Abstract
Twelve different bacteria–yeast combinations were tested for determination of their ability to biodegrade diesel oil. The cell surface properties of the bacterial and yeast strains were correlated with the type of carbon source used in the experiments. The highest biodegradation of diesel oil after 7 days was obtained for the following combinations: Aeromonas hydrophila MR4–Yarrowia lipolytica EH 56 (87 %) and Xantomonas maltophila MRP7–Candida maltosa EH15 (90 %). Degradation performances of 10 of 12 combinations were enhanced by the presence of rhamnolipids. The highest increases were observed for A. hydrophila MR4–C. maltosa EH15 (from 34 to 67 %), A. hydrophila MR4–C. maltosa EH60 (from 47 to 76 %) and for Pseudomonas stutzeri MR7–C. maltosa EH60 (from 29 to 79 %). The addition of rhamnolipids to the system reduces the removal time of diesel oil from the contaminated water and changes the microbial adhesion to hydrocarbons. Modification of the cell surface of the tested strain during biodegradation is a very important factor determining the removal of hydrophobic compounds.
Keywords: Biodegradation, Combinations, Hydrophobicity, Diesel oil, Yeast
Introduction
Crude oil is a potential source of soil and water contamination. Petroleum hydrocarbons cause serious environmental problems, which lead to disruption in the ecosystem and cause serious health problems. Particularly dangerous are large scale accidental spills.
Biodegradation of a hydrocarbon source is directly related to the structure of the carbon source, enzymatic cell activity and the bioavailability of xenobiotics [1, 2]. Using biological combinations in order to remove existing pollutions is possible as microorganisms are capable degrading hydrocarbon compounds to less harmful chemical substances. Many reports show bacterial ability in crude oil degradation by single or mixed bacterial strains [3]. The rate and extent of hydrocarbon biodegradation may be determined by the type of soil and hydrocarbon mixtures. However, bacterial and yeast mixtures are also used in the biodegradation process. Horakova and Nemec [4] showed that the presence of the Yarrowia lipolytica strain had a significant positive influence on crude oil degradation when used in combination with Pseudomonas than degradation by Pseudomonas alone. Our previous research results indicated that the positive effect of using the mixed yeast–bacteria mixture is mainly attributed to the yeast [5]. In the bioremediation process, it is very important to focus on the cell surface properties of microorganisms and their biodegradation features. Moreover, the addition of surfactants enhances the desorption and solubilization of organic compounds by increasing their bioavailability [6]. However, the addition of surfactants to the system may cause an increase in hydrocarbon degradation, not only by the solubilization, but also depends on the sum of the chemical molecular interactions between hydrocarbon and the surfactant [7]. Different synthetic, as well as natural surfactants could be used in the bioremediation process. Some of the bacterial strains could produce their own biosurfactant, for example the Pseudomonas aeruginosa strain produces rhamnolipids biosurfactant.
Surfactants may also contribute to changes in the cell surface properties [8, 9].
The aim of this research was to estimate the effectiveness of the diesel oil biodegradation process with microorganism combinations. Each of three bacterial strains: Aeromonas hydrophila MR4, Xanthomonas maltophila MP4, P. stutzeri MP7 was mixed with one of four yeast strains: Candida maltosa EH15 or EH60, Y. lipolytica EH56 or H465. Biodegradation of diesel oil was determined after 7 and 14 days. The study also focused on the efficiency analysis of the removal of aliphatic fractions present in diesel oil. The influence of the external addition of biosurfactant on diesel oil biodegradation was examined. Furthermore, the cell surface properties of the studied microorganisms were tested during growth on several different carbon sources with the aim of checking their influence on surface modifications.
Materials and Methods
Microorganisms and Growth Conditions
Three bacterial strains isolated from soil contaminated with crude oil: A. hydrophila MR4, P. stutzeri MR7 and X. maltophila MRP7 were used in experiments. The identification of the bacterial strains were performed using biochemical tests ID 32 GN (prod. bio-Merieux, France) and molecular techniques. The yeast strains: C. maltosa EH15 and EH60 as well as Y. lipolytica EH59 and 425 were also used in experiments [10]. The mineral culture medium used throughout the studies consisted of (g/l): Na2HPO4·2H2O 7.0, KH2PO4 2.8, NaCl 0.5, NH4Cl 1.0, MgSO4·7H2O 0.01, FeSO4·7H2O 0.001, MnSO4·4H2O 0.0005, ZnCl2 0.00064, CaCl2·6H2O 0.0001, BaCl2 0.00006, CoSO4·7H2O 0.000036, CuSO4·5H2O 0.000036, H3BO3 0.00065, EDTA 0.001, and HCl 37 % 0.0146 ml [11]. For bacteria and yeast stock cultures yeast extract (0.3 g l−1) was added. Stock cultures were prepared in a 250 ml Erlenmeyer flask containing 50 ml of medium. Next, a loop full of cells from an agar plate was added to the flask with medium. After approximately 24 h a 3–5 ml of this liquid culture was used for the inoculation of the final culture to reach an OD of ca. 0.1 (this corresponds to 107–108 cells ml−1).
Chemicals
The hydrocarbon and other fine chemicals employed in this study were of the highest purity grade, produced by Merck (Germany). Rhamnolipids was obtained from Jeneil Biosurfactant Company, USA (JBR 425, content 25 % of rhamnolipids).
Biodegradation Test
Diesel oil was used as a carbon source (2 % w/v) for microorganism biodegradation. The influence of rhamnolipids on diesel oil biodegradation was also tested. Surfactants were used at 120 mg l−1. This quantity of surfactants was experimentally chosen earlier. Experiments were performed in Erlenmeyer flasks containing 100 ml of culture medium. Experimental samples contained: diesel oil, a culture medium and 5 ml of bacteria stock cultures (to reach an OD of ca. 0.1). Each experiment was repeated five times to attain an accuracy of ±3.8 %. Diesel oil content was determined as described in detail by Kaczorek et al. [11].
Chromatographic Analysis
The biodegradation of the aliphatic hydrocarbon fraction was also estimated using Gas Chromatography (GC). Qualitative and quantitative analysis were done with a GC with flame ionization detector (GC-FID); analyses were carried out using a HP 5890II GC with an autosampler, capillary column 50 % cyanopropylmethyl–50 % phenylmethyl polisiloxane DB-225 (Agilent Technologies), was used a film thickness of 0.25 μm, 30 m × 0.25 mm I.D. Helium was used as the carrier gas at a flow-rate of 1.5 ml min−1 and a head pressure of 90 kPa. The injector and detector temperature was 300 °C. The column temperature was held at 60 °C for 1 min, then ramped at 10 °C min−1 to 220 °C where it was held for 10.5 min.
Microbial Adhesion to Hydrocarbons
Microbial surface properties were assessed using a microbial adhesion to the hydrocarbon method (MATH). Cells in exponential phase were centrifuged at 8,000×g for 5 min and washed twice with a mineral culture medium. The cells were then re-suspended in the medium to fit an optical density of ca. 1.0 (Ao). Optical density was measured at 600 nm on the Shimadzu UV–Visible Spectrophometer. Next, 500 μl of hexadecane was added to 5 ml of microbial suspension and vortexed for 2 min. After 10 min the optical density of the aqueous phase was measured (A1). MATH was calculated as [1−A1)/A0] 100 % [12]. Bacterial and yeast strains were grown on different carbon sources: hexadecane, diesel oil, glucose, rhamnolipids and the diesel oil–rhamnolipids system. Each experiment was repeated three times to attain an accuracy of ±3 %.
Results and Discussion
Influence of Carbon Source on the Cell Surface Properties of Bacterial and Yeast Strains
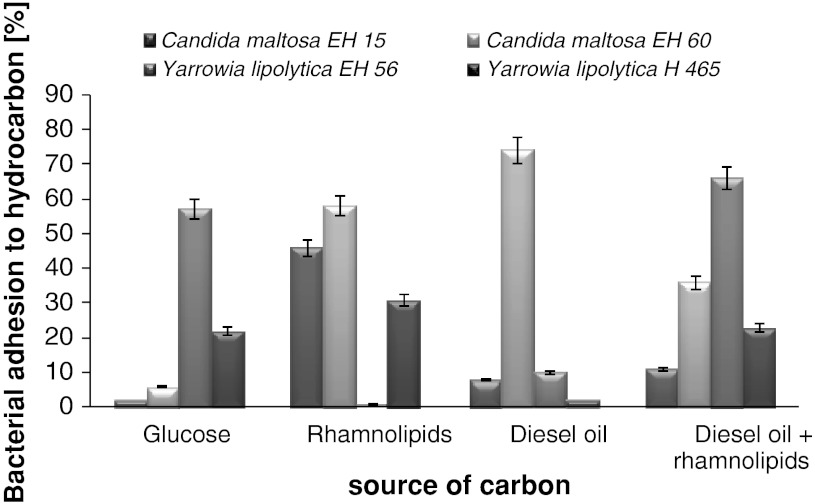
The cell surface properties of the tested bacterial and yeast strains were correlated with the type of the carbon source used in the experiments. For the yeast strains the highest hydrophobicity was observed for C. maltosa EH60–74 % grown on diesel oil (Fig. 1). The rest of the yeast strains displayed hydrophilic features in the tested systems. The rhamnolipids addition to the diesel oil system caused an increase in hydrophobicity of the yeast strains, except the C. maltosa EH60 strain. In this case a significant decrease in hydrophobicity from 74 (diesel oil) to 36 % (diesel oil and rhamnolipids) was noticed. The cell surface hydrophobicity (CSH) could have influence on hydrocarbon biodegradation. Chrzanowski et al. [13] showed that for yeasts with high initial hydrophobicity the best results of hydrocarbon biodegradation after rhamnolipids addition were observed at the decrease of the cell surface hydrophobicity.
Fig. 1.
Influence of carbon source on yeast strain hydrophobicity (process carried out over 7 days; surfactant concentration 120 mg l−1)
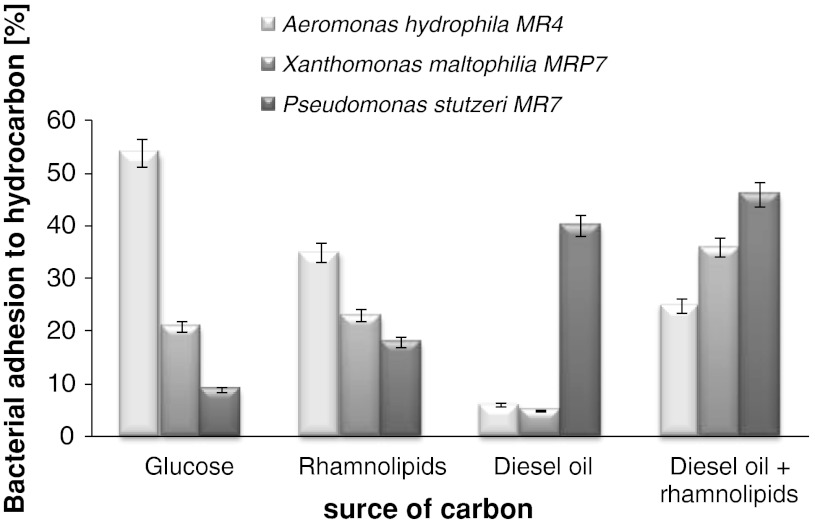
The addition of rhamnolipids to diesel oil system caused an increase of hydrophobicity of all tested bacterial strains (Fig. 2). Furthermore the A. hydrophila MR4 strain was characterized by the highest hydrophobicity during growth on the hydrophilic carbon sources–glucose and rhamnolipids (54, 35 % respectively). Moreover, when hydrophobic cells dominated the population in the diesel oil system, the addition rhamnolipids to that system caused a decrease in hydrophobic properties of the tested strains.
Fig. 2.
Influence of carbon source on bacterial strain hydrophobicity (process carried out over 7 days; surfactant concentration 120 mg l−1)
According to Zhong et al. [9] dirhamnolipid adsorption on the microbial cell depends on the microbial source and the physiological conditions of the cell. Cell surface properties are very important factors limiting hydrocarbon uptake by microorganisms. Al-Tahhan et al. [14] described the extraction of lipopolysaccharide from the surface resulted in the increase of CSH. However, Obuekwe et al. [15] demonstrated that the population of P. aeruginosa consists of cells with different CSH which affects hydrocarbon degradation. This resulted in the use of potential capacity to utilize different carbon sources found in petroleum by this bacterial strain.
Diesel Oil Biodegradation by Microorganism Combinations
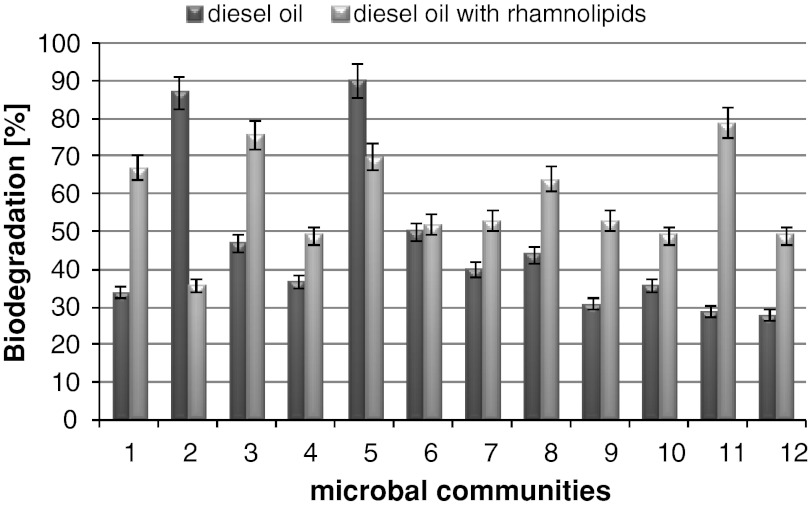
Among tested bacteria-yeast combinations, the highest diesel oil biodegradation was obtained: A. hydrophila MR4–Y. lipolytica EH56–87 % and X. maltophilia MRP7–C. maltosa EH15–90 % (Fig. 3).
Fig. 3.
Comparison of biodegradation by 12 combinations of bacterial-yeast strains after 7 days of experiment in system with and without addition of rhamnolipids. Microorganisms combinations: (1) A. hydrophila MR4–C. maltosa EH15, (2) A. hydrophila MR4–Y. lipolytica EH56, (3) A. hydrophila MR4–C. maltosa EH60, (4) A. hydrophila MR4–Y. lipolytica EH465, (5) X. maltophilia MRP7–C. maltosa EH15, (6) X. maltophilia MRP7–Y. lipolytica EH56, (7) X. maltophilia MRP7–C. maltosa EH60, (8) X. maltophilia MRP7–Y. lipolytica EH465, (9) P. stutzeri MR7–C. maltosa EH15, (10) P. stutzeri MR7–Y. lipolytica EH56, (11) P. stutzeri MR7–C. maltosa EH60, (12) P. stutzeri MR7–Y. lipolytica EH465
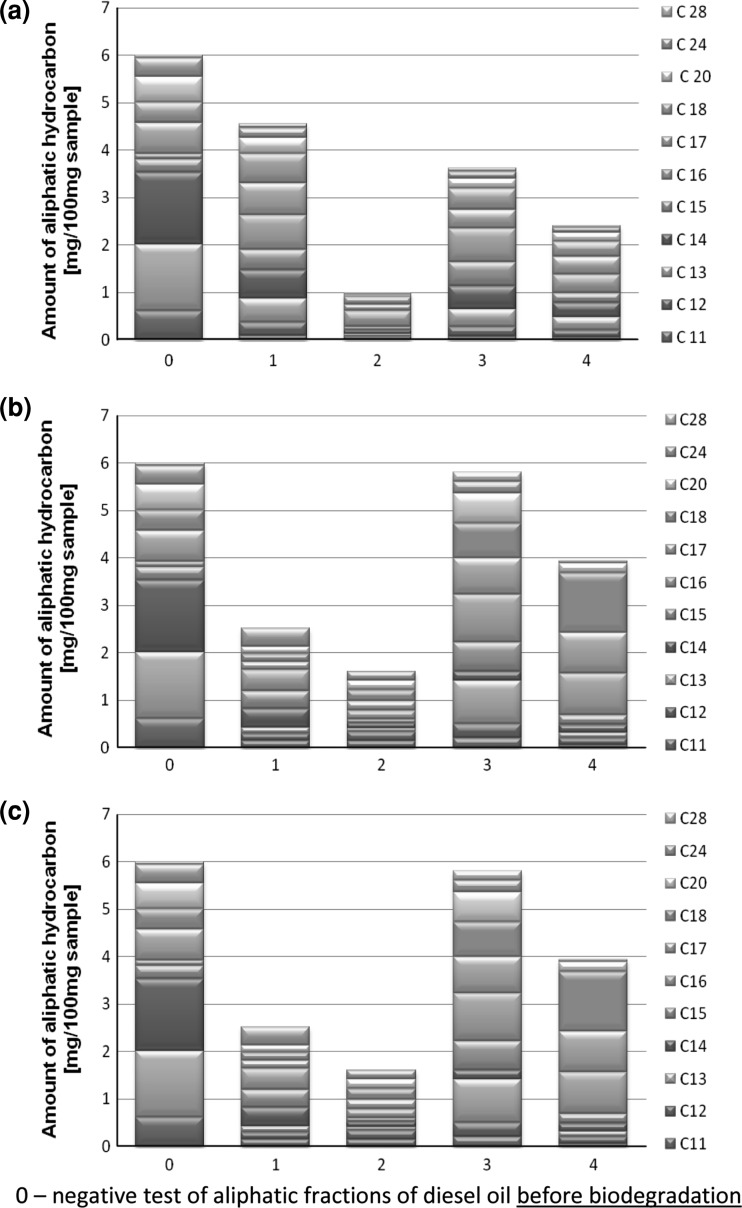
GC analyses of the saturated aliphatic hydrocarbons fraction displayed a different degradation pattern (Fig. 4). The biggest removal of the C11 to the C28 fraction of hydrocarbons was observed for the combination X. maltophilia MRP7–C. maltosa EH15. Moreover, similar results were noticed for Y. lipolytica EH56 with three tested bacterial strains (Fig. 4a–c). The combinations of C. maltosa EH60 with bacterial strains characterized the lowest biodegradation of aliphatic hydrocarbons.
Fig. 4.
Contents of aliphatic fraction of carbon C11 to C28 after 7 days of diesel oil biodegradation: (1) C. maltosa EH15, (2) Y. lipolytica EH56, (3) C. maltosa EH60, (4) Y. lipolytica EH465
However, for the C. maltosa EH15 yeast strain, the biodegradation of the aliphatic compounds depended on the bacterial strain in different combinations. In this case, the best microorganism combination was X. maltophilia MRP7–C. maltosa EH15 (Fig. 4b) and the worst A. hydrophila MR4–C. maltosa EH15 (Fig. 4a); for example for the combinations A. hydrophila MR4 with yeast strains the fractions from C11 to C14 and from C20 to C28 were the fastest removed (Table 1).
Table 1.
Results of GC analysis of aliphatic fraction of carbons from C11 to C28 after 7 days of diesel oil biodegradation by A. hydrophila MR4 with combinations of yeast strain, without and without rhamnolipids
| Combinations A. hydrophila MR4 with yeast strains | Amount of aliphatic fractions of hydrocarbons of diesel oil after biodegradation (mg/100 mg sample) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C20 | C24 | C28 | |
| Without rhamnolipids | |||||||||||
| Negative control test—aliphatic fractions of diesel oil before biodegradation | 0 | 0.614 | 1.406 | 1.505 | 0.277 | 0.124 | 0.654 | 0.419 | 0.542 | 0.399 | 0.061 |
| C. maltosa EH 15 | 0.095 | 0.283 | 0.505 | 0.592 | 0.426 | 0.725 | 0.675 | 0.619 | 0.344 | 0.203 | 0.083 |
| Y. lipolytica EH 56 | 0 | 0.075 | 0.053 | 0.082 | 0.072 | 0.326 | 0.135 | 0.168 | 0.047 | 0.005 | 0.026 |
| C. maltosa EH 60 | 0.073 | 0.200 | 0.385 | 0.472 | 0.507 | 0.714 | 0.398 | 0.433 | 0.222 | 0.136 | 0.072 |
| Y. lipolytica EH465 | 0.054 | 0.144 | 0.280 | 0.302 | 0.214 | 0.385 | 0.376 | 0.306 | 0.195 | 0.109 | 0.046 |
| With rhamnolipids | |||||||||||
| C. maltosa EH 15 | 0 | 0 | 0 | 0 | 0.039 | 0 | 0.042 | 0.007 | 0.001 | 0.007 | 0.040 |
| Y. lipolytica EH 56 | 0 | 0 | 0 | 0 | 0.033 | 0.024 | 0.039 | 0 | 0 | 0.055 | 0 |
| C. maltosa EH 60 | 0 | 0 | 0 | 0 | 0.028 | 0.025 | 0.067 | 0.002 | 0.007 | 0.026 | 0.057 |
| Y. lipolytica EH465 | 0 | 0 | 0 | 0 | 0 | 0.052 | 0.012 | 0 | 0 | 0.013 | 0 |
Our results indicated that the kind of bacterial and yeast strain in combinations have an influence on the biodegradation of the saturated aliphatic fraction. Bouchez et al. [16] demonstrated that soil combinations possessed a wider variety of strains that were capable of compensating the competitive inhibition between PAH, as well as specialized strains that mineralized potentially inhibitory PAH metabolites produced by cometabolism. Moreover, Jacques et al. [17] observed that microbial combinations consisting of five bacterial strains mineralized high percentages of anthracene, phentathrene and pyrene in the soil within a relatively short period.
The n-alkanes disappear relatively faster compared with other compounds, such as branched alkanes and aromatics because of their structure. During diesel oil biodegradation the amount of lighter hydrocarbons increases due to degradation of the heavier once [18].
Influence of Rhamnolipids on Diesel Oil Biodegradation
The results of diesel oil biodegradation indicated that for ten combinations the addition of rhamnolipids had a positive influence (Fig. 3).
The highest increases in diesel oil biodegradation after the addition of rhamnolipids were observed for three combinations: A. hydrophila MR4–C. maltosa EH15 (from 34 to 67 %), for A. hydrophila MR4–C. maltosa EH60 (from 47 to 76 %), and for P. stutzeri MR7–C. maltosa EH60 (from 29 to 79 %). The addition of rhamnolipids to the diesel oil system caused an almost total removal of the aliphatic fraction of hydrocarbons (Table 2; Figs. 3, 5). The negative results of the addition of the rhamnolipids were observed for two mixtures: A. hydrophila MR4–Y. lipolytica EH56 and X. maltophilia MRP7–C. maltosa EH15. In these cases, diesel oil biodegradation decreased from 87 to 36 % for the combination A. hydrophila MR4–Y. lipolytica EH56 and for the mixture of X. maltophilia MRP7–C. maltosa EH15 decreased from 90 to 70 %. This outcome could be caused by the fact that the former combinations used in the first order the rhamnolipids as a carbon source. Goudar et al. [19] indicated that inhibition of hydrocarbon biodegradation after the addition of surfactants is caused by competitive degradation.
Table 2.
The influence of rhamnolipids on the biodegradation process by Psedomonas stutzeri MR7 after 7 and 14 days
| Strains | Biodegradation of diesel oil without rhamnolipids (%) | Biodegradation of diesel oil with rhamnolipids (%) | ||
|---|---|---|---|---|
| 7 days | 14 days | 7 days | 14 days | |
| P. stutzeri MR7 | 33 ± 2.1 | 45 ± 1.7 | 50 ± 1.8 | 62 ± 2.5 |
| P. stutzeri MR7–C. maltosa EH 15 | 31 ± 1.1 | 33 ± 2.3 | 53 ± 2.5 | 91 ± 3.6 |
| P. stutzeri MR7–Y. lipolytica EH 56 | 36 ± 0.9 | 50 ± 2.3 | 49 ± 2.4 | 61 ± 2.8 |
| P. stutzeri MR7–C. maltosa EH 60 | 29 ± 1.2 | 72 ± 1.4 | 79 ± 1.3 | 93 ± 3.8 |
| P. stutzeri MR7–Y. lipolytica EH 465 | 28 ± 1.3 | 50 ± 1.3 | 49 ± 3.1 | 78 ± 3.3 |
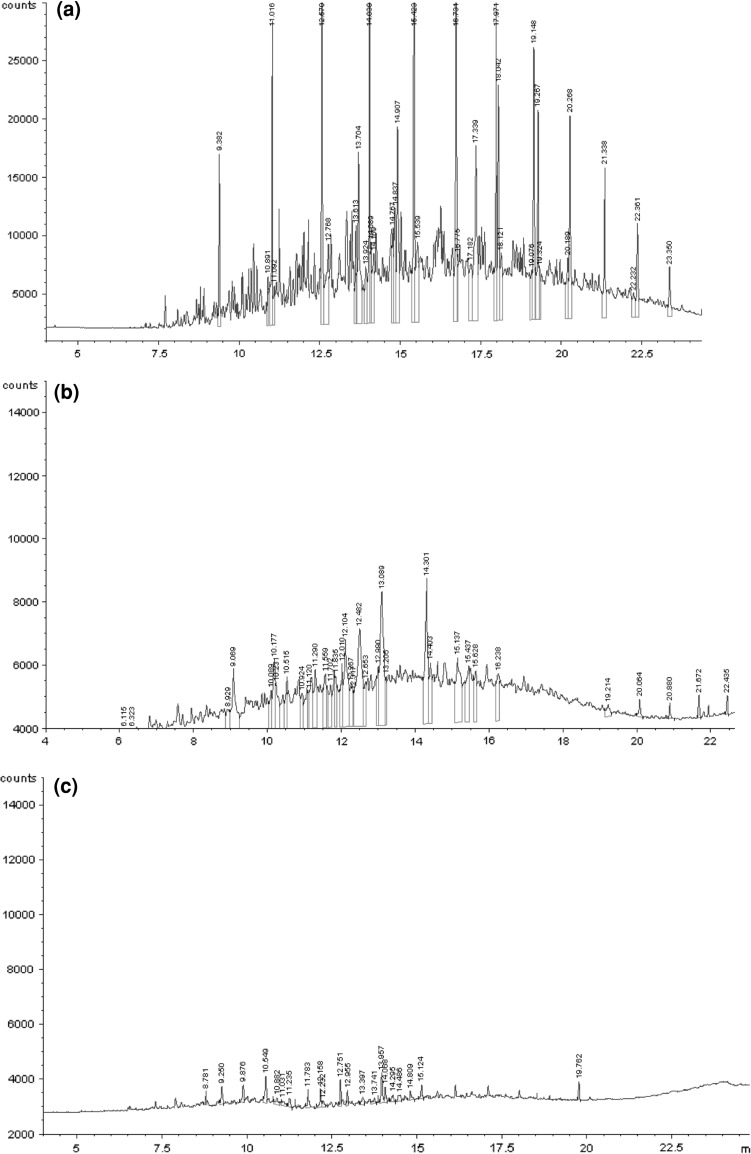
Fig. 5.
Chromatograms of diesel oil after biodegradation by A. hydrophila MR4–C. maltosa EH15, 7 days of experiments, a diesel oil before biodegradation, b diesel oil after biodegradation, c diesel oil after biodegradation in the rhamnolipids system
Generally, hydrophobic organic hydrocarbons due to low water solubility have limited bioavailability to microorganisms. Surfactants increases solubility and dispersion of hydrocarbons, as well as causes changes in microorganisms cell surface properties [6]. Many surfactants are tested for their potential application in hydrocarbon bioremediation. Choice of the kind and amount of surfactants is very important as they can be environmentally friendly. Lai et al. [20] demonstrated that rhamnolipids improve total petroleum hydrocarbon (TPH) removal for soil contaminated in comparison with synthetic surfactants. TPH removal efficiency increases with an increase in rhamnolipids concentration. Many literature reports show the positive effects after the addition of rhamnolipids to the system which significantly improves both aliphatic and aromatic biodegradation [21]. Nevertheless, the addition of rhamnolipids could also have a negative effect. In such cases, biosurfactants do not enhance the removal of contaminants from the environment [22]. Rhamnolipids could be adsorbed on the cell surface of a bacterial strain. Our previous research indicated that the structure of monolayer adsorption is dependent on the type of surfactant. In the case of rhamnolipids, the adsorption tendency is greater than for saponins and Triton X-100 [11].
The extension of time of the biodegradation process to 14 days augmented diesel oil biodegradation by the tested combinations (Table 2). The most significant difference was observed for P. stutzeri MR7–C. maltosa EH60, where the biodegradation of diesel oil increased from 29 % after 7 days to 72 % after 14 days. This positive effect was also observed for the diesel oil–rhamnolipids systems. In this system the best biodegradation was displayed by the combination P. stutzeri MR7–C. maltosa EH15. Diesel oil biodegradation increased from 53 % after 7 days of the experiment to 91 % after 14 days and only system P. stutzeri MR7–Y. lipolytica EH56 gave comparable results to the bacterial system. Other systems allowed to achieve significantly higher diesel oil biodegradation. The rhamnolipids addition to the system causes faster decomposition of diesel oil.
Conclusion
The selection of bacterial and yeast strains used in combinations in the biodegradation process seems to be very important. The addition of the rhamnolipids had a different influence on the biodegradation process of diesel oil in the different microorganism combinations. The positive influence of the addition of biosurfactant was observed for 10 combinations. In the case of the two other microbial mixtures, the negative effect could be caused by using rhamnolipids as a source of the carbon in the first order. Rhamnolipids reduced the time of hydrocarbon biodegradation, which is very important for removing pollutants from the environment. Cell surface hydrophobicity of tested strains is correlated with the kind of carbon used in experiments. Moreover, the carbon source modifies cell surface properties of bacterial and yeast strains in different ways. The increase in cell hydrophobicity caused diesel oil to be more effectively removed from the system.
Acknowledgments
This study was supported by Grant No. N N304 163337, Polish Ministry of Science and Higher Education, years 2009–2011 and DS 32/045/2011.
References
- 1.Margesin R, Hämmerle M, Tscherko D. Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: effects of hydrocarbon concentration, fertilizers and incubation time. Microb Ecol. 2007;53:259–269. doi: 10.1007/s00248-006-9136-7. [DOI] [PubMed] [Google Scholar]
- 2.Allen JP, Atekwana EA, Atekwana EA, Duris JW, Werkema DD, Rossbach S. The microbial community structure in petroleum-contaminated sediments corresponds to geophysical signatures. Appl Environ Microbiol. 2007;73:2860–2870. doi: 10.1128/AEM.01752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palittapongarnpim M, Pokethitiyook P, Upatham ES, Tangbanluekal L. Biodegradation of crude oil by soil microorganisms in the tropic. Biodegradation. 1998;9:83–90. doi: 10.1023/A:1008272303740. [DOI] [PubMed] [Google Scholar]
- 4.Horakova DMV, Nemec M. RC1 Consortium for soil decontamination: its preparation and use. Remediation engineering of contaminated soils. In: Wise DL, Tranto DJ, Cichon EJ, Injang HI, Stottmeister U, editors. Remediation engineering of contaminated soils. New York: Marcel Dekker Inc; 2000. pp. 357–372. [Google Scholar]
- 5.Kaczorek E, Chrzanowski Ł, Pijanowska A, Olszanowski A. Yeast and bacteria cell hydrophobicity and hydrocarbon biodegradation in the presence of natural surfactants: ramnolipides and saponins. Bioresour Technol. 2008;99:4285–4291. doi: 10.1016/j.biortech.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Volkering F, Breurke AM, van Andel JG, Rulkens WH. Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 1995;61:1699–1705. doi: 10.1128/aem.61.5.1699-1705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YT, Lee JF, Hung ChH. PAH biodegradation in surfactant-water systems based on the theory of cohesive energy density (CED) J Chem Technol Biot. 2007;82:442–452. doi: 10.1002/jctb.1684. [DOI] [Google Scholar]
- 8.Yuan XZ, Ren FY, Zeng GM, Zhong H, Fu HY, Liu J, Xu XX. Adsorption of surfactants on a Pseudomonas aeruginosa strain and the effect on cell surface lypohydrophilic property. Appl Microbiol Biotechnol. 2007;76:1189–1198. doi: 10.1007/s00253-007-1080-z. [DOI] [PubMed] [Google Scholar]
- 9.Zhong H, Zeng GM, Yuan XZ, Fu HY, Huang GH, Ren FY. Adsorption of dirhamnolipid on four microorganisms and the effect on cell surface hydrophobicity. Appl Microbiol Biotechnol. 2007;77:447–455. doi: 10.1007/s00253-007-1154-y. [DOI] [PubMed] [Google Scholar]
- 10.Chrzanowski Ł, Bielicka-Daszkiewicz K, Owsianiak M, Aurich A, Kaczorek E, Olszanowski A. Phenol and n–alkanes (C12 and C16) utilization: influence on yeast cell surface hydrophobicity. World J Microbiol Biotechnol. 2008;24:1943–1949. doi: 10.1007/s11274-008-9704-8. [DOI] [Google Scholar]
- 11.Kaczorek E, Urbanowicz M, Olszanowski A. The influence of surfactants on cell surface properties of Aeromonas hydrophila during diesel oil biodegradation. Colloids Surf B. 2010;81:363–368. doi: 10.1016/j.colsurfb.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. doi: 10.1111/j.1574-6968.1980.tb05599.x. [DOI] [Google Scholar]
- 13.Chrzanowski Ł, Kaczorek E, Pijanowska A, Olszanowski A. The relation between rhamnolipid adsorption on yeats and bacterial strains, hydrophobicity and hydrocarbon biodegradation. Fresen Environ Bull. 2006;15:682–686. [Google Scholar]
- 14.Al-Tahhan RA, Sandrin TS, Bodour AA, Maier RM. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl Environ Microbiol. 2000;66:3262–3268. doi: 10.1128/AEM.66.8.3262-3268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obuekwe CO, Al-Jadi ZK, Al-Saleh ES. Comparative hydrocarbon utilization by hydrophobic and hydrophilic variants of Pseudomonas aeruginosa. J Appl Microbiol. 2008;105:1876–1887. doi: 10.1111/j.1365-2672.2008.03887.x. [DOI] [PubMed] [Google Scholar]
- 16.Bouchez M, Blanchet D, Bardin V, Haeseler F, Vandecasteele J-P. Efficiency of defined strains and of soil communities in the biodegradation of polycyclic aromatic hydrocarbon (PAH) mixtures. Biodegradation. 1999;10:423–429. doi: 10.1023/A:1008382030604. [DOI] [PubMed] [Google Scholar]
- 17.Jacques RJS, Okeke BC, Bento FM, Teixeira AS, Peralba MCR, Camargo FAO. Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour Technol. 2008;99:2637–2643. doi: 10.1016/j.biortech.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Gallego JLR, Loredo J, Llamas JF, Vazguez F, Sanchez J. Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation. 2001;12:325–335. doi: 10.1023/A:1014397732435. [DOI] [PubMed] [Google Scholar]
- 19.Goudar C, Strevett K, Grego J. Competetive substrate biodegradation during surfactant-enhanced remediation. J Environ Eng. 1999;125:1142–1148. doi: 10.1061/(ASCE)0733-9372(1999)125:12(1142). [DOI] [Google Scholar]
- 20.Lai CC, Huang YCh, Hong Y, Chang JS. Biosurfactant-enhanced removal of total petroleum hydrocarbon from contaminated soil. J Hazard Mater. 2009;167:609–614. doi: 10.1016/j.jhazmat.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Rahman KSM, Rahman TJ, Kourkoutoas Y, Petsas I, Marchant R, Banat IM. Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol. 2003;90:159–168. doi: 10.1016/S0960-8524(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 22.Mata-Sandoval JC, Karns J, Torrents A. Influence of rhamnolipids and Triton X-100 on the biodegradation of three pesticides in aqueous phase and soil slurries. J Agric Food Chem. 2001;49:3296–3303. doi: 10.1021/jf001432w. [DOI] [PubMed] [Google Scholar]