Abstract
Objective
This study was to investigate the variables in bone marrow harvesting procedure and individual donor factors which can potentially affect the yield of mesenchymal stromal cells (MSC).
Methods
We determined the yield of MSC from bone marrow under different clinical conditions by comparing the MSC colony numbers from: (1) donors of different ages; (2) healthy donors and patients with leukemia; (3) bone marrow aspirated at different time points during marrow harvesting; (4) bone marrow harvested by different needles.
Results
During the process of harvesting, the number of MSC significantly decreased with increase number of aspiration, from 675/ml at the initial decreased to 60/ml after 100 ml bone marrow aspirated, and 50/ml after 200 ml bone marrow aspirated. The number of MSC retrieved from leukemia patients (99/ml bone marrow) was significantly lower than that of healthy donors (708/ml bone marrow). However, there was no significant difference in growth rate. There was no significant age-related difference of MSC yielded from donors <55 years. And there was no significant difference in MSC number between the samples from single end-holed needle and those from multiple-side-hole needle.
Conclusion
The optimal bone marrow samples for MSC collection should be obtained earlier in the process of harvesting procedure. Bone marrow from donors <55 years was equally good as MSC sources. The autologous MSC from leukemia patients can be utilized for in-vitro MSC expansion.
Key words: Mesenchymal stromal cell, CFU-F, Bone marrow aspiration, Leukemia
INTRODUCTION
Bone marrow aspiration usually contains mesenchymal stromal cells (MSC) which represent an important cellular component of the bone marrow microenvironment. MSC is a heterogeneous cellular population consisting of mesenchymal stem cells and previous studies showed that the MSC plays important role in supporting hematopoietic stem cells (HSC) engraftment after transplantation[1,2]. Moreover, recent studies support the potential role of MSC as an immune modulator, and MSC play important role in prevention and treatment of graft-versus-host disease (GVHD)[3-12]. These MSC applied clinically were all in-vitro expanded. How to obtain an adequate amount of MSC within a reasonable limited time frame is crucial for the success of MSC treatment in-vivo. While finding the optimal culture conditions in expanding MSC in vitro is an important issue, how to improve the yield of MSC from proper collection of bone marrow samples is equally vital. This can help to reduce the culture time and chance of contamination, therefore making the expanding process efficient and less costly. Few evidence based data are currently available to guide the clinicians how to select the appropriate technique and donor for the purpose of MSC collection. The current study focused on two specific goals: (1) to determine the variables in bone marrow harvesting procedure which can potentially affect the yield of MSC; (2) to investigate the individual donor factors such as age, gender and disease status which can influence the initial MSC harvest quality.
MATERIALS AND METHODS
Patients
Bone marrow samples were obtained from 29 healthy bone marrow transplantation donors, and 19 patients with leukemia. The samples of all leukemic patients are obtained at diagnosis before commencement of treatment. They were diagnosed by standard techniques including morphology, cytochemical staining, immunophenotyping and cytogenetic analysis. The leukemic patients included 6 acute lymphoblastic leukemia (ALL), 6 acute myeloid leukemia (AML), 4 chronic myeloid leukemia (CML), and 3 chronic lymphocytic leukemia (CLL). All bone marrow samples were obtained from the posterior superior iliac crest (PSIC) under either local or general anesthesia. This study was performed with written informed consents under the approval of the Combined Internal Review Board (Ethical Committee) of the University of Hong Kong and The Hong Kong West Cluster of Hospital of Hospital Authority.
Isolation and Culture of MSC
Heparinized bone marrow samples were mixed with 2 volumes of phosphate buffered saline (PBS) and density separated by Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden). Mononuclear cells (MNC) were collected from the interface and washed twice with PBS. The washed cells were resuspended in MSC medium consisting of Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mmol/L L-glutamine. The cells were plated at 106 cells/100 mm dish. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2. After 24 h, non-adherent cells were removed by changing medium and the plate was washed twice with PBS. Medium was replaced every 3-4 day thereafter. When the culture were near confluence, cells were detached by 0.05% trypsin/25 mmol/L EDTA solution and re-plated at a density of 2×105 cells/75 cm2 flask as first passage. Then the phenotype of the first passage cells was assayed by flow cytometry. The doubling time of MSC was calculated according to the increase of cell number at the end of first passage and the culture time. Selected in vitro expanded MSC from healthy donors and leukemia patients were subjected to immunophenotypic analysis and differentiation testing as we previously reported[13].
MSC Colony Assay
To evaluate the MSC colony (also known as CFU-F, colony forming unit-fibroblast) forming frequency, 106 mononuclear cells from bone marrow samples were plated at 100-mm dish in 10 milliliter medium consisting of Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG) supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mmol/L L-glutamine. The medium was changed after 3 days and then twice a week. After incubation for 14 days at 37°C in 5% humidified CO2, the cells were washed with PBS and stained with 0.5% Crystal Violet in methanol for 5-10 min at room temperature. After a final rinse in tap water, colonies were counted using a standard inverted microscope. Clusters of cells were considered to be a colony if they contained 50 or more spindle cells. All evaluations were performed in duplicate and expressed as mean values. The frequency of MSC expressed as CFU-F per 106 MNC, the number of MSC expressed as CFU-F per milliliter bone marrow.
Effect of Repeated Bone Marrow Aspiration from the Same Donor on the Yield of MSC
To investigate whether the number of MSC was kept at the same level during the whole process of harvesting bone marrow, MSC colony assays were done with bone marrow samples from different aspiration of 20 bone marrow donors. The median age of the donors was 34 years (range from 4 to 55 years). There were 10 male donors and 10 female donors. During the harvest procedure, samples from the first aspiration, the tenth aspiration and the twentieth aspiration from the ipsilateral site of the same donor were collected. We followed the standard practice of changing the puncture sites on the bony surface within the same area of the skin puncture for each aspiration. Each aspiration contained 10 ml of bone marrow; the number of MSC was investigated at the three time points after an estimated amount of marrow was aspirated. The study time points were: (1) at the beginning; (2) after 100 ml of bone marrow was harvested (10th aspiration); and (3) after 200 ml bone marrow was harvested (20th aspiration).
Effect of Different Aspiration Needles on the Yield of MSC
We compared the yields of bone marrow derived MSC by using multiple side-holed needle (DePuy Spine 3-hole aspirator needle, DePuy International Ltd., Raynham, Massachusetts, USA) and conventional bone marrow end-holed needle without side-holes (Figure 1). Bone marrow aspiration was performed by both types of needle in a single healthy donor. We performed bone marrow aspirates from the designated bone marrow transplantation donor similar to our routine clinical practice without additional unnecessary punctures. In details, at first, Depuy needle was advanced into the intramedullary cavity at left PSIC, and the conventional needle was advanced into the intramedullary cavity at the right PSIC. Bone marrow 10 ml was aspirated from both sides and 2 ml were saved for analysis, the needles were then removed and the marrow was unloaded into sterile bottles with heparin. Each needle was then again advanced to the contralateral sides. That means Depuy needle to the right and conventional needle to the left PSIC. Another 10 ml of bone marrow were aspirated from each as in the first round and 2 ml each were sent for analysis. Then third and fourth round of aspirations were done in similar alternated fashion. Then the eight bone marrow samples were investigated for mononuclear cell number and MSC colonies number.
Figure 1.

Comparing conventional single end-holed needle (upper) and multiple side-holed DePuy Needle (lower).
Statistical Analysis
Both median and normalized mean were used to describe the average level. Wilcoxon test and unpaired t-test was used to detect the differences of average between 2 groups. A significant level of P<0.05 with 2-tailed will be used for all analyses. Both Parson correlation and Spearman correlation were used to detect any correlation between age and MSC yielding.
RESULTS
Comparing Yield of MSC from Same Donors after Repeated Bone Marrow Aspirations
The results of 60 samples from 20 donors demonstrated that the number of both MNC and MSC decreased significantly after repeated aspirations (Table 1). The median yield of MSC colonies decreased as the aspiration volume increased. There was a 66% decrease, from 87 per million MNC in the first aspiration down to only 30 per million MNC in the tenth aspiration. The median yield of MSC colonies (22 per million MNC) in the twentieth aspiration showed 74% drop as compared to the first aspiration. The difference between the first aspiration and the tenth aspiration was significant (P<0.05). Using the median number of MSC colonies per unit volume of bone marrow as assessment, there was a 91% decrease from the first aspiration (675 per milliliter bone marrow) to the tenth aspiration (60 per milliliter bone marrow). The median number of MSC colonies in the twentieth aspiration (50 per milliliter bone marrow) showed a 93% decrease from the first aspiration and a 17% decrease from the tenth aspiration. The differences between the first and the tenth aspiration, also between the tenth and the twentieth aspiration were both significant (P<0.01, and P<0.05 respectively).
Table 1. The cell harvest from bone marrow of different aspirated volume.
| CFU-F (/106 MNC) | MNC (106/ml) | CFU-F (/ml) | |||
|---|---|---|---|---|---|
| 1st aspiration | |||||
| Mean | 84 | 12 | 849 | ||
| Median | 87 | 8 | 675 | ||
| Range | 1-1080 | 1-51 | 11-2440 | ||
| 10th aspiration | |||||
| Mean | 47 | 10 | 391 | ||
| Median | 30* | 5 | 60** | ||
| Range | 1-820 | 1-85 | 1-2285 | ||
| 20th aspiration | |||||
| Mean | 54 | 4 | 126 | ||
| Median | 22 | 2**# | 50**# | ||
| Range | 1-274 | 0.3-20 | 1-820 | ||
*P<0.05 versus 1st aspiration; **P<0.01 versus 1st aspiration; #P<0.05 versus 10th aspiration; CFU-F: colony forming unit-fibroblast; MNC: mononuclear cells.
Comparing Yield of MSC from Healthy Donors of Different Ages & Sex
The median frequency of MSC colonies of male and female were 107 (range from 1 to 428) units per million MNC and 46 (range from 4 to 150) units per million MNC respectively (P=0.12). The median number of MSC colonies of male and female was 902 (range from 9 to 2525) units per milliliter bone marrow and 430 (range from 34 to 2440) units per milliliter bone marrow respectively (P=0.85). Both Parson correlation and Spearman correlation analysis showed that age was not correlative to the MSC yielding, therefore we did not separate the age group for the analysis. Figure 2 showed the median frequency and number of MSC colonies of 29 donors (median age 31 years, from 1-52 years) plotted against age.
Figure 2.
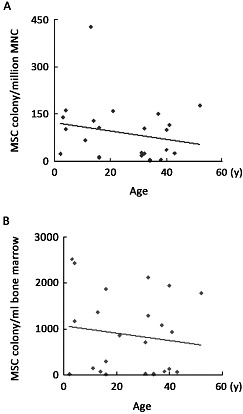
Correlation of MSC colony with donors’ age and gender. MSC colony numbers of 29 donors were plotted against their age. No correlation between MSC colonies with age was noted.
Comparing Yield of MSC from Leukemia Patients and Healthy Donors
There was no notable difference in the microscopic morphology of the MSC between those derived from leukemia patients or healthy donors. With the immuno- phenotyping of the MSC defined as positive for CD29, CD105 and HLA-A, B, C, but negative for CD14, CD34 and CD45 as previously described (13), we did not find any differences between the MSC derived from either the healthy donors or leukemia patients. MSC colonies in the donor group and in the leukemia group were compared (Figure 3). The median yield of MSC colonies among leukemia patients was 3 (the normalized mean was 12, range from 1 to 67) per million MNC. It was significantly lower than that of the healthy bone marrow donors (median 99, the normalized mean was 98, range from 1 to 428 per million MNC), (P<0.01). The median number of MSC colonies derived from leukemia patients and healthy donors were 126 (the normalized mean was 414, range from 1 to 2946) and 708 (the normalized mean was 861, range from 9 to 2525) per milliliter bone marrow respectively. The number of MSC colony per unit volume of bone marrow derived from healthy donors was also significantly higher than that of the leukemia patients (P<0.05). However, when cultured in vitro, the mean doubling time of MSC from 7 healthy donors and 10 leukemia patients were 2.067 days and 2.071 days respectively, so there was no significant difference in the growth rate between MSC derived from healthy donors and leukemia patients (Figure 4).
Figure 3.
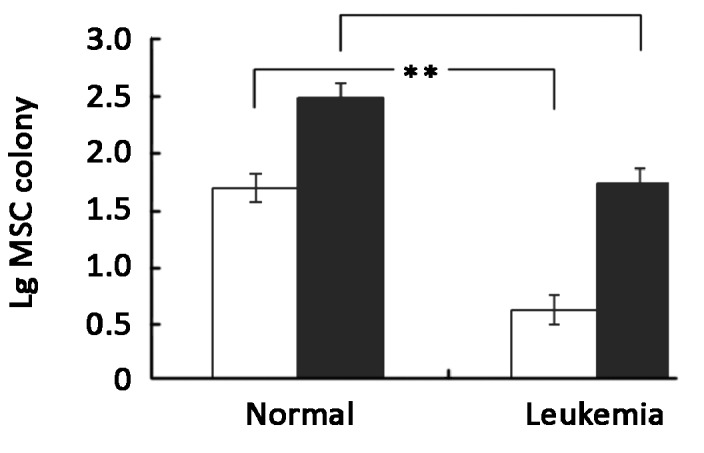
MSC colony number from bone marrow of leukemia patients (n=21) and healthy donors (n=29). Results were represented as mean ±SEM of common logarithm transformation of MSC colony number. □ Represented common logarithm transformation of MSC colony per million MNC. ■ Represented common logarithm transformation of MSC colony per milliliter bone marrow. *P<0.05, **P<0.01.
Figure 4.
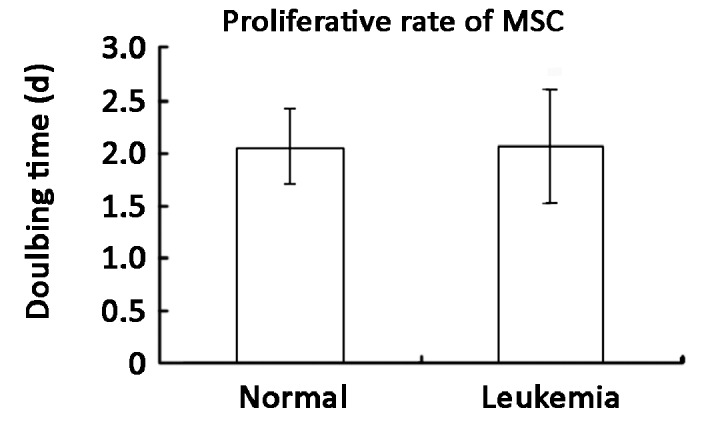
Growth rate of MSC from leukemia patients (n=10) and healthy donors (n=7). Results were represented as mean ±SEM. There was no significant difference between the two groups.
Effect of Different Aspiration Needle on the Yield of MSC
A mean of 5.38×106 MNC was contained in each 1 milliliter of bone marrow aspirated by the DePuy multiple side-holed needle, compared with 3.34×106 MNC/per ml of bone marrow aspirated by the conventional single end-holed needle. The results of the 4 samples from the DePuy needle aspiration yielded a mean of 17.76 MSC colonies/milliliter bone marrow, and a mean of 11.35 MSC colonies/milliliter bone marrow was yielded by the conventional needle aspiration. Though with apparently higher yield by number, after statistical analysis, the multiple side-holed needle did not show any significant benefit in harvesting either MNC or MSC as compared to the conventional single end-holed needle (Figure 5).
Figure 5.
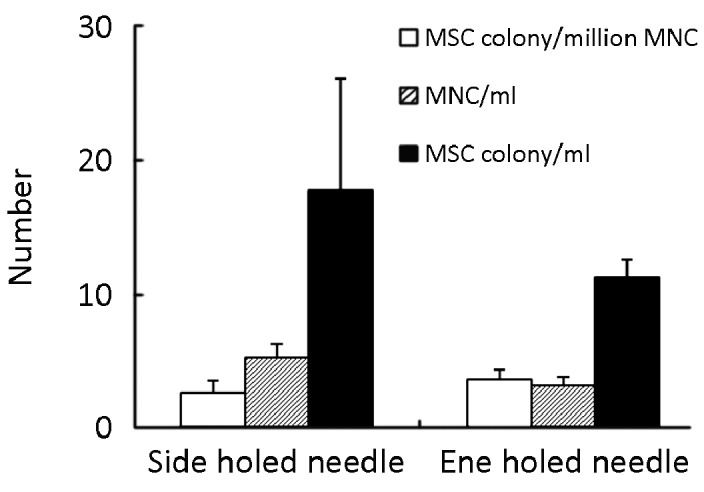
MSC colony number from bone marrow samples aspirated by the multiple side-holed needle and the conventional end-holed needle (n=4). Results were represented as mean ±SEM. There was no significant difference between the two kinds of needles.
DISCUSSION
Our data provided basic information on various factors that may influence the yield of MSC from either healthy donors or leukemia patients. During the process of bone marrow harvesting for BMT, the number of MSC significantly decreased after repeated aspirations. It can not be explained solely by peripheral blood dilution[14] because the extent of MSC decrease was out of the proportion to the drop in the MNC count. Previous studies only documented the relative number of MSC derived from bone marrow aspiration of small volume, and reported about one MSC colony can be derived from 105 bone marrow nucleated cells[15, 16] or 100-2300 MSC colony can be yielded from one ml of bone marrow[17]. Our results showed at the beginning of harvest, the number of MSC was within the range of previous study. When the harvested bone marrow volume exceeded 100 milliliters, the median number of MSC dropped to <10% of that of the initial aspiration. Two insights could be gained from this observation. The first one is that by simply increasing the volume of aspiration within a small operating area may not help in improving the yield of MSC. Secondly, the actual number of donor’s MSC recruited and transplanted to the recipients in the conventional bone marrow transplantation (BMT) setting may be much less than what we actually expected. Our data also indicated that bone marrow aspirated at the initial phase of the harvest process is the most optimal sample for isolating and expanding MSC.
In our study, no correlation was observed between the yield of MSC and the age or gender of the donors. Previous studies on the age-related effect on the number of MSC were conflicting with one another[18-27]. The only two studies on the number of MSC colony derived from healthy human subjects showed that there is no significant decrease among old (age range 66-78 years) individuals[23, 27]. Our data on healthy human donors was similar to these two reports, and clearly indicated that there was no age-related change in MSC number from a cohort of human donors younger than 55 years.
The yield of MSC from patients with leukemia was significantly lower than that of healthy donors. After MSC was removed from its untoward microenvironment and cultured separately in vitro, the growth rate of MSC derived from leukemia patients was similar with the healthy donors. Several groups demonstrated that the yield of MSC colonies in patients with acute leukemia was significantly lower than that of normal group[28-30]. However, they all evaluated the number of MSC by counting the MSC colony per million nucleated cells. Since the total nuclear cell number of the bone marrow in leukemia patients may be markedly elevated due to the presence of excessive number of leukemic blasts. The actual absolute number of MSC per unit volume may therefore be under-estimated. Indeed, we showed MSC number was lower than healthy donors even by counting per milliliter bone marrow. In addition, the results supports the suppression of MSC colonies in leukemia patients was possibly through humoral factors secreted by leukemia cells[30, 31]. Such suppressive effect might be reversible by removing the leukemic cells from the system. Our finding was in line with 2 recently published studies showing that the biological characteristics of MSC derived from both childhood acute leukemia and adult acute myeloid leukemia were similar to the MSC derived from normal marrow[32, 33]. Our result supported that the decreased yield of MSC in bone marrow of leukemia patients was not caused by any intrinsic changes in MSC, but was rather due to the adverse factors found in the leukemic micro-environment in vivo. These information indicated that for leukemia patients undergoing HSC transplantation, the residual autologous MSC from the recipients remained normal. Due to the small sample size and heterogeneity of leukemia patient group included in our analysis, our findings have to be confirmed with future studies.
Although MSC can be derived from other sources including cord blood and adipose tissue, bone marrow is considered as the most abundant source of MSC. However, even for bone marrow, the absolute number of MSC remains low and therefore, it is a limiting factor in applying MSC based therapy clinically. There is limited available information on the optimal method to collect MSC from the bone marrow currently. There are theoretical claims that needle with multiple side-holes can improve the efficiency of harvesting MSC from bone marrow as compared to the conventional single end-holed needle. We compared the number of marrow derived MSC obtained by these 2 forms of needles and found that there was no significant difference in the yield of either MSC or MNC. The comparison was only done on one donor. In future studies, more different individuals should be included. Tanikawa et al.[34] evaluated the yield of nucleated cells in bone marrow harvested by aspiration needles with or without side-holes and also showed no difference between the two kinds of needles. Another study also showed there was no significant difference between the needle with and without side holes in related to the number of NMC and CD34+ cells obtained from bone marrow. However, they demonstrated that the needle with side holes can reduce the harvest time to about half of the needle without side holes[35]. We therefore suggest that the conventional single end-holed needle may serve the same purpose as the more expensive multiple side-holed needle in harvesting bone marrow for MSC.
In summary, there are many factors influencing the number of MSC obtained from bone marrow aspiration. Bone marrow aspirated at the initial phase of the harvest process is the most optimal sample for isolating and expanding MSC. To increase the yield of MSC, increasing the volume of aspiration within a small puncture area may not be helpful. With the potential increase of clinical application of MSC, most cost effective means in obtaining MSC for in vitro expansion is important. Our study hence provided some background information on these aspects and will help to design an evidence-based working protocol for future MSC related projects.
REFERENCES
- 1.Fibbe WE, Noort WA. Mesenchymal stem cells and hematopoietic stem cell transplantation. Ann N Y Acad Sci 2003; 996:235-44 [DOI] [PubMed] [Google Scholar]
- 2.Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 2000; 7: 358-63 [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105:1815-22 [DOI] [PubMed] [Google Scholar]
- 4.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105:2214-9 [DOI] [PubMed] [Google Scholar]
- 5.Chung NG, Jeong DC, Park SJ, et al. Cotransplantation of marrow stromal cells may prevent lethal graft-versus-host disease in major histocompatibility complex mismatched murine hematopoietic stem cell transplantation. Int J Hematol 2004; 80:370-6 [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363:1439-41 [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Ringdén O.Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11:321-34 [DOI] [PubMed] [Google Scholar]
- 8.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 2004; 33:597-604 [DOI] [PubMed] [Google Scholar]
- 9.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11:389-98 [DOI] [PubMed] [Google Scholar]
- 10.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006; 81:1390-7 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy 2009; 11:278-88 [DOI] [PubMed] [Google Scholar]
- 12.von Bonin M, Stölzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant 2009; 43:245-51 [DOI] [PubMed] [Google Scholar]
- 13.Li J, Law HK, Lau YL, et al. Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. Br J Haematol 2004; 127:326-34 [DOI] [PubMed] [Google Scholar]
- 14.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am 1997; 79:1699-709 [DOI] [PubMed] [Google Scholar]
- 15.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human-bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 1980; 56:289-301 [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-7 [DOI] [PubMed] [Google Scholar]
- 17.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol 1999; 27:1460-6 [DOI] [PubMed] [Google Scholar]
- 18.Lebedinskaia OV, Gorskaia IuF, Shuklina Elu, et al. Age changes in the numbers of stromal precursor cells in the animal bone marrow. Morfologiia 2004; 126:46-9 [PubMed] [Google Scholar]
- 19.Scutt A, Kollenkirchen U, Bertram P.Effect of age and ovariectomy on fibroblastic colony-forming unit numbers in rat bone marrow. Calcif Tissue Int 1996; 59:309-10 [DOI] [PubMed] [Google Scholar]
- 20.Bergman RJ, Gazit D, Kahn AJ, et al. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res 1996; 11:568-77 [DOI] [PubMed] [Google Scholar]
- 21.Egrise D, Martin D, Vienne A, et al. The number of fibroblastic colonies formed from bone marrow is decreased and the in vitro proliferation rate of trabecular bone cells increased in aged rats. Bone 1992; 13: 355-61 [DOI] [PubMed] [Google Scholar]
- 22.Bellows CG, Pei W, Jia Y, et al. Proliferation, differentiation and self-renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech Ageing Dev 2003; 124:747-57 [DOI] [PubMed] [Google Scholar]
- 23.Justesen J, Stenderup K, Eriksen EF, et al. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int 2002; 71:36-44 [DOI] [PubMed] [Google Scholar]
- 24.Oreffo RO, Bennett A, Carr AJ, et al. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol 1998; 27:415-24 [DOI] [PubMed] [Google Scholar]
- 25.Oreffo RO, Bord S, Triffitt JT. Skeletal progenitor cells and ageing human populations. Clin Sci (Lond) 1998; 94:549-55 [DOI] [PubMed] [Google Scholar]
- 26.Brockbank KG, Ploemacher RE, van Peer CM. An in vitro analysis of murine hemopoietic fibroblastoid progenitors and fibroblastoid cell function during aging. Mech Ageing Dev 1983; 22:11-21 [DOI] [PubMed] [Google Scholar]
- 27.Stenderup K, Justesen J, Eriksen EF, et al. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res 2001; 16:1120-9 [DOI] [PubMed] [Google Scholar]
- 28.Katsuno M, Hirata J, Kaneko S, et al. Serial studies of bone marrow-derived fibroblastoid colony-forming cells and granulocyte/macrophage precursor cells in patients with acute leukemia. Acta Haematol 1986; 76:185-91 [DOI] [PubMed] [Google Scholar]
- 29.Nagao T, Yamauchi K, Komatsuda M.Serial in vitro bone marrow fibroblast culture in human leukemia. Blood 1983; 61:589-92 [PubMed] [Google Scholar]
- 30.Nara N, Jinnai I, Imai Y, et al. Reduction of granulocyte-macrophage progenitor cells (CFU-C) and fibroblastoid colony-forming units (CFU-F) by leukemic cells in human and murine leukemia. Acta Haematol 1984; 72:171-80 [DOI] [PubMed] [Google Scholar]
- 31.Nagao T, Yamauchi K, Komatsuda M, et al. Inhibition of human bone marrow fibroblast colony formation by leukemic cells. Blood 1983; 62:1261-5 [PubMed] [Google Scholar]
- 32.Wu LP, Chen FX, Lu HM. Biological characteristics of bone marrow-derived mesenchymal stem cells in children with acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009; 17:734-8 [PubMed] [Google Scholar]
- 33.Yin WJ, Yang PD, Huang YZ, et al. Comparison of biological characteristics of bone marrow mesenchymal stem cells in acute myeloid leukemia with those from non-leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009; 17:395-9 [PubMed] [Google Scholar]
- 34.Tanikawa S, Sakamaki H, Mori S, et al. Relationship between the presence of side-holes in bone marrow aspiration needle and the number of harvested bone marrow mononuclear cells. Rinsho Ketsueki 1997; 38:1249-53 [PubMed] [Google Scholar]
- 35.Lannert H, Able T, Becker S, et al. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplant 2008; 42:443-7 [DOI] [PubMed] [Google Scholar]


