Abstract
The production of many Pseudomonas aeruginosa virulence factors and secondary metabolites is regulated in concert with cell density by quorum sensing (QS). Therefore, strategies designed to inhibit QS are promising for the control of diseases. Here, we succeeded in isolating soil bacteria (56 out of 7,000 isolates) capable of inhibiting violacein production by Chromobacterium violaceum CV026. We focused on an isolate identified as a Pseudomonas sp. based on its 16S rRNA nucleotide sequence. A partially purified inhibitor factor(s) derived from culture supernatants consisted of at least three major components by HPLC analysis. A more highly purified preparation (16 μg/ml) specifically inhibited rhl-controlled pyocyanin and rhamnolipid production by wild type P. aeruginosa PAO1 (PAO1) and a QS double mutant PAO-MW1, without affecting growth. A significant inhibitory effect on elastase, protease and biofilm was also observed. These results provide compelling evidence that the inhibitor(s) interferes with the QS system. The identities of the inhibitors remain to be established.
Keywords: Pseudomonas aeruginosa, Quorum sensing, Rhl system, Screening
Introduction
The production of virulence factors by many Gram-negative bacteria is regulated in a cell density-dependent manner known as quorum sensing (QS). Pseudomonas aeruginosa is one of the major causes of nosocomial infections. It possesses two main QS systems designated las and rhl. The rhl system is regulated by the las system in a hierarchical manner at the transcriptional and posttranscriptional levels. N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL) and N-butanoyl-l-homoserine lactone (BHL) act as autoinducers in each system, respectively. Autoinducers accumulate as cell density increases. When a threshold is reached, the autoinducers activate the receptors LasR and RhlR, which triggers a regulatory cascade [1].
Abuse of antibiotics in pharmacotherapy has made it crucial to find alternatives to currently available drugs. Because the QS system plays an important role in the virulence of diverse bacterial species, inhibition of this system may attenuate their pathogenicity. Since the first demonstration of a natural QS inhibitor (QSI), halogenated furanones from a marine alga Delisea pulchra [2], a large number of these compounds have been identified from both chemical synthesis programs [3] and natural sources [4]. The rhl system is also critical for the regulation of some key virulence factors such as pyocyanin and rhamnolipid. Compounds that inhibit the rhl system have been identified in diverse organisms, including medicinal plants [5], edible food [6], marine sponges [7], seaweeds [8] and even bacteria themselves [9].
Soil bacteria are a rich and promising source of natural products, making them a focus for antimicrobial drug discovery. In contrast, a few studies, to our knowledge, have devoted to the isolation anti-QS compounds from soil bacteria [10]. The goal of the present study was to attempt to isolate new and perhaps more powerful QS inhibitors from soil bacteria using a new QSI screening system. We succeeded in identifying QS inhibitors from cultures of a P. aeruginosa isolate that hold promise for clinical use.
Materials and Methods
Bacterial Strains and Culture Conditions
Chromobacterium violaceum strain CV026 which is deficient in the production of C6-homoserine lactone was used as reporter stain. With addition of external short chain HSL, it can produce purple pigmentation [11]. P. aeruginosa PAO1 is wild-type. PAO-MW1 (lasI/rhlI) is deficient in the production of both the signaling molecules, but responds to them [12]. They were grown in LB medium (tryptone 1 %, yeast extract 0.5 %, NaCl 1 %). Soil isolates were maintained on R2A media. (yeast extract 0.05 %, tryptone 0.05 %, asein acid hydrolysates 0.05 %, glucose 0.05 %, starch 0.05 %, sodium pyruvate 0.03 %, KH2PO4 0.03 %, MgSO4 0.005 %, agar 1.5 %). For quantitative assays, either PPB (Pepton 2 %, MgCl2 0.14 %, K2SO4 1 %, Glycerol 2 %) or PTSB (Polypeptone 5 %, Tryptic Soy Broth 0.25 %) was used. For plasmid maintenance, CV026 was cultured in the presence of 50 μg/ml kanamycin.
Soil Sample Collection and Isolation of Bacteria
Soil samples were collected from Jimei tidal flat, beach of Jiulong River, Fujian; Baicheng, Jiling; Zhangzhou River, Fujian and Qubaqiao wetland, Tibet, China. We acquired samples at the surface, removing the subsurface soil and large particles and placed the inner soil in sterile plastic bags. Each sample (5 g) was suspended in 20 ml of PBS in a sterile conical flask with glass beads. Soil suspensions were shaken for 2 h, spread on the plate and incubated at room temperature for 24 h. Pure colonies were obtained by repetitive dilution streaking and stored in 20 % glycerol at −20 °C.
Preliminary Screening for QSI Activity
In preliminary color screens for QSI activity, we used the biomonitor CV026 for short-chain AHL inhibitor detection. CV026 was cultured in LB broth plus 50 μg/ml kanamycin with shaking overnight. Total 15 ml of melted LB agar medium (50 °C) mixed with 15 μl overnight culture and 20 μM BHL were poured over the plates. Test organisms were dotted on the center or corner of the plate, incubated at 28 °C and observed for inhibition zones. A ring of colorless but viable cells around the growing test colony proved to indicate QSI.
Secondary Screening of the Putative Inhibitory Factors
Putative inhibitory strains were then cultivated in R2A medium. Five hundred milliliters of culture was collected by centrifugation and the supernatant was extracted twice with an equal volume of ethyl acetate. After removal of the organic phase solvent, the extract was sterilized by filtered through a 0.45 μm membrane filter, diluted to 0.5 ml by methanol and stored at −20 °C. Holes were bored using a small stainless puncher in LB agar plate containing CV026 and 20 μM BHL. A 20 μl aliquot of extract was placed into each hole. Methanol was used as the negative control. These plates were then incubated at 28 °C for 24 h.
Phylogenetic Characterization
DNA extraction from the strongest inhibition strain was used as templates. Amplification of the 16S rDNA gene was performed with the universal forward (27F 5′-AGAGTTTGATCMTGGCTCAG-3′) and reverse (1492R 5′-TACGGYTACCTTGTTACGACTT-3′) primers. The complete 16S rDNA gene sequence was determined by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. Sequence data were compared with the GenBank nucleotide sequence database. The tree was calculated with partial 16S rRNA gene sequences (1,076 bp) by using the maximum composite likelihood implemented in Mega 5.0 (http://www.megasoftware.net/).
Bioassay of Culture Supernatant Extracts
The LB agar plate was cut into separate slices (7 mm in width). Test samples were added onto the end of the slices. A fresh culture of the reporter strain CV026 was spotted (0.4 μl) progressively on slices. The blank (methanol) and negative controls (7.5 μl methanol and 5 μl of 200 μM BHL) were used to confirm that the reporter strains could be induced only by the QS autoinducers BHL. These plates were incubated at 28 °C overnight to observe the color change. The distance from the first induced colored colony to the origin of the isolated inoculated in each agar slice was measured and the inhibition ability of the anti-QS was estimated.
Fractionation of Inhibitory Factors by Bioassay
The strongest inhibition strain was cultivated in 70 l LB medium batches and the culture supernatants were extracted as described above. The extract was subjected to a bioassay-guided fractionation scheme. It was first fractionated by passage through a silica column with three different eluents sequentially (4:1 ethyl acetate/petroleum ether, ethyl acetate, methanol) yielding three active fractions as determined by the plate bioassay. Positive fractions were collected. The mixtures were then loaded onto a silica column with an ethyl acetate/petroleum ether (2:1) mobile phase and were eluted using an ethyl acetate gradient.
The active subfractions were collected and further fractionated by thin-layer chromatography (TLC). As a control, an aliquot of 1 μl sample was diluted and spotted onto a TLC plate; the remainder of the concentrated sample was applied to a preparative TLC plate as a continuous line. Two plates were chromatographed in the same chamber using ethyl acetate/petroleum ether (3:1) as the mobile phase. After chromatography, the preparative plate was kept in a sealed box at 4 °C, and the control analysis plate was then incubated with addition of 15 ml LB agar containing CV026 strain and 20 μl BHL for 24 h. Following incubation of the control TLC plate, the matrix in the regions of the preparative plate corresponding to the inhibitory regions of control TLC plate were scraped off, combined, and extracted three times with ethyl acetate. The combined extracts were centrifuged, filtered, dried and weighed 10.8 mg.
The final product was analyzed by HPLC at 254 nm using a reverse-phase C18 column (Zorbax ODS 250 × 4.6 mm) that was eluted with a mixture of water:methanol (15:85) at a flow rate of 1 ml/min. The effect of extract on bacterial cell proliferation was determined according to a published procedure [9]. The growth curve of PAO1 and PAO-MW1 were determined for up to 24 h.
Antimicrobial Assays
The effect of extracts on cell proliferation was determined by monitoring individual strain growth curve of CV026, PAO1 and PAO-MW1. CV026 was cultured in 28 °C, PAO1 and PAO-MW1 in 37 °C. After overnight cultivation, LB cultures were adjusted to an OD600 of 0.05 with fresh medium, the PAO-MW1 was supplemented with 100 nM OdDHL and 2 uM of BHL, and divided into 3 ml aliquots, to which methanol (control) or a series of gradient extract was added. The OD600 was monitored at 2 h intervals until a final time point of 20 h.
Assays for Virulence Factors
The final product was assayed for anti-QS. The pyocyanin assay [12] is based on its absorbance at 520 nm. Protease activity was measured using the azocasein method [10]. Elastase activity was assessed using elastin-congo red as described [13]. Rhamnolipid was monitored by absorbance at 421 nm of orcinol-treated supernatants [14].
Biofilm Formation Assay
PAO1 and its mutant PAO-MW1 were incubated with a plastic coverslip (Thermanox plastic, Thermo Fisher Scientific Co., Ltd) in a 24-well plate containing 1 ml LB medium at 37 °C for 24 h [1]. After incubation, planktonic bacteria were discarded, and the biofilms formed on the coverslips were washed three times with distilled water and collected. Each coverslip was added with 100 μl crystal violet (1 % in water) and incubated for 15 min at room temperature. The excessive crystal violet was then discarded, and stained biofilms were washed three times with 600 μl of distilled water. Ethanol (95 % in water) was added to the stained biofilms (600 μl), and OD600 was read. Each value is a mean of triplicate measurements ± standard deviation. Data are not statistically different (P < 0.05).
Results
Screening of Soil Bacteria
We isolated 56 strains from 7,000 that exhibited anti-QS activity without inhibiting growth (Fig. 1a). The compounds, which inhibited the production of violacein in the screening assay, were found to possess rhl-inhibiting activity similar to that of other bacterial species including P. aeruginosa. Less than 2 % of the total strains expressing significant QSI activity were found to significantly inhibit the growth of CV026.
Fig. 1.
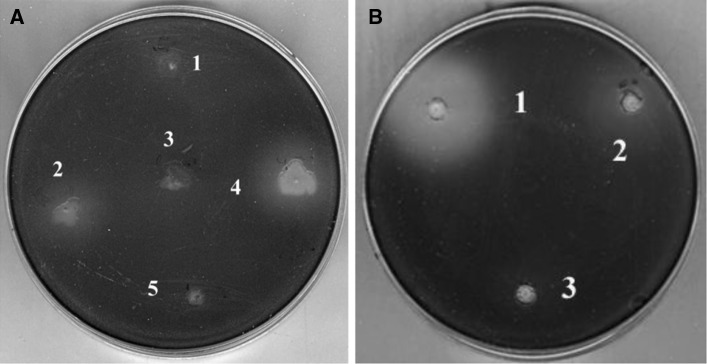
Agar plate screening assay for soil bacteria expressing anti-QS activity. a The diffuse, lightly colored areas surrounding the wells indicate inhibition of violacein production. Strains (2) 3999 and (4) 4001 caused varying degrees of inhibition, but they did not affect the growth of CV026. Strains (1) 3998, (3) 4000, and (5) 4002 showed no inhibitory activity. b Inhibition of violacein production by CV026 in a secondary screen. (1) strain 4001, (2) strain 3999, (3) methanol as a negative control. The extract from 4001 showed stronger inhibitory activity than that of 3999. Violacein production was not affected by methanol
The 29 most active, putative QSIs-producing strains were selected for further screening. These bacterial cultures were concentrated, extracted, and tested using the agar plate assay for secondary screening (Fig. 1b). Strain 4001 (Fig. 1a) was chosen for further research because it produced the largest colorless zone and had little effect on the growth of the indicator strain.
Phylogenetic Characterization
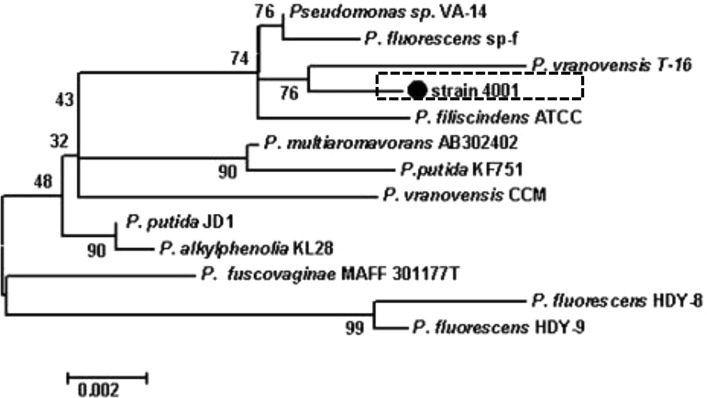
The 16S rDNA gene sequence of strain 4001 was determined and deposited in GenBank (Accession Number JF708119). This bacterium is phylogenetically closely related to Pseudomonas (Fig. 2), and shares more than 99 % nucleotide sequence identity with P. vranovensis strain T-16 (HQ202845) isolated from the Western Kunlun Mountains, Pseudomonas sp. VA-14 (DQ984205), and P. fluorescens strain sp-f (DQ256083).
Fig. 2.
Phylogeny of strain 4001. Phylogenetic maximum-parsimony tree calculated using partial 16S rRNA gene sequences. Maximum-parsimony (1,000 resamplings) bootstrap values are provided for relevant groups. The bar indicates 0.002 % sequence divergence. The numbers provide support for the robustness of the adjacent nodes
Purification of Inhibitors
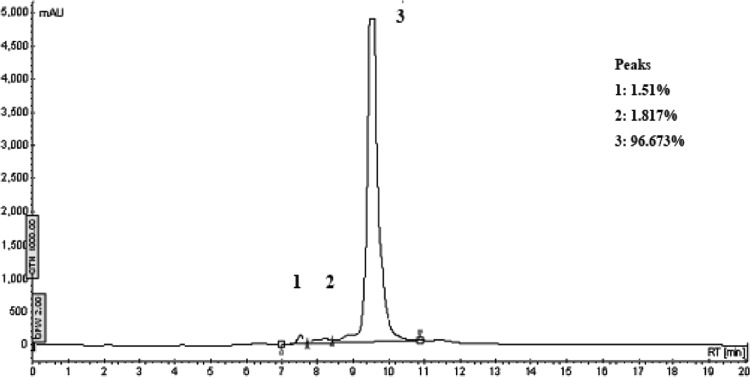
The bacterial extract was fractionated using a silica column yielding three major peaks designated Fr. 1, Fr. 2 and Fr. 3. Only Fr. 1 exhibited QSI activity and did not block cell growth at the tested concentrations (Fig. 3). There are 19 subfractions in Fr. 1 separated by further gradient silica column. Among them, subfractions F6 to F12 inhibited violacein production by further fraction. To identify the inhibitory compound(s), subfractions F6 to F12 were collected and subjected to preparative TLC. Tailing was observed on the TLC plate as shown in Fig. 4. The active component, the entire spot and the tail, collected from the preparative TLC plate as the final product of the purification. Reversed-phase HPLC analysis revealed a major peak (96.673 %) (Fig. 5) detected by its absorbance at 254 nm [15]. This major peak, designated Peak 3, was subjected to further analysis. The percentages of the combined absorbance of the peaks were as follows: (1) 1.51 %, (2) 1.817 %, and (3) 96.673 % (Fig. 5).
Fig. 3.
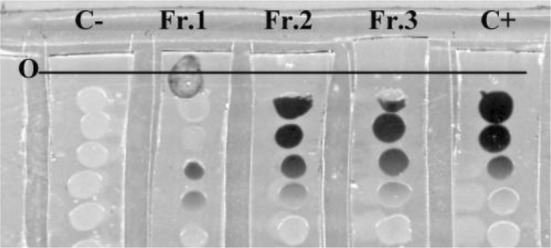
Bioassay of samples fractionated by silica gel chromatography. Samples separated by chromatographic were added to the front of the line of CV026 line dotted on LB agar stripes and incubated overnight. Methanol (5 μl) was added in lane C as negative control. In lane C+, 2.5 μl BHL (200 μM) and 2.5 μl methanol was added as positive control. Lane Fr. 1, Fr. 2, Fr. 3 contained 2.5 μl of the indicated fraction and 2.5 μl BHL (200 μM). O marks the position where samples were added. The potential QSI activity of Fr. 1 (pigment inhibition) was highest compared with Fr. 2 and Fr. 3, which produced little inhibitory activity
Fig. 4.
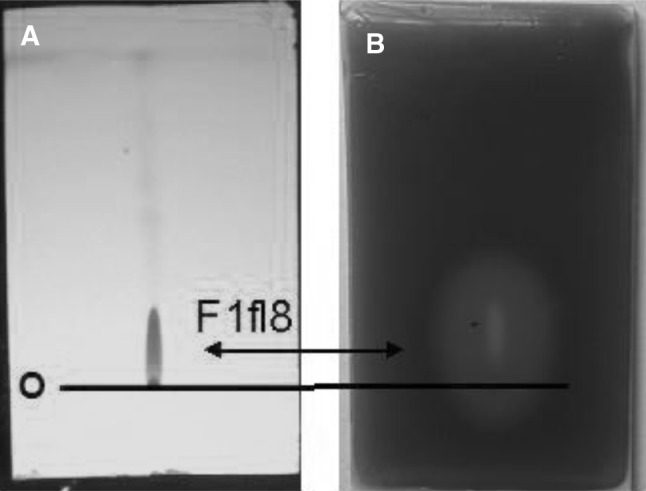
TLC bioassay. a A total of eight spots were visualized on the plate by their fluorescence at 254 nm. b. The control plate was incubated with CV026, Only fraction F1fl8 was active. The “O” marks the position of the origin
Fig. 5.
HPLC analysis of TLC fractions. The HPLC elution profile of the active fraction from the TLC separation (254 nm). Three subfractions were detected. mAU arbitrary unit. The percentages of the combined absorbance of the peaks were as follows: (1) 1.51 %, (2) 1.817 %, and (3) 96.673 %
Growth Inhibition Analysis
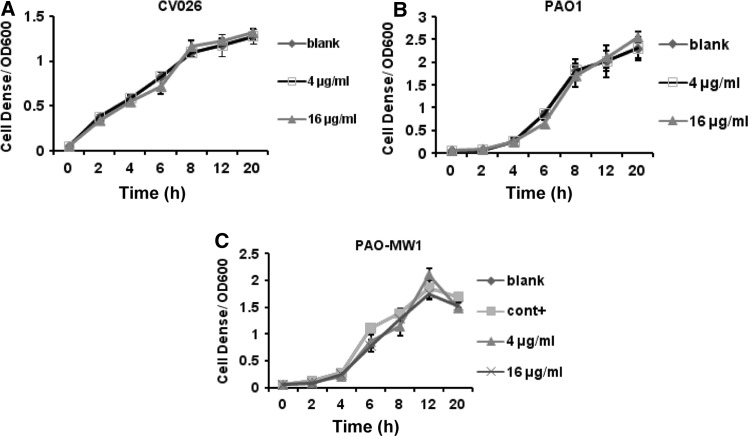
For growth inhibition analysis, the bacterial cultures were treated with the Peak 3 extracts at the tested concentration of 4 and 16 μg/ml. It did not show any significant variation in the cell density of PAO1, PAO-MW1 and CV026 (Fig. 6a, b, c). The assessment on the growth effect of the PAO1 and its mutant PAO-MW1 revealed that the final product of extract had little affection in bacterial growth, and the stationary phase was reached approximately 10 h after final extract addition in all samples. These results confirmed that the inhibition of QS-dependent phenotypic expressions in PAO1, PAO-MW1 and CV026 by bacterial extract was not due to antibiotic activity, but due to QSI activity.
Fig. 6.
Effect of the final extract on growth of CV026, PAO1 and PAO-MW1. Extracts were added at 0 h. Blank control of CV026 (a), PAO1 (b) and PAO-MW1 (c) were set by addition of methanol, positive control of PAO-MW1 was set by addition of 100 nmol/l of OdDHL, 2 μmol/l BHL and methanol. For PAO1, no positive control was set. Each value is a mean of triplicate measurements ± standard deviation
Assay of Peak 3 for Inhibition of Virulence Factor Production
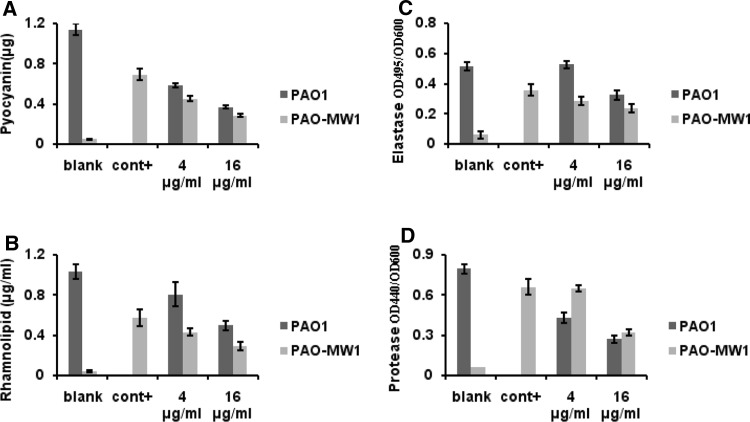
Peak 3 had little effect on the growth of PAO1 or PAO-MW1. Both strains reached stationary phase and at the same cell density in the presence or absence of Peak 3. To test the efficacy of Peak 3 to inhibit the production of QS-regulated virulence factors, we measured the levels of several extracellular virulence factors controlled by rhl and las systems separately or together using PAO1 and PAO-MW1. The control experiment showed that 100 nM OdDHL and 2 μM BHL were required for consistent induction of virulence factor activity in PAO-MW1. When the concentration of Peak 3 at 16 μg/ml, its effects on QS-controlled virulence factors exhibited that pyocyanin production was reduced by 68.8 % in PAO1 and 58.9 % in PAO-MW1; The inhibitory effect was observed in rhamnolipid production assay (53.7 % in PAO1 and 38.2 % in PAO-MW1) (Fig. 7a, b); Elastase activity was reduced by 39.2 % in PAO1 and 62.5 % in PAO-MW1 (Fig. 7c); Inhibition of protease production was about 68.7 % in PAO1 and 57.1 % in PAO-MW1 (Fig. 7d).
Fig. 7.
Bioassay of the purified inhibitor. Affect of serial dilutions of the final extract on pyocyanin production (a), rhamnolipid (b), elastase (c) and protease activity (d) in PAO1 and PAO-MW1
Final Extract has Some Effect on Biofilm Formation
The Pseudomonas aeruginosa is highly tolerant to antibiotic treatment largely due to the biofilm [16]. Since QS system was found to be essential for creation of mature, differentiated biofilms [17], we also tested if the final extract of strain 4001 exhibited a similar inhibition effect on biofilm formation. When PAO1 and PAO-MW1 were grown in the presence of extract at the concentration of 16 μg/ml, formation of biofilm was dropped to about 72.5 % in PAO1 and 50.1 % in PAO-MW1 (Fig. 8).
Fig. 8.
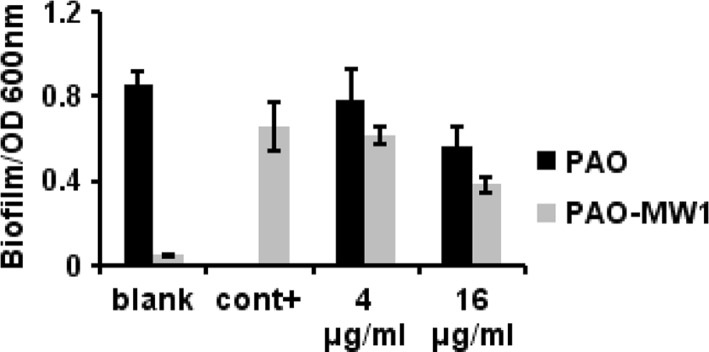
Biotest of final extract on biofilm formation. Measurements of serial dilutions of the purified extract on biofilm formation in PAO1 and PAO-MW1. Blank control of PAO-MW1 and PAO1 were set by addition of methanol, positive control of PAO-MW1 was set by addition of 100 nmol/l of OdDHL, 2 μmol/l BHL and methanol. For PAO1, no positive control was set. Each value is a mean of triplicate measurements ± standard deviation. Data are not statistically different (P < 0.05)
Discussion
Up to 11 % of the P. aeruginosa genome is subjected to QS-dependent regulation [18]. Inhibition of QS offers a new hope for combating multi-antibiotic-resistant bacteria, because it does not impose harsh selective pressure for the development of resistance. Our goal here was to identify new QS inhibitors. For this purpose, we screened a library of approximately 7,000 soil bacteria, and in our initial screen, we found that there was approximately 1 % strains exhibiting significant anti-QS activity without inhibiting the growth of an indicator strain. Some strains produced growth inhibitors and warrant further study to identify potentially useful new antibiotics. The strain with the highest anti-QS activity, designated 4001, due to its better growth characteristics in the media for selection and culture, is phylogenetically most closely related to the genus Pseudomonas based on nucleotide sequence analysis of 16S rRNA genes. Interestingly, we found that several nearest neighbors with more than 99 % 16S rRNA gene sequence identity to strain 4001 were originally isolated from soil. Pseudomonas spp. have attracted great attention due to their high capacity to produce secondary metabolites of biotechnological interest, such as antibiotics, enzymes, and siderophores [19]. Therefore, we were not surprised to discover that strain 4001 inhibited QS.
The HPLC analysis of the final extract suggested that it might contain three or even more compounds. We decided to focus our efforts on the predominant peak (96.673 % of A254 eluted) designated as Peak 3. Molecules with similar retention times were also detected. Tailing on the TLC plate and the adsorption by the silica column, suggest the existence of molecules containing free amino groups or highly polar side groups. Silica chromatography is problematic for obtaining good yields making it difficult to obtain sufficient quantities of inhibitory factors for structural studies. Identifying Peak 3 is our highest research priority. The final yield (10.8 mg) under the growth and purification conditions described here was satisfactory considering that QS inhibitors are usually present at only nanomolar concentrations or lower in culture media. However, greater yields are clearly required for detailed structure–function analysis.
A number of isolated QS inhibitors repress the QS system at milli- to micromolar concentrations [2]. The inhibitory activity of Peak 3 could be detected at concentrations as low as 2 μg/ml, and significantly reduced the levels of QS-controlled virulence factors without a reduction in growth. Although an appropriate positive control was not available, it is reasonable to assume that the specific inhibitory activity of Peak 3 will increase when it is more highly purified.
Peak 3 affected virulence factors controlled by las and rhl system to different extents, at levels that did not inhibit the growth of our P. aeruginosa indicator strains. Because macromolecules were removed during the extraction, the concentration-dependent inhibitory effect of Peak 3 leads us to believe that there are specifically competing against autoinducers of QS system. Compounds that interfere with both rhl and las systems will be helpful in treatment of nosocomial infections.
Although effectively inhibited kinds of virulence factors, the extract didn’t affect the biofilm formation obviously. QS is thought to be involved mainly in this last stage, differentiation, of the biofilm formation [20]. QS mutants of P. aeruginosa have been shown to form thick and flat biofilms while wild type strains produce biofilms with canals and mushroom structures characteristic of mature biofilms. However, the inhibition to the biofilm formation by the final extract was not obvious, suggesting the difficulty of biofilm treatment. Recent study also shows that in P. aeruginosa, QS system is not the sole mechanism by which biofouling is controlled [21]. Under certain conditions, lasI mutants of P. aeruginosa can still form biofilms [22]. This may be help to explain our result. We will withhold further speculation on the mechanism of action until the active compounds are purified.
Acknowledgments
We are thankful for the funds provided by National Natural Science Foundation of China under Grant 81273409 and 90813010. Many thanks also to Professor Lianhui Zhang (IMCB, Singapore) and Professor Mark Willcox (UNSW, Australia) for providing the biomonitor strains.
Contributor Information
Lianhui Wang, Email: wlhui@fudan.edu.cn.
Lixing Weng, Email: lxweng@njupt.edu.cn.
References
- 1.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:253–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 3.Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob Agents Chemother. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gram L, de Nys R, Maximilien R, Givskov M, Steinberg P, Kjelleberg S. Inhibitory effects of secondary metabolites from the red alga Delisea pulchra on swarming motility of Proteus mirabilis. Appl Environ Microbiol. 1996;62:4284–4287. doi: 10.1128/aem.62.11.4284-4287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiba Krishnan, Yin WF, Chan KG. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium Aromaticum) bud extract. Sensors. 2012;12(4):4016–4030. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Høiby N, Bjarnsholt T, Givskov M (2012) Food as a source for QS inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl Environ Microbiol. doi:10.1128/aem.05992-11 [DOI] [PMC free article] [PubMed]
- 7.Kim J, Kim Y, Seo Y, Park S. Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnol Bioprocess Eng. 2007;12(3):308–311. doi: 10.1007/BF02931109. [DOI] [Google Scholar]
- 8.Skindersoe M, Ettinger-Epstein P, Rasmussen T, Bjarnsholt T, de Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol. 2008;10(1):56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- 9.Daglia M, Stauder M, Papetti A, Signoretto C, Giusto G, Canepari P, Pruzzo C, Gazzani G. Isolation of red wine components with anti-adhesion and anti-biofilm activity against Streptococcus mutans. Food Chem. 2009;119(3):1182–1188. doi: 10.1016/j.foodchem.2009.08.037. [DOI] [Google Scholar]
- 10.Romero M, Martin-Cuadrado AB, Roca-Rivada A, Cabello AM, Otero A. Quorum quenching cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol Ecol. 2011;75(2):205–217. doi: 10.1111/j.1574-6941.2010.01011.x. [DOI] [PubMed] [Google Scholar]
- 11.McLean RJC, Pierson Iii LS, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods. 2004;58(3):351–360. doi: 10.1016/j.mimet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Prince RW, Cox CD, Vasil ML. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sbarra AJ, Gilfillan RF, Bardawil WA. A plate assay for elastase. Nature. 1960;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- 14.Pearson J, Pesci E, Iglewski B. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179(18):5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, El Jaziri M, Baucher M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl Environ Microbiol. 2010;76(1):243–253. doi: 10.1128/AEM.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Kievit TR. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11(2):279–288. doi: 10.1111/j.1462-2920.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 18.Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamei Y, Isnansetyo A. Lysis of methicillin-resistant Staphylococcus aureus by 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int J Antimicrob Agents. 2003;21(1):71–74. doi: 10.1016/S0924-8579(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 20.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 21.Sakuragi Y, Kolter R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J Bacteriol. 2007;189:5383–5386. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purevdorj B, Costerton JW, Stoodley P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2002;68:4457–4464. doi: 10.1128/AEM.68.9.4457-4464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]