidal | Pentagonal, irregular | Pentagonal, irregular | Polygonal, irregular |
| Crystal size (l × w, μm) | 2.20 ± 0.18 × 1.35 ± 0.01 | 1.76 ± 0.16 × 1.13 ± 0.09 | 1.72 ± 0.09 × 1.11 ± 0.02 | 1.40 ± 0.11 × 0.98 ± 0.01 |
| NaCl (%) tolerance | 6 | 7 | 8 | 13 |
| Na-acetate (M) tolerance | 0.3 | 0.25 | 0.25 | 0.25 |
| Antibiotic sensitivity | abcd | abcd | abcd | abcd |
| Antibiotic resistance | e | e | e | e |
| Gram stain, catalase, tributyrin hydrolysis, protease, starch hydrolysis, lecithinase, methyl red, nitrate reduction, Vogues–Proskauer test, sucrose fermentation, cellobiose utilization, esculin fermentation2 | + | + | + | + |
| Anaerobic growth, oxidase, chitinase, mannose utilization, salicin fermentation, indole production, arginine dihydrolase3, urease4 | – | – | – | – |
| Pellicle formation, tween esterase, cholesterol utilization | – | ± | + | + |
| Acid and gas production from glucose, trehalose, fructose, glycerine, glycogen, galactose, cellobiose, sorbitol, mannitol, maltose, inositol5 | + | + | + | + |
| Acid gas from arabinose | – | – | – | – |
| Acid and gas production from raffinose, rhamnose, tween esterase, cholesterol utilization | – | ± | + | + |
| Acid and gas production from xylose, dulcitol and lactose | – | + | +6 | – |
| Genus | Bacillus | Bacillus | Bacillus | Bacillus |
| Species | thuringiensis | thuringiensis | thuringiensis | thuringiensis |
| Subspecies (tentative) | wahuensis | sotto | sotto | israelensis |
1C = circular, W = white, M = metallic, GW = gummy white, CW = creamish white, BW = brownish white, R = raised, E = entire, U = undulate, L = lobate, G = gummy; 2Negative for TB142; 3Positive for TB142; 4± for TB19 and 142; 5Negative for TB142; 6± for xylose, arabinose, adonitol and rhamnose; a = Streptomycin, norfloxacin (10 μg/disc), vancomycin (30 μg/disc), polymyxin (300 U/disc); b = erythromycin (15 μg/disc); c = bacitracin (10 U/disc); d = triplesulph (300 μg/disc); e = penicillin G (10 U/disc), ampicillin/sulbactam (10/10 μg/disc), trimethoprim (30 μg/disc); + = positive result; − = negative result; Results were recorded from all isolates of each subspecies
Fig. 4.
Phylogram of B. thuringiensis strains and related species based on 16S rRNA gene sequence
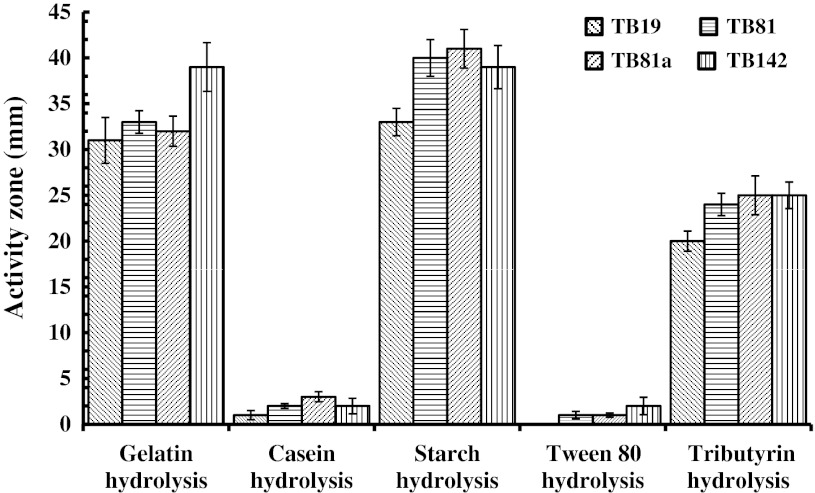
Osmotic stress tolerance (7 %) of TB81 (Table 2) could be attributed to its exposure to the abiotic stresses in effluent polluted soil having moderate osmoticum, alkalinity, lower oxygen tension, metal contamination, organic enrichment etc. [4, 17]. However, salt tolerance of the Bt of all soils complemented the proposition that osmotolerant Bt was widespread in rice soils [1]. Nevertheless, NaCl (13 %) tolerance of TB142 of the non-saline and mesophilic CRRI soil would be possible due to their exposure to organic manuring (FYM) and fertilizers which would also induce osmotic stress tolerance. However, lower (7 %) salt tolerance of TB81 (Table 2) of effluent polluted (stressed) soil from Choudwar than TB142 of the mesophilic soil of CRRI suggested that salinity tolerance is independent and intrinsic character of Bt [1]. The Bt isolates produced diverse extracellular enzymes viz. proteases, amylase and lipases but failed to hydrolyze pectin and chitin (Table 2; Fig. 5) which supported that endospore forming bacteria, 93 % mesophiles, thermophiles and the stress tolerant organisms would produce extracellular proteases [31] and would be an efficient PGP bacteria [1]. Besides, Bt is also known to increase water use efficiency in different plants [32]. So, the alkali and osmotolerant Bt with combination of hydrolytic enzyme activities could be used for leather and food processing, pollutant recycling, as well as, for PGP in mesophilic, alkaline and saline ecologies [29].
Fig. 5.
Extracellular enzyme activities of the B. thuringiensis isolates
Acknowledgments
The authors thank AMAAS, ICAR for financial support to carry out the research.
References
- 1.Das J, Dangar TK. Microbial population dynamics, especially stress tolerant Bacillus thuringiensis, in partially anaerobic rice field soils during post-harvest period of the Himalayan, island, brackish water and coastal habitats of India. World J Microbiol Biotechnol. 2008;24:1403–1410. doi: 10.1007/s11274-007-9620-3. [DOI] [Google Scholar]
- 2.Zhou Y, Choi YL, Sun M, Yu Z. Novel roles of Bacillus thuringiensis to control plant diseases. Appl Microbiol Biotechnol. 2008;80:563–572. doi: 10.1007/s00253-008-1610-3. [DOI] [PubMed] [Google Scholar]
- 3.Rao AV. Microbiological productivity of soils as influenced by textile industrial effluents. In: Gupta IC, Joshi DC, Kumar D, editors. Industrial waste waters and environmental pollution. Jodhpur: Scientific Publishers; 2000. pp. 80–85. [Google Scholar]
- 4.Wynne G, Maharaj D, Buckley C. Cleaner production in the textile industry—lessons from the Danish experience. Durban: School of Chemical Engineering, University of Natal; 2001. p. 3. [Google Scholar]
- 5.Raddadi N, Belaouis A, Tamagnini I, Hansen BM, Hendriksen NB, Boudabous A, Cherif A, Daffonchio D. Characterization of polyvalent and safe Bacillus thuringiensis strains with potential use for biocontrol. J Basic Microbiol. 2008;48:1–11. doi: 10.1002/jobm.200890001. [DOI] [PubMed] [Google Scholar]
- 6.Garg VK, Kaushik P. Influence of textile mill wastewater irrigation on the growth of sorghum cultivars. Appl Ecol Environ Res. 2008;6:1–12. [Google Scholar]
- 7.Ogugbue CJ, Sawidis T. Assessment of bio elimination and detoxification of phenothiazine dye by Bacillus firmus in synthetic wastewater under high salt conditions. J Appl Sci. 2011;11:2886–2897. doi: 10.3923/jas.2011.2886.2897. [DOI] [Google Scholar]
- 8.Babayan M, Javaheri M, Tavassoli A, Esmaeilian Y. Effects of using wastewater in agricultural production. Afr J Microbiol Res. 2012;6:1–6. [Google Scholar]
- 9.Gupta PK. Methods in environmental analysis water, soil and air. New Delhi: Agrobios; 2004. pp. 242–245. [Google Scholar]
- 10.Needleman BA, Wander MM, Shi GS. Organic carbon extraction efficiency in chloroform fumigated and non-fumigated soils. Soil Sci Soc Am J. 2001;65:731–1733. [Google Scholar]
- 11.Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall; 1973. [Google Scholar]
- 12.Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;70:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 13.Smibert R, Krieg NR. Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood W, Krieg E, editors. Methods for general and molecular bacteriology. Washington, DC: American Society for Microbiology; 1995. pp. 607–654. [Google Scholar]
- 14.Logan NS, De Vos P. Genus I. Bacillus. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwing W, Rainey FA, Schleifer KH, Whitman WB, editors. Bergey’s manual of systematic bacteriology. New York: Springer; 2009. pp. 21–128. [Google Scholar]
- 15.Kinnear PR, Gray CD. SPSS for Windows made simple, Release 10. Sussex: Psychology Press; 2000. [Google Scholar]
- 16.Mohabansi NP, Tekade PV, Bawankar SV. Physico-chemical and microbiological analysis of textile industry effluent of Wardha region. Water Res Dev. 2011;1:40–44. [Google Scholar]
- 17.O’Connor GA, Elliott HA, Bastian RK. Degraded water reuse: an overview. J Environ Qual. 2008;37:S157–S168. doi: 10.2134/jeq2007.0459. [DOI] [PubMed] [Google Scholar]
- 18.Sarayu K, Sandhya S. Potential of facultative microorganisms for biotreatment of textile wastewater. Envis Centre Newslett. 2009;7:6. [Google Scholar]
- 19.Yusuff RO, Sonibare JA. Characterization of textile industries effluents in Kaduna, Nigeria and pollution implications. Global Nest Int J. 2004;6:212–221. [Google Scholar]
- 20.Bhaskar R, Begam MS, Sundaram S. Characterization and reuse of textile effluent treatment plant waste sludge in clay bricks. J Univ Chem Technol Metal. 2006;41:473–478. [Google Scholar]
- 21.Singh A, Agrawal M. Effects of municipal waste water irrigation on availability of heavy metals and morpho-physiological characteristics of Beta vulgaris L. J Environ Biol. 2010;31:727–736. [Google Scholar]
- 22.Bajwa R, Khokhar I, Mukhtar I, Mushtaq S. Isolation and identification of filamentous fungi from different industrial effluents. Plant Product Res J. 2010;14:32–35. [Google Scholar]
- 23.Francis I, Holsters M, Vereecke D. The Gram-positive side of plant–microbe interactions. Environ Microbiol. 2010;12:1–12. doi: 10.1111/j.1462-2920.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 24.Liesack W, Schnell S, Revsbech NP. Microbiology of flooded rice paddies. FEMS Microbiol Rev. 2000;24:625–645. doi: 10.1111/j.1574-6976.2000.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 25.Reichardt W, Briones A, de Jesus R, Padre B. Microbial population shifts in experimental rice systems. Appl Soil Ecol. 2001;17:151–163. doi: 10.1016/S0929-1393(01)00122-6. [DOI] [Google Scholar]
- 26.SubbaRao NS. Soil microorganisms and plant growth. New Delhi: Oxford IBH Publication; 2007. [Google Scholar]
- 27.Moat AG, Foster JW, Spector MP. Microbial physiology. New York: Wiley–Liss Inc.; 2002. [Google Scholar]
- 28.Saravanamoorthhyl MD, Ranjitha Kumari BD. Study of textile effluent on physiological and biochemical changes in two peanut varieties. In: Jayabalan N, editor. Plant biotechnology. New Delhi: APH Publishing Corporation; 2006. pp. 293–301. [Google Scholar]
- 29.Venugopal M, Saramma AV. An alkaline protease from Bacillus circulans BM 15, newly isolated from a mangrove station: characterization and application in laundry detergent formulations. Indian J Microbiol. 2007;47:298–303. doi: 10.1007/s12088-007-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polyanskaya LM, Ivanov KE, Guzev VS, Zvyagintsev DG. Estimation of abundance dynamics of Gram-negative bacteria in soil. Microbiology. 2008;77:760–764. doi: 10.1134/S0026261708060143. [DOI] [PubMed] [Google Scholar]
- 31.Gorlach-Lira K, Coutinho HDM. Population dynamics and extracellular enzymes activity of mesophilic and thermophilic bacteria isolated from semi-arid soil of northeastern Brazil. Brazilian J Microbiol. 2007;38:135–141. doi: 10.1590/S1517-83822007000100028. [DOI] [Google Scholar]
- 32.Barrow JR, Lucero ME, Rayes-Vera I, Havstad KM. Do symbiotic microbes have a role in plant evolution, performance and response to stress? Commun Integr Biol. 2008;1:69–73. doi: 10.4161/cib.1.1.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]