Abstract
Biological hydrogen (H2) production by dark and photo-fermentative organisms is a promising area of research for generating bioenergy. A large number of organisms have been widely studied for producing H2 from diverse feeds, both as pure and as mixed cultures. However, their H2 producing efficiencies have been found to vary (from 3 to 8 mol/mol hexose) with physiological conditions, type of organisms and composition of feed (starchy waste from sweet potato, wheat, cassava and algal biomass). The present review deals with the possibilities of enhancing H2 production by integrating metabolic pathways of different organisms-dark fermentative bacteria (from cattle dung, activated sludge, Caldicellulosiruptor, Clostridium, Enterobacter, Lactobacillus, and Vibrio) and photo-fermentative bacteria (such as Rhodobacter, Rhodobium and Rhodopseudomonas). The emphasis has been laid on systems which are driven by undefined dark-fermentative cultures in combination with pure photo-fermentative bacterial cultures using biowaste as feed. Such an integrative approach may prove suitable for commercial applications on a large scale.
Keywords: Biowaste, Dark-fermentation, Hydrogen, Mixed culture, Photo-fermentation
Introduction
Hydrogen (H2) has been recognized as fuel for the future due to its high efficiency (122 kJ/g) and eco-friendly nature in comparison to fossil fuels [1, 2]. Biological H2 production (BHP) process has been widely studied under dark- and photo-fermentative conditions. With these approaches the yields of H2 have been quite low in comparison to the theoretically achievable values of 4 and 8 mol/mol of glucose under dark and photo-fermentative conditions, respectively [2–7]. Quite a few research efforts have been made to overcome the limitations of these processes. It has been realized that in order to recover maximum H2 from the organic matter, it is necessary to further use the end-products of the dark fermentative process, especially volatile fatty acids (VFA). It is possible to convert VFAs to H2 by photosynthetic bacteria. The potential of exploiting these processes in various combinations have been reviewed to some extent [7–10]. However a large number of hurdles still seem to persist such as: (i) in the dark-fermentative process—(a) relatively lower H2 yield (b) the need for strict anaerobic conditions for high H2 producers, and (c) thermodynamic instability of the process at higher H2 concentrations, and (ii) during the photo-fermentative process—(a) sensitivity of the H2 production process to nitrogen content of the feed (b) effect to light intensity and duration of radiation under outdoor (sunlight) and indoor (artificial light sources) conditions, and (c) types of bioreactors required for H2 production [2, 11–14].
High cost of the feed and operational conditions is the major limiting factor of BHP. Most basic studies have been carried out on simple and complex sugars as feed material [15–21]. For circumventing the issues related to cost of the feed, biowastes of diverse origins especially agricultural, food and fruit processing industries, and those of municipal markets have been suggested as cheap and renewable alternatives [2, 22–28]. Although, the amount of H2 generated from different biowastes encourages one to pursue this route however, it demands quite a bit of optimization at different stages [29–34]. Instead of dwelling on optimization efforts being made on individual parameters of BHP process, an emerging proposal is to combine the dark- and photo-fermentative H2 producing organisms [7, 10, 35, 36]. The efforts in this direction have been targeted on the following combinations: (i) using defined dark- and photo-fermentative H2 producing organisms in a sequential manner in two independent stages (ii) using undefined dark-fermentative H2-producers along with defined photosynthetic organisms in two stages (iii) using the two types of BHP processes into a single stage, and (iv) using effluent from a dark-fermentative process (not necessarily a H2 production reactor) and exploiting photo-fermentative bacteria for their H2 producing abilities [33, 37–48]. In our recent efforts, we have emphasized only on using defined bacterial cultures in a sequential manner and evaluate it with respect to their individual H2 producing abilities from pure substrates and biowastes [7]. In the present work, we are concentrating our efforts on studies conducted using undefined dark fermentative H2 producing culture combinations and exploitation of effluent from dark-fermentative process by photosynthetic organisms using biowaste as feed.
Biological Hydrogen Production
Integrative Two Stage Dark- and Photo-Fermentative Sequential Hydrogen Production
The physiology and metabolic activities of bacteria vary significantly under dark- and photo-fermentative conditions. The efficiency depends primarily on the types of enzymes involved in H2 evolution. Under dark-fermentative conditions, hydrogenase and nitrogenase are the major enzymes responsible for this process [2, 49]. In the overall conversion of feed to H2, a few intermediates are also generated, such as VFAs and alcohols. The efficiency of the dark-fermentative H2 evolution process is governed by VFAs (Eqs. 1–4), such that acetic acid generation can lead to an additional 4 mol of H2 whereas butyric acid is expected to generate 2 mol of H2/mol of substrate. Lactic acid and ethanol are considered to be counter-productive to H2 evolution process [2, 28]. The intermediates of the dark-fermentative BHP, such as acetic and butyric acid can be taken up by photosynthetic organisms to generate additional H2 (Eqs. 5–6) [45, 46, 50, 51].
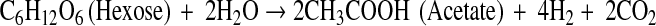 |
1 |
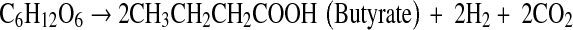 |
2 |
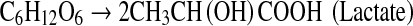 |
3 |
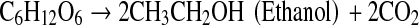 |
4 |
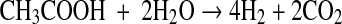 |
5 |
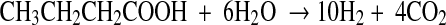 |
6 |
Using the organisms present in activated sludge enriched for dark-fermentative H2-producers, along with photosynthetic organisms such as Rhodobacter sphaeroides, Rhodopseudomonas palustris and undefined photosynthetic bacteria, it has been possible to achieve 2.86–6.07 mol H2/mol hexose [34, 52], over an incubation period ranging from 1 to 6 days of dark-fermentation followed by 5–14 days of photo-fermentative phase [53, 54]. In most of the cases, the temperature of 31–37 °C has been found to be optimal during the dark phase and 30 °C during the light phase (Table 1). In these cases, starchy wastes have been employed, which had originated from wheat, rice and cassava (Table 1). In other studies, cattle dung, dairy manure and mixed cultures in combinations with Rhodobacter capsulatus, R. palustris, and R. sphaeroides, and their combinations have been shown to yield 3.40–7.15 mol H2/mol hexose [33, 38, 43, 47, 55, 56]. In these cases, starchy wastes, cheese whey and water hyacinth have been fermented for quite long periods 2–10 days of the dark phase followed by 11–21 days of the light period and exceptionally it was 90/100 days under repeated batch culture [47]. In a few other combinations of dark and photosynthetic bacteria, Caldicellulosiruptor, Clostridium, Klebsiella, Lactobacillus and Thermotoga in association with R. capsulatus, R. sphaeroides, Rhodobium marinum and R. palustris have been used for H2 production (Table 1). These integrative approches of two stage H2 production have proved effective as most of them have lead to yields up to 7.2 mol/mol hexose [30]. In dark-fermentative BHP, the H2 yields are quite low in most cases and exceptionally it is posible to achieve a value of 3.8 mol H2/mol hexose [57]. In contrast, the two stage integrative approach is much more effective and exceptionally only it falls around 2.8–3.9 mol/mol hexose [45, 51, 58]. A summary of the results of yields of ≥7.0 mol H2/mol hexose reveals that it has been achieved with combinations such as (i) mixed culture—R. palustris and water hyacinth (10 g/l) [33], (ii) Clostridium butyricum—R. sphaeroides—algal biomass (starch at the rate of 5 g/l) [31], (iii) C. butyricum and Enterobacter aerogenes—R. sphaeroides/Rhodobacter sp. and sweet potato starch (5–10 g/l) [30, 59].
Table 1.
Integration of dark- and photo-fermentative bacteria in a sequential two stage hydrogen production from biowastes
| Organisms | Substrate (concentration: g/l) | Process parameters (in dark/light phase) | H2 yield (mol/mol hexose) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dark-fermentative | Photo-fermentative | Reactor capacity (l) | pH | Temp. (°C) | IPc (days) | Culture mode | |||
| Activated sludge | Rhodobacter sphaeroides | Wheat starch (20.0) | 0.31/0.31 | 6.8/7.1 | 37/30 | 3/8 | Batch/Batch | 4.55 | [20] |
| Mixed strains (R. sphaeroides) | Wheat starch (20.0) | 0.31/0.31 | 6.8/7.5 | 37/30 | –d/14 | Batch/Batch | 3.81 | [53] | |
| Rhodopseudomonas palustris | Cassava starch (10.0) | 0.30/0.30 | 7.0/7.0 | 35/30 | 6/5 | Batch/Batch | 2.86h | [52] | |
| Mixed PSBa | Rice straw (50.0) | 0.30/0.10 | 6.5/7.0 | 35/30 | 1/– | Batch/Batch | 5.18 | [54] | |
| Cassava starch (10.4) | 0.30/0.10 | 6.3/7.0 | 31/30 | 3/8 | Batch/Batch | 6.07 | [34] | ||
| Cattle dung compost | R. sphaeroides | Cassava starch (18.0) | 0.038/0.038 | 6.8/7.0 | 37/30 | 4/8 | Batch/Batch | 6.51 | [38] |
| Food waste (20.0) | 0.038/0.038 | 6.8/7.0 | 37/30 | 4/8 | Batch/Batch | 5.40 | |||
| Dairy manure microflora | R. sphaeroides | Corncob (Sugar, 10.0) | 0.60–25/0.32 | 7.0/7.0 | 36/35 | –/12 | Batch and CSTRe/Batch | 6.59 | [43] |
| Mixed culture | R. palustris | Cheese whey (COD, 30.0) | 1.0/0.25 | 7.5/6.9 | 55/31 | –/21 | CSTR (HRTf–24h)/Batch | 5.00h | [55] |
| Water hyacinth (10.0) | 0.30/0.30 | 7.0/7.0 | 35/30 | 2–5/11 | Batch/Batch | 7.15 | [33] | ||
| Rhodobacter capsulatus | Potato starch (50.0–100.0) | 0.25/0.30 | 6.8/7.0 | 37/28 | 10–12/25 | Batch/Batch | 5.60 | [56] | |
| R. capsulatus and R. sphaeroides | Starch (20.0) | 0.50/100 cm3 (dimensions) | 6.8/6.4 | 37/28 | 90/100 | Batchg/Batchg | 3.40–5.30 | [47] | |
| Clostridium butyricum | R. sphaeroides | Algal biomass (Starch, 5.0) | 0.15/0.15 | 6.8/7.0 | 37/30 | 2/15 | Batch/Batch | 8.30 | [31] |
| R. palustris | Starch (17.0) | 0.20/1.00 | 7.5/7.1 | 37/32 | 6–32/– | Batch/Batch and CSTR/CSTR (HRT–12/24h) | 3.09 | [37] | |
| Rhodobacter sp. | Starch (5.0) | TTb/TT | 7.0/6.5 | 30/30 | –/– | Batch | 3.60 | [68] | |
| C. butyricum and Enterobacter aerogenes | R. sphaeroides | Sweet potato starch (Starch, 5.0) | 0.25/TT | 5.3/7.5 | 37/35 | 10/30 | Batchg/Batchg | 7.00 | [59] |
| Rhodobacter sp. | Sweet potato starch (Starch, 10.0) | 0.25/TT | 5.3/7.5 | 37/35 | 13/30 | Batchg/Batchg | 6.70–7.20 | [30] | |
| Clostridium acetobutylicum and Escherichia coli | R. capsulatus | Date palm fruits and sucrose (5.0) | 2.00/2.00 | 7.3/7.0 | 30/30 | 3/7 | Batch/Batch | 3.90 | [58] |
| Thermotoga neapolitana | R. capsulatus | Miscanthus (Sugar, 10.0) | 2.00/0.105 | 7.0/6.5 | 80/30 | 3/10 | Batch/Batch | 4.50h | [57, 60] |
| Caldicellulosiruptor saccharolyticus | R. capsulatus | Potato steam peels (Sugar, 15.0) | 2.00/0.055 | 6.8/6.4 | 72/30 | –/– | Batch/Batch | 3.91 | [51] |
| Sugar beet molasses (Sucrose, 15.0) | 1.00/0.055 | 6.9/6.6 | 72/30 | 3/12 | Batch/Batch | 5.70h | [32, 46] | ||
| 1.00/0.055 | 6.9/6.7 | 72/30 | 3/7 | Batch/Batch | 6.85h | [32] | |||
| 2.00/0.055 | 6.8/6.4 | 72/30 | –/– | Batch/Batch | 5.81 | [51] | |||
| R. capsulatus, R. palustris and R. sphaeroides | Potato steam peels (Sugar, 15.0) | 2.0/0.055 | 6.4/6.4 | 72/30 | –/6 | Batch/Batch | 2.87–3.39h | [45] | |
| Klebsiella oxytoca | R. palustris | Sugarcane bagasse (50.0) | 0.14/0.14 | 7.0/7.0 | 37/30 | –/– | Batch/Batch | 4.14h | [44] |
| Lactobacillus amylovorus | Rhodobium marinum | Algal biomass (Starch, 4.05) | 0.07/0.07 | 7.0/6.5 | 30/30 | 6/6 | Batch/Batch | 5.40 | [29] |
aPhoto synthetic bacteria
bTest tube with dimension of 2.4 × 20 cm2
cIncubation period
dValues not given
eContinuous stirred tank reactor
fHydraulic retention time
gBoth in batch and repeated batch cultures
hValues converted from the original data
A perusal of Table 1 allows us to draw a few conclusions on the significance of the roles of photosynthetic organisms in influencing H2 yields in the integrative BHP process. Here, it can be observed that photo-fermentative organisms can utilize different biowastes to produce high H2 yields—(i) R. sphaeroides could evolve 3.81–8.30 mol H2/mol hexose (ii) R. capsulatus yielded 3.90–6.85 mol H2/mol hexose, and (iii) R. palustris was also effective in generating up to 7.15 mol H2/mol hexose [31–33, 53, 58]. In view of the effective working of the photosynthetic partners in the integrated BHP process, the observed variations in H2 yields can be assigned to the dark-fermentative H2-producers. Dark-fermentative bacteria present in the activated sludge were relatively less effective in producing H2 in comparison to those present in cattle dung. Among the defined dark fermentative bacteria, C. butyricum alone or in association with E. aerogenes was quite consistent in yielding 7–8 mol H2/mol hexose, along with R. sphaeroides as the photo-fermentative partner [31, 59]. H2 yields did not vary when R. capsulatus was used in association with a wide range of dark-fermentative H2-producers [47, 51, 57, 58, 60]. In contrast to R. sphaeroides, the combination of C. butyricum and R. palustris did not prove to be the most effective H2-producing culture combination [37].
Integrative Single Stage Dark and Photo-Fermentative Hydrogen Production
In contrast to subjecting feed material to dark- and photo-fermentative bacteria under two different sets of conditions, attempts have been made to combine the two (Table 2). Combination of activated sludge (as source of dark-fermentative H2-producers) with R. sphaeroides has resulted in H2 yield of 0.3–3.4 mol/mol hexose [39, 61–63]. The variation in H2 yields could be assigned to differences in substrate concentration, inoculum ratios, light intensities, etc. [39, 63]. In most of the reports, batch and fed-batch mode of reactors have been employed. The best results of 3.4 mol H2/mol hexose were reported when wheat starch was used at the rate of 5 g/l with an inoculum ratio of 1:3 (of dark/photo-fermentative bacteria) in continuous mode (periodic feed) [63].
Table 2.
Integration of dark- and photo-fermentative bacteria in a single stage hydrogen production from biowastes
| Organisms | Substrate (concentration: g/l) | Process parameters | H2 Yield (mol/mol hexose) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum ratioa | Light intensityc | Reactor capacity (L) | pH | Temp. (°C) | IPh | Culture mode | ||||
| Activated sludge + Rhodobacter sphaeroides | Wheat starch (5.0) | 1:3 | 5,000 lux | 0.25 | 7.0 | 30 | –i | Continuousk (HRTl–8) | 3.40 | [63] |
| Wheat starch (5.95) | 1:2 | 10,000 lux | 0.31 | 7.5 | 30 | 8 | Batch | 1.45 | [39] | |
| Wheat starch (10.0) | 1:2 | 5,000 lux | 2.00 | 7.5 | 30 | 10 | Fed-batch | 1.32o | [61] | |
| Wheat starch (12.8) | 1:2 | 6,000 lux | 0.31 | 7.5 | 30 | 12 | Batch | 0.28–0.36o | [62] | |
| Activated sludge + Rhodobacter sp. and Rhodopsedomonas palustris | Wheat starch (2.5) | 1:7 | 9,500 lux | 0.31 | 7.5 | 30 | 8 | Batch | 1.05 | [64] |
| Wheat starch (5.0) | 1:7 | 9,500 lux | 0.31 | 7.3 | 30 | 13 | Batch | 1.16 | [65] | |
| Wheat starch (20.0) | 1:2 | 9,500 lux | 2.00 | 7.5 | 30 | 11 | Combined fed-batch | 0.43o | [42] | |
| Activated sludge + Rhodospirillum rubrum | Cassava starch (COD, 20) | 1:1 | 6,000d candela/m2 | 0.075 | 7.2 | 30 | – | Batch | 340p | [71] |
| Lactobacillus amylovorus + Rhodobium marinum | Algal biomass (Starch, 4.05) | 0.5:0.6 | 330 W/m2 | 0.07 | 6.5 | 30 | 16 | Batch | 7.30 | [29] |
| Clostridium beijerinkii + R. sphaeroides | Wheat starch (5.0) | 1:3.9 | 10,000 lux | 7.63 | 7.3 | 32 | – | AHBm (HRT-6) | 0.60 | [41] |
| Wheat starch (12.8) | 1:2 | 6,000 lux | 0.31 | 7.5 | 30 | 12 | Batch | 0.12–0.15o | [62] | |
| Clostridium butyricum + Rhodobacter sp. | Starch (5.0) | 2:3 | 5,000 lux | TTf | 6.5 | 30 | 8–40j | Bacth and fed-batchn | 4.50–6.60 | [68] |
| Citrobacter freundii and Enterobacter aerogenes + R. palustris | Sugar cane effluent (Sugar, 7.9) | 1:1:1b | NAe | 10–100g | 7.0 | 37 | 2 | Batch | 2.76 | [66] |
| Vibrio fluvialis + Rhodobium marinum | Algal biomass (Starch, 4.05) | 2:1 | 330 W/m2 | 0.07 | 7.0 | 30 | 9 | Batch | 6.20 | [67] |
aDark/light organisms
bCo-cultures
cContinuous light
dDark and light periods of 12 h each also
eNot applicable
fTest tube with dimension of 2.4 × 20 cm2
gReactor dimensions in m3
hIncubation period in days
iValues not given
jDifferent sets of experiment
kPeriodic feed
lHydraulic retention time in days
mAnnular-hybrid bioreactor
nRepeated
oValues converted from the original data
pml H2/g COD
In other experiments, low H2 yields in the range of 1.05–1.16 mol/mol hexose were recorded on substituting R. sphaeroides with Rhodobacter sp. and R. palustris combination, along with activated sludge and wheat starch (as feed) [64, 65] and quite high yield of 2.76 mol/mol hexose with pure culture [66]. It allowed one to conclude the superiority of R. sphaeroides as a photo-fermentative partner. The high H2 yielding capacity of R. sphaeroides was negatively affected when combined with Clostridium beijerinckii as the dark-fermentative partner–resulting in low H2 yield of 0.6 mol/mol hexose [41]. R. marium proved to be an effective H2-producer, which resulted in high yields of 7.3 mol/mol hexose with Lactobacillus amylovorus and 6.2 mol/mol hexose with Vibrio fluvialis [29, 67]. Incidentally, in spite of being such highly effective H2-poducers, L. amylovorus and V. fluvialis have not been pursued since their initial reports.
Perspectives
Among the different worries which loom large are the pollution due to burning of fossil fuels and their limited resources. Although biohydrogen has been identified as a clean alternative to ever polluting fossil fuels, however, in order to establish biohydrogen as a non-polluting energy carrier it is imperative to carry out innovative research. At present, the struggle is on to look for cheap sources of feed and robust microbes for commercial scale H2 production. The need stems from the fact that BHP is regarded as inefficient due to low yields. Theoretically 12 mol of H2 can be generated from each mol of glucose. However, in practice, H2 yields are stagnant, such that a maximum of 3.8 mol/mol glucose has been shown as the achievable limit with either dark- or photo-fermentative routes by a limited number of bacteria. It was however realized quite soon that H2 yields can be enhanced by combining the two metabolic routes. Here, VFAs especially acetic acid and butyric acid generated as the end products of dark fermentative H2-production process can be subjected to photo-fermentative bacteria. Theoretically, acetic acid can be converted to generate 4 mol of H2 [50]. Such that a H2 yield of 12 mol/mole glucose can be achieved by employing an integrative approach–dark followed by photo-fermentation [7]. The need is to optimize the various process parameters and thus improve the efficiency of the organisms. Since, bacteria exist largely as complex communities, they create conditions such that ecological selection persists and the most productive system prevails. Taking advantage of the abilities of the bacteria to occur as mixed cultures and as consortia, it is desirable to select bacteria which are compatible to each other and exploit their natural abilities to accomplish our purpose. Facultative anaerobes such as Bacillus and Enterobacter have abilities to produce H2 in quantities which are quite comparable to those produced by strict anaerobic (Clostridium). They however offer additional advantages in terms of their abilities to survive in the presence of O2 during the initial stage of anaerobic biodegradation and produce H2 efficiently. They also offer an added feature by quenching O2 in cases where Clostridium may be the associated H2-producer [68, 69]. In case of photo-fermentation, light intensity is a major requirement for most metabolic activities. During photo-fermentative BHP, nitrogenase enzyme requires energy for the H2 production, which is provided by the light energy conversion to ATP [70]. It has been shown that increase in the light energy does enhance BHP [39], although exceptionally it may not prove effective [71]. We can design complex communities consisting of robust and self stabilizing populations. This syntrophic association must be managed for the sustainable development. It is envisaged that the feasibility of these two stage processes can be established by combining it with microalgae photosynthesis processes, which is likely to enhance overall H2 production by utilizing CO2 produced in the previous stages [37]. From a commercial point of view, it may be necessary to integrate other processes such as bioplastic and methane production in it [4, 7, 17, 72–75].
Acknowledgments
The authors wish to thank Director of CSIR-Institute of Genomics and Integrative Biology and Department of Biotechnology (DBT) Biology for providing the necessary funds, facilities and moral support. SKSP is thankful to CSIR for granting Research Associate Fellowship.
References
- 1.Kalia VC (2007) Microbial treatment of domestic and industrial wastes for bioenergy production. Appl Microbiol (e-Book). National Science Digital Library NISCAIR, New Delhi, India. http://nsdl.niscair.res.in/bitstream/123456789/650/1/DomesticWaste.pdf
- 2.Kalia VC, Purohit HJ. Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol. 2008;35:403–419. doi: 10.1007/s10295-007-0300-y. [DOI] [PubMed] [Google Scholar]
- 3.Kalia VC, Lal S, Ghai R, Mandal M, Chauhan A. Mining genomic databases to identify novel hydrogen producers. Trends Biotechnol. 2003;21:152–156. doi: 10.1016/S0167-7799(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domíguez-Espinosa R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004;22:477–485. doi: 10.1016/j.tibtech.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Levin DB, Pitt L, Love M. Biohydrogen production: prospects and limitations to practical application. Int J Hydrogen Energy. 2004;29:173–185. doi: 10.1016/S0360-3199(03)00094-6. [DOI] [Google Scholar]
- 6.Lee HS, Vermaas WFJ, Rittmann BE. Biological hydrogen production: prospects and challenges. Trends Biotechnol. 2010;28:262–271. doi: 10.1016/j.tibtech.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Patel SKS, Kumar P, Kalia VC. Enhancing biological hydrogen production through complementary microbial metabolisms. Int J Hydrogen Energy. 2012 [Google Scholar]
- 8.Hallenbeck PC, Ghosh D, Skonieczny MT, Yargeau V. Microbiological and engineering aspects of biohydrogen production. Indian J Microbiol. 2009;49:48–59. doi: 10.1007/s12088-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallenbeck PC, Ghosh D. Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 2009;27:287–297. doi: 10.1016/j.tibtech.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Argun H, Kargi F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: an overview. Int J Hydrogen Energy. 2011;36:7443–7459. doi: 10.1016/j.ijhydene.2011.03.116. [DOI] [Google Scholar]
- 11.Kotay SM, Das D. Biohydrogen as a renewable energy resource—prospects and potentials. Int J Hydrogen Energy. 2008;33:258–263. doi: 10.1016/j.ijhydene.2007.07.031. [DOI] [Google Scholar]
- 12.Hallenbeck PC. Fermentative hydrogen production: principles, progress and prognosis. Int J Hydrogen Energy. 2009;34:7379–7389. doi: 10.1016/j.ijhydene.2008.12.080. [DOI] [Google Scholar]
- 13.Dasgupta CN, Gilbert JJ, Lindblad P, Heidorn T, Borgvang SA, Skjanes K, Das D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int J Hydrogen Energy. 2010;35:10218–10238. doi: 10.1016/j.ijhydene.2010.06.029. [DOI] [Google Scholar]
- 14.Hallenbeck PC, Abo-Hashesh M, Ghosh D. Strategies for improving biological hydrogen production. Bioresour Technol. 2012;110:1–9. doi: 10.1016/j.biortech.2012.01.103. [DOI] [PubMed] [Google Scholar]
- 15.Carere CR, Kalia V, Sparling R, Cicek N, Levin DB. Pyruvate catabolism and hydrogen synthesis pathway genes of Clostridium thermocellum ATCC 27405. Indian J Microbiol. 2008;48:252–266. doi: 10.1007/s12088-008-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan C-M, Fan Y-T, Zhao P, Hou H-W. Fermentative hydrogen production by the newly isolated Clostridium beijerinckii Fanp3. Int J Hydrogen Energy. 2008;33:5383–5391. doi: 10.1016/j.ijhydene.2008.05.037. [DOI] [Google Scholar]
- 17.Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Ren N-Q, Liu B-F, Ding J, Xie G-J. Hydrogen production with R. faecalis RLD-53 isolated from freshwater pond sludge. Bioresour Technol. 2009;100:484–487. doi: 10.1016/j.biortech.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.deVrije T, Budde MAW, Lips SJ, Bakker RR, Mars AE, Claassen PAM. Hydrogen production from carrot pulp by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int J Hydrogen Energy. 2010;35:13206–13213. doi: 10.1016/j.ijhydene.2010.09.014. [DOI] [Google Scholar]
- 20.Patel SKS, Purohit HJ, Kalia VC. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrogen Energy. 2010;35:10674–10681. doi: 10.1016/j.ijhydene.2010.03.025. [DOI] [Google Scholar]
- 21.Ghosh D, Hallenbeck PC. Fermentative hydrogen yields from different sugars by batch cultures of metabolically engineered Escherichia coli DJT135. Int J Hydrogen Energy. 2009;34:7979–7982. doi: 10.1016/j.ijhydene.2009.08.004. [DOI] [Google Scholar]
- 22.Kalia VC, Jain SR, Kumar A, Joshi AP. Fermentation of biowaste to hydrogen by Bacillus licheniformis. World J Microbiol Biotechnol. 1994;10:224–227. doi: 10.1007/BF00360893. [DOI] [PubMed] [Google Scholar]
- 23.Kalia VC, Joshi AP. Conversion of waste biomass (pea-shell) into hydrogen and methane through anaerobic digestion. Bioresour Technol. 1995;53:165–168. doi: 10.1016/0960-8524(95)00077-R. [DOI] [Google Scholar]
- 24.Sonakya V, Raizada N, Kalia VC. Microbial and enzymatic improvement of anaerobic digestion of waste biomass. Biotechnol Lett. 2001;23:1463–1466. doi: 10.1023/A:1011664912970. [DOI] [Google Scholar]
- 25.Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzyme Microb Technol. 2006;38:569–582. doi: 10.1016/j.enzmictec.2005.09.015. [DOI] [Google Scholar]
- 26.Balat H, Kirtay E. Hydrogen from biomass present–scenario and future prospects. Int J Hydrogen Energy. 2010;35:7416–7426. doi: 10.1016/j.ijhydene.2010.04.137. [DOI] [Google Scholar]
- 27.Levin DB, Chahine R. Challenges for renewable hydrogen production from biomass. Int J Hydrogen Energy. 2010;35:4962–4969. doi: 10.1016/j.ijhydene.2009.08.067. [DOI] [Google Scholar]
- 28.Patel SKS, Singh M, Kumar P, Purohit HJ, Kalia VC. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 29.Kawaguchi H, Hashimoto K, Hirata K, Miyamoto K. H2 production from algal biomass by a mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus. J Biosci Bioeng. 2001;91:277–282. doi: 10.1263/jbb.91.277. [DOI] [PubMed] [Google Scholar]
- 30.Yokoi H, Maki R, Hirose J, Hayashi S. Microbial production of hydrogen from starch-manufacturing wastes. Biomass Bioenergy. 2002;22:389–395. doi: 10.1016/S0961-9534(02)00014-4. [DOI] [Google Scholar]
- 31.Kim M-S, Baek J-S, Yun Y-S, Sim S-J, Park S, Kim S-C. Hydrogen production from Chlamydomonas reinhardtii biomass using a two-step conversion process: anaerobic conversion and photosynthetic fermentation. Int J Hydrogen Energy. 2006;31:812–816. doi: 10.1016/j.ijhydene.2005.06.009. [DOI] [Google Scholar]
- 32.Ozgur E, Mars AE, Peksel B, Louwerse A, Yucel M, Gunduz U, Claassen PAM, Eroglu I. Biohydrogen production from beet molasses by sequential dark and photo-fermentation. Int J Hydrogen Energy. 2010;35:511–517. doi: 10.1016/j.ijhydene.2009.10.094. [DOI] [Google Scholar]
- 33.Su H, Cheng J, Zhou J, Song W, Cen K. Hydrogen production from water hyacinth through dark- and photo-fermentation. Int J Hydrogen Energy. 2010;35:8929–8937. doi: 10.1016/j.ijhydene.2010.06.035. [DOI] [Google Scholar]
- 34.Cheng J, Su H, Zhou J, Song W, Cen K. Hydrogen production by mixed bacteria through dark- and photo-fermentation. Int J Hydrogen Energy. 2011;36:450–457. doi: 10.1016/j.ijhydene.2010.10.007. [DOI] [Google Scholar]
- 35.Redwood MD, Beedle MP, Macaskie LE. Integrating dark and light bio-hydrogen production strategies: towards the hydrogen economy. Rev Environ Sci Biotechnol. 2009;8:149–185. doi: 10.1007/s11157-008-9144-9. [DOI] [Google Scholar]
- 36.Guwy AJ, Dinsdale RM, Kim JR, Massanet-Nicolau J, Premier G. Fermentative biohydrogen production systems integration. Bioresour Technol. 2011;102:8534–8542. doi: 10.1016/j.biortech.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 37.Lo Y-C, Chen S-D, Chen C-Y, Huang T-I, Lin C-Y, Chang J-S. Combining enzymatic hydrolysis and dark-photo fermentation processes for hydrogen production from starch feedstock: a feasibility study. Int J Hydrogen Energy. 2008;33:5224–5233. doi: 10.1016/j.ijhydene.2008.05.014. [DOI] [Google Scholar]
- 38.Zong W, Yu R, Zhang P, Fan M, Zhou Z. Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenergy. 2009;33:1458–1463. doi: 10.1016/j.biombioe.2009.06.008. [DOI] [Google Scholar]
- 39.Argun H, Kargi F. Effects of light source, intensity and lighting regime on bio-hydrogen production from ground wheat starch by combined dark and photo-fermentations. Int J Hydrogen Energy. 2010;35:1604–1612. doi: 10.1016/j.ijhydene.2009.12.033. [DOI] [Google Scholar]
- 40.Argun H, Kargi F. Photo-fermentative hydrogen gas production from dark fermentation effluent of ground wheat solution: effects of light source and light intensity. Int J Hydrogen Energy. 2010;35:1595–1603. doi: 10.1016/j.ijhydene.2009.12.040. [DOI] [Google Scholar]
- 41.Argun H, Kargi F. Bio-hydrogen production from ground wheat starch by continuous combined fermentation using annular-hybrid bioreactor. Int J Hydrogen Energy. 2010;35:6170–6178. doi: 10.1016/j.ijhydene.2010.03.132. [DOI] [Google Scholar]
- 42.Kargi F, Ozmihci S. Effects of dark/light bacteria ratio on bio-hydrogen production by combined fed-batch fermentation of ground wheat starch. Int J Hydrogen Energy. 2010;34:869–874. doi: 10.1007/s10295-009-0679-8. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Guo L, Liu F. Enhanced bio-hydrogen production from corncob by a two-step process: dark- and photo-fermentation. Bioresour Technol. 2010;101:2049–2052. doi: 10.1016/j.biortech.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Li Q, Dieudonne M, Cong Y, Zhou J, Long M. Enhanced H2 gas production from bagasse using adhE inactivated Klebsiella oxytoca HP1 by sequential dark-photo fermentations. Bioresour Technol. 2010;101:9605–9611. doi: 10.1016/j.biortech.2010.07.095. [DOI] [PubMed] [Google Scholar]
- 45.Afsar N, Özgür E, Gürgan M, Akköse S, Yücel M, Gündüz U, Eroglu I. Hydrogen productivity of photosynthetic bacteria on dark fermenter effluent of potato steam peels hydrolysate. Int J Hydrogen Energy. 2011;36:432–438. doi: 10.1016/j.ijhydene.2010.09.096. [DOI] [Google Scholar]
- 46.Androga DD, Ozgur E, Eroglu I, Gunduz U, Yucel M. Amelioration of photofermentative hydrogen production from molasses dark fermenter effluent by zeolite-based removal of ammonium ion. Int J Hydrogen Energy. 2012 [Google Scholar]
- 47.Laurinavichene TV, Belokopytov BF, Laurinavichius KS, Khusnutdinova AN, Seibert M, Tsygankov AA. Towards the integration of dark- and photo-fermentative waste treatment. 4. Repeated batch sequential dark- and photofermentation using starch as substrate. Int J Hydrogen Energy. 2012;37:8800–8810. doi: 10.1016/j.ijhydene.2012.01.132. [DOI] [Google Scholar]
- 48.Özkan E, Uyar B, Özgür E, Yücel M, Eroglu I, Gündüz U. Photofermentative hydrogen production using dark fermentation effluent of sugar beet thick juice in outdoor conditions. Int J Hydrogen Energy. 2012;37:2044–2049. doi: 10.1016/j.ijhydene.2011.06.035. [DOI] [Google Scholar]
- 49.Das D, Dutta T, Nath K, Kotay SM, Das AK, Veziroglu TN. Role of Fe-hydrogenase in biological hydrogen production. Curr Sci. 2006;90:1627–1637. [Google Scholar]
- 50.Fang HHP, Liu H, Zhang T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int J Hydrogen Energy. 2005;90:785–793. doi: 10.1016/j.ijhydene.2004.12.010. [DOI] [Google Scholar]
- 51.Özgür E, Afsar N, Vrije T, Yücel M, Gündüz U, Classen PAM, Eroglu I. Potential use of thermophile dark fermentation effluents in photofermentative hydrogen production by Rhodobacter capsulatus. J Clean Prod. 2010;18:S23–S28. doi: 10.1016/j.jclepro.2010.02.020. [DOI] [Google Scholar]
- 52.Su H, Cheng J, Zhou J, Song W, Cen K. Improving hydrogen production from cassava starch by combination of dark and photo fermentation. Int J Hydrogen Energy. 2009;34:1780–1786. doi: 10.1016/j.ijhydene.2008.12.045. [DOI] [Google Scholar]
- 53.Argun H, Kargi F, Kapdan IK, Oztekin R. Light fermentation of dark fermentation effluent for bio-hydrogen production by different Rhodobacter species at different initial volatile fatty acid (VFA) concentrations. Int J Hydrogen Energy. 2008;33:7405–7412. doi: 10.1016/j.ijhydene.2008.09.059. [DOI] [Google Scholar]
- 54.Cheng J, Su H, Zhou J, Song W, Cen K. Microwave-assisted alkali pretreatment of rice straw to promote enzymatic hydrolysis and hydrogen production in dark- and photo-fermentation. Int J Hydrogen Energy. 2011;36:2093–2101. doi: 10.1016/j.ijhydene.2010.11.021. [DOI] [Google Scholar]
- 55.Azbar N, Dokgoz FTC. The effect of dilution and l-malic acid addition on bio-hydrogen production with Rhodopseudomonas palustris from effluent of an acidogenic anaerobic reactor. Int J Hydrogen Energy. 2010;35:5028–5033. doi: 10.1016/j.ijhydene.2009.10.044. [DOI] [Google Scholar]
- 56.Laurinavichene TV, Belokopytov BF, Laurinavichius KS, Tekucheva DN, Seibert M, Tsygankov AA. Towards the integration of dark- and photo-fermentative waste treatment. 3. Potato as substrate for sequential dark fermentation and light-driven H2 production. Int J Hydrogen Energy. 2010;35:8536–8543. doi: 10.1016/j.ijhydene.2010.02.063. [DOI] [Google Scholar]
- 57.de Vrije T, Bakker RR, Budde MAW, Lai MH, Mars AE, Claassen PAM. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol Biofuels. 2009;2:12. doi: 10.1186/1754-6834-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abd-Alla MH, Morsy FM, El-Enany A-WE. Hydrogen production from rotten dates by sequential three stages fermentation. Int J Hydrogen Energy. 2011;36:13518–13527. doi: 10.1016/j.ijhydene.2011.07.098. [DOI] [Google Scholar]
- 59.Yokoi H, Saitsu A, Uchida H, Hirose J, Hayashi S, Takasaki Y. Microbial hydrogen production from sweet potato starch residue. J Biosci Bioeng. 2001;91:58–63. doi: 10.1263/jbb.91.58. [DOI] [PubMed] [Google Scholar]
- 60.Uyar B, Schumacher M, Gebicki J, Modigell M. Photoproduction of hydrogen by Rhodobacter capsulatus from thermophilic fermentation effluent. Bioprocess Biosyst Eng. 2009;32:603–606. doi: 10.1007/s00449-008-0282-9. [DOI] [PubMed] [Google Scholar]
- 61.Ozmihci S, Kargi F. Effects of starch loading rate on performance of combined fed-batch fermentation of ground wheat for bio-hydrogen production. Int J Hydrogen Energy. 2010;35:1106–1111. doi: 10.1016/j.ijhydene.2009.11.048. [DOI] [Google Scholar]
- 62.Ozmihci S, Kargi F. Comparison of different mixed cultures for bio-hydrogen production from ground wheat starch by combined dark and light fermentation. J Ind Microbiol Biotechnol. 2010;37:341–347. doi: 10.1007/s10295-009-0679-8. [DOI] [PubMed] [Google Scholar]
- 63.Sagnak R, Kargi F. Photo-fermentative hydrogen gas production from dark fermentation effluent of acid hydrolyzed wheat starch with periodic feeding. Int J Hydrogen Energy. 2011;36:4348–4353. doi: 10.1016/j.ijhydene.2011.01.033. [DOI] [Google Scholar]
- 64.Argun H, Kargi F, Kapdan IK. Effects of the substrate and cell concentration on hydrogen production from ground wheat by combined dark and light fermentation. Int J Hydrogen Energy. 2009;34:6181–6188. doi: 10.1016/j.ijhydene.2009.05.130. [DOI] [Google Scholar]
- 65.Argun H, Kargi F, Kapdan IK. Hydrogen production by combined dark and light fermentation of ground wheat solution. Int J Hydrogen Energy. 2009;34:4305–4311. doi: 10.1016/j.ijhydene.2009.03.033. [DOI] [Google Scholar]
- 66.Vatsala TM, Raj SM, Manimaran A. A pilot-scale study of biohydrogen production from distillery effluent using defined bacterial co-culture. Int J Hydrogen Energy. 2008;33:5404–5415. doi: 10.1016/j.ijhydene.2008.07.015. [DOI] [Google Scholar]
- 67.Ike A, Murakawa T, Kawaguchi H, Hirata K, Miyamoto K. Photoproduction of hydrogen from raw starch using a Halophilic bacterial community. J Biosci Bioeng. 1999;88:72–77. doi: 10.1016/S1389-1723(99)80179-0. [DOI] [PubMed] [Google Scholar]
- 68.Yokoi H, Mori S, Hirose J, Hayashi S, Takasaki Y. H2 production from starch by mixed culture of Clostridium butyricum and Rhodobacter sp M-19. Biotechnol Lett. 1998;20:895–899. doi: 10.1023/A:1005327912678. [DOI] [Google Scholar]
- 69.Hung C-H, Cheng C-H, Guan D-W, Wang S-T, Hsu S-C, Liang C-M, Lin C-Y. Interactions between Clostridium sp. and other facultative anaerobes in a self-formed granular sludge hydrogen-producing bioreactor. Int J Hydrogen Energy. 2011;36:8704–8711. doi: 10.1016/j.ijhydene.2010.06.010. [DOI] [Google Scholar]
- 70.Boran E, Özgür E, Yücel M, Gündüz U, Eroglu I. Biohydrogen production by Rhodobacter capsulatus in solar tubular photobioreactor on thick juice dark fermenter effluent. J Clean Prod. 2012;31:150–157. doi: 10.1016/j.jclepro.2012.03.020. [DOI] [Google Scholar]
- 71.Reungsang A, Sangyoka S, Chaiprasert P, Imai T. Factors affecting hydrogen production from Cassava wastewater by a co-culture of anaerobic sludge and Rhodospirillum rubrum. Pak J Biol Sci. 2007;10:3571–3577. doi: 10.3923/pjbs.2007.3571.3577. [DOI] [PubMed] [Google Scholar]
- 72.Kalia VC, Chauhan A, Bhattacharyya G, Rashmi Genomic databases yield novel bioplastic producers. Nat Biotechnol. 2003;21:845–846. doi: 10.1038/nbt0803-845. [DOI] [PubMed] [Google Scholar]
- 73.Kumar T, Singh M, Purohit HJ, Kalia VC. Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol. 2009;106:2017–2023. doi: 10.1111/j.1365-2672.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 74.Singh M, Patel SKS, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel SKS, Singh M, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of Bacillus spp. from glucose in two stage system. Indian J Microbiol. 2011;51:418–423. doi: 10.1007/s12088-011-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


