Abstract
Objective
To investigate the associations between the different breast cancer subtypes and survival in Chinese women with operable primary breast cancer.
Methods
A total of 1538 Chinese women with operable primary breast cancer were analyzed in this study, the median follow-up was 77 months. Estrogen receptor (ER), progesterone receptor (PR), and HER2 status were available for these patients.
Results
Luminal A (ER+ and/or PR+, HER2-) had a favorable disease-free survival (DFS) and overall survival (OS) compared with other subtypes in the entire cohort. Using the luminal A as a reference, among the patients with lymph node positive disease, HER2+ (ER-, PR-, HER2+) had the worst DFS (hazard ratio, HR=1.80, 95% CI 1.11 to 2.91, P=0.017) and luminal B (ER+ and/or PR+, HER2+) had the worst OS (HR=2.27, 95% CI 1.50 to 3.45, P<0.001); among the patients with lymph node negative disease, triple-negative (ER-, PR-, HER2-) had the worst DFS (HR=2.21, 95% CI 1.43 to 3.41, P<0.001), whereas no significant difference in DFS between HER2+ and luminal B or luminal A was observed.
Conclusion
As compared with luminal A, luminal B and HER2+ have the worst survival in patients with lymph node positive disease, but this is not the case in patients with lymph node negative disease; triple-negative subtype has a worse survival in both lymph node positive and lymph node negative patients.
Key words: Breast cancer, Subtypes, Disease-free survival, Overall survival
INTRODUCTION
Breast cancer is a heterogeneous disease and gene expression analysis has identified several distinct subtypes with different prognosis and response to treatment[1,2]. However, the molecular subtyping determined by cDNA microarrays does not have an accepted standard and its technically demanding limits its wide application in clinical practice[3], Fortunately the intrinsic gene expression microarray categorization is well correlated with the immunohistochemistry classification according to estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth receptor2 (HER2)[4-6]. Many researchers therefore classified breast cancer patients into four subtypes based on the expression of ER, PR and HER2: luminal A (ER+ and/or PR+, HER2-); luminal B (ER+ and/or PR+, HER2+); HER2+ subtype (ER-, PR-, HER2+) and triple-negative subtype (ER-, PR-, HER2-). Previous studies suggested that patients with luminal A have a favorable prognosis when compared with patients with luminal B, HER2+ or triple-negative subtype[4,7,8].
It is well documented that axially lymph node involvement is an independent unfavorable factor in breast cancer[9], however, lymph node positive breast cancer patients represent approximately 40% of entire patients in most population studies, the majority of patients exhibit lymph node negative disease. Given the important prognosis of lymph node status, few studies currently are available for investigating the associations between breast cancer subtypes and survival according to lymph node status. Therefore, the purpose of this study was to investigate the associations between the different subtypes and survival in Chinese women with operable primary breast cancer, and we further investigated the associations stratified by lymph node status.
MATERIALS AND METHODS
Study Population
A total of 1538 patients with operable primary breast cancer (stage I-III) with available data for ER, PR, and HER2 status were selected from the pool of 2459 consecutive breast cancer patients treated at Peking University Cancer Hospital from December 1994 to December 2003. The ER, PR, and HER2 status were not significantly different between the cohort of 1538 patients and the entire of 2459 patients (data not shown). Tumor size was defined as the maximum tumor diameter measured on the tumor specimens at the time of operation. Patients received radical mastectomy, modified radical mastectomy, or breast conserving surgery; the axillary lymph nodes were routinely dissected at least at levels I and II, and lymph node metastasis was determined based on histological examination. The majority of patients in this cohort received adjuvant chemotherapy (cyclophosphamide, methotrexate, and fluorouracil regimen, anthracycline- based or paclitaxel-based regimen) or sequential chemotherapy and endocrine therapy, patients with ER and/or PR positive tumors usually received adjuvant tamoxifen treatment (20 mg/day) for 5 years after chemotherapy or surgery. No patients in this cohort received trastuzumab therapy. The follow-up data were available for 1521 (98.9%) patients, with a median follow-up of 77 months. This study was approved by the Research and Ethical Committee of Peking University School of Oncology.
Hormone Receptors
ER and PR status of 1129 cases was determined by using a dextran-coated charcoal assay as previously described[10].[3H]-estradiol(Amersham, Buckinghamshire, United Kingdom) and [3H]-R5020 (Dupont New England Nuclear, Boston, MA) were used as the labeled ligands for ER and PR analysis, respectively. Specimens containing at least 10 fmol/mg of protein were considered ER or PR positive. ER and PR expression in the remaining 409 cases were assessed by immunohisto- chemical assay using an ER-specific antibody raised against the N-terminal of ER epitope (clone:1D5, Zymed, South Francisco, CA; dilution 1:100) and a PR specific antibody (clone: 1A6, Zymed; dilution 1:100) respectively. ER or PR immunostaining was considered positive when 10% of tumor cells showed positive nuclear staining. HER2 expression was determined using an immunohisto- chemistry assay as described previously using an HER2 specific antibody (clone CB-11, Zymed; dilution 1:100), only the membrane staining was scored, the scoring for HER2 staining was graded as follows: no staining or staining observed in less than 10% of tumor cells was given a score 0; faint/barely perceptible staining and a moderate staining detected in at least 10% of tumor cells was scored as 1 and 2 respectively; strong complete observed in at least 10% of tumor cells was scored as 3, FISH testing of HER2 gene amplification was not routinely performed in our institute. So only a score of 3 was considered as HER2 positive.
Statistical Analysis
The differences in clinicopathologic characteristics between the breast subtypes were determined using Pearson χ2 test. Disease-free survival (DFS) was defined as the time from date of diagnosis to first recurrence (local or distant) or death from breast cancer without a recorded relapse. Overall survival (OS) was defined as the time from date of diagnosis to death with any causes. Patients who were alive at the last follow-up were censored at the last follow-up date. Survival curves were derived from Kaplan-Meier estimates, and the curves were compared by log-rank tests. A Cox-proportional hazard model was applied to estimate the hazard ratios for DFS and OS between the breast subtypes in a multivariate analysis. The most common subtype luminal A (ER+ and/or PR+, HER2-) was used as a reference. All statistical tests were two-sided, and P values less than 0.05 were considered as statistically significant. The statistical analyses were performed using SPSS 16.0 software.
RESULTS
Patient Characteristics
The clinicopathologic characteristics in this cohort of 1538 patients are present in Table1. Three hundreds fifty-three patients developed local recurrence or distant metastases, and 235 patients died during the follow-up period. We classified the study population into four subtypes based on the ER, PR and HER2 status, of these 1538 patients, 955 (62.1%) were luminal A, 171 (11.1%) were luminal B, 115 (7.5%) were HER2+ subtype, and 297 (19.3%) were triple-negative(Table1). Differences in baseline characteristics between the four subtypes are present in Table 2, Patients with luminal A or HER2+ subtype were more likely to have small tumor (P<0.001). Patients with luminal B subtype were more likely to be younger(P=0.009), and have a big tumor(P<0.001). No significant difference in lymph node status was found between the four subgroups. Patients with HER2+ or triple-negative were more likely to received chemotherapy(P<0.001), patients with luminal A or luminal B subtype were more likely to received chemotherapy plus endocrine therapy or endocrine therapy alone (P<0.001) (Table2).
Table 1. Patient Characteristics.
| Characteristics | n | % |
|---|---|---|
| Total | 1538 | |
| Age(years) | ||
| ≤50 | 768 | 49.9 |
| >50 | 770 | 50.1 |
| Tumor size(cm) | ||
| ≤2 | 822 | 53.4 |
| >2 | 713 | 46.4 |
| Unknown | 3 | 0.2 |
| Lymph nodes | ||
| Positive | 633 | 41.2 |
| Negative | 884 | 57.5 |
| Unknown | 21 | 1.3 |
| ER | ||
| Positive | 983 | 63.9 |
| Negative | 555 | 36.1 |
| PR | ||
| Positive | 776 | 50.5 |
| Negative | 762 | 49.5 |
| HER2 | ||
| Positive | 286 | 18.6 |
| Negative | 1252 | 81.4 |
| Subtypes | ||
| Luminal A | 955 | 62.1 |
| Luminal B | 171 | 11.1 |
| HER2 | 115 | 7.5 |
| Triple-negative | 297 | 19.3 |
| Adjuvant therapy | ||
| C | 684 | 44.5 |
| C+E | 579 | 37.6 |
| E | 195 | 12.7 |
| No treatment | 45 | 2.9 |
| Unavailable | 35 | 2.3 |
ER, estrogen receptor; PR, Progesterone receptor;
HER2, human epidermal growth factor receptor 2; C, chemothrapy;
E, endocrine therapy; C=E, chemotherapy plus endocrine therapy.
Table 2. Baseline characteristics by breast cancer subtypes.
| Characteristics | n | Luminal A |
Luminal B |
HER2 |
Triple-negative |
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Age (years) | ||||||||||
| ≤50 | 768 | 489 | 51.2 | 99 | 57.9 | 52 | 45.2 | 128 | 43.1 | 0.009 |
| >50 | 770 | 466 | 48.8 | 72 | 42.1 | 63 | 54.8 | 169 | 56.9 | |
| Tumor size (cm) | ||||||||||
| ≤2 | 822 | 543 | 57.0 | 75 | 43.9 | 66 | 57.9 | 138 | 46.5 | 0.001 |
| >2 | 713 | 410 | 43.0 | 96 | 56.1 | 48 | 42.1 | 159 | 53.5 | |
| Unknown | 3 | |||||||||
| Lymph nodes | ||||||||||
| Positive | 633 | 395 | 42.0 | 73 | 42.9 | 48 | 42.1 | 117 | 40.1 | 0.927 |
| Negative | 884 | 546 | 58.0 | 97 | 57.1 | 66 | 57.9 | 175 | 59.9 | |
| Unknown | 21 | |||||||||
| Adjuvant therapy | ||||||||||
| C | 684 | 334 | 35.8 | 59 | 35.3 | 79 | 71.2 | 212 | 72.4 | <0.001 |
| C+E | 579 | 431 | 46.2 | 85 | 50.9 | 23 | 20.7 | 40 | 13.7 | <0.001 |
| E | 195 | 152 | 16.3 | 20 | 12.0 | 2 | 1.8 | 21 | 7.2 | <0.001 |
| No treatment | 45 | 15 | 1.6 | 3 | 1.8 | 7 | 6.3 | 20 | 6.8 | <0.001 |
| Unavailable | 35 | |||||||||
C: chemotherapy; E: endocrine therapy; C+E: chemothrapy plus endocrine therapy.
Five-year DFS and OS in the Four Breast Cancer Subtypes
The 5-year DFS and 5-year OS in the entire study population were 80.3% and 87.0%, respectively. Patients with luminal A subtype had significantly better 5-year DFS and OS than did patients with any other subtypes, while patients with luminal B subtype had a worse 5-year DFS and OS as compared with other subtypes (Table 3 and Figure1 A, 1B). We then stratified the patients with lymph node status, among patients with lymph node positive disease, patients with HER2+, luminal B, or triple-negative subtype had a significant worse 5-year DFS and OS than did patients with luminal A subtype (Table 3, Figure 1C, 1D); on the other hand, among patients with lymph node negative disease, 5-yaer DFS and OS were not significantly different in patients with HER2+ subtype compared with patients with luminal A subtype, whereas patients with triple-negative had a worse 5-DFS and OS than did patients with luminal A subtype (Table 3, Figure 1E, 1F). Patients with luminal B had a worse 5-year OS compared with patients with luminal A subtype, but not significant difference in 5-year DFS was observed between the two subtypes (Table 3, Figure 1E, 1F).
Table 3. The 5-year disease-free survival (DFS) and 5-year overall survival (OS).
| Subtypes | n | 5-year DFS |
5-year OS |
||
|---|---|---|---|---|---|
| %±SE | P | %±SE | P | ||
| Total | 1521 | 80.3±1.0 | 87.0±0.9 | ||
| Luminal A | 946 | 83.7±1.2 | (ref.) | 90.4±1.0 | (ref.) |
| Luminal B | 170 | 73.5±3.4 | 0.005 | 76.1±3.4 | <0.001 |
| HER2 | 114 | 76.7±4.1 | 0.065 | 82.1±3.7 | 0.011 |
| Triple-negative | 291 | 74.4±2.6 | <0.001 | 84.0±2.2 | 0.003 |
| Lymph node positive | |||||
| Luminal A | 390 | 70.9±2.4 | (ref.) | 80.5±2.1 | (ref.) |
| Luminal B | 72 | 53.5±6.0 | 0.002 | 57.7±6.0 | <0.001 |
| HER2 | 47 | 53.9±7.7 | 0.022 | 60.3±7.6 | 0.003 |
| Triple-negative | 114 | 59.7±4.7 | 0.032 | 70.0±4.4 | 0.026 |
| Lymph node negative | |||||
| Luminal A | 543 | 92.6±1.1 | (ref.) | 97.1±0.7 | (ref.) |
| Luminal B | 97 | 88.3±3.3 | 0.257 | 89.2±3.2 | 0.002 |
| HER2 | 66 | 92.1±3.4 | 0.509 | 96.8±2.2 | 0.568 |
| Triple-negative | 172 | 84.7±2.8 | <0.001 | 93.3±1.9 | 0.015 |
*Follow-up date available; SE, standard error
Figure 1.
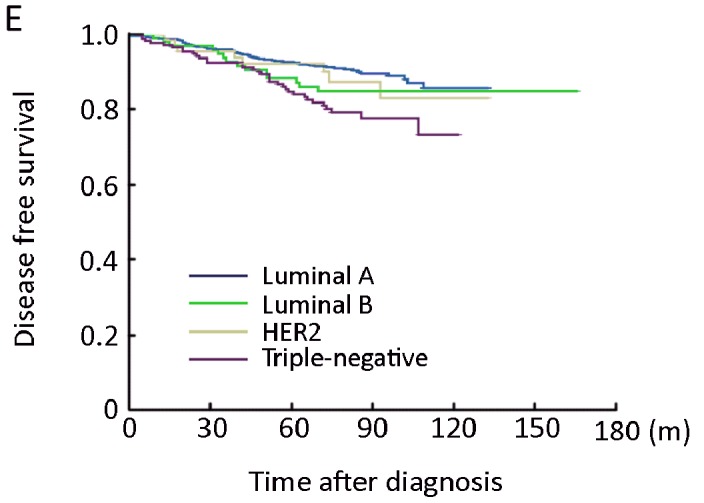
Kaplan-Meier estimate of disease-free survival (DFS) (A) and overall survival (OS) (B)according to breast cancer subtypes in the entire of study population; DFS (C) and OS (D) according to breast cancer subtypes in the lymph-node positive patients; DFS (E) and OS (F) according to breast cancer subtypes in the lymph-node negative patients
Breast Cancer Subtypes and Survival in Multivariate Analysis
Patients with luminal B, HER2+, or triple-negative subtype had a worse DFS and OS than did patients with luminal A in the entire study population in a multivariate analysis after adjusted for age, tumor size, lymph node status, and adjuvant therapy, with triple-negative subtype being the worst one in DFS (hazard ratio, HR=1.79, 95% CI 1.36 to 2.37. P<0.001), and luminal B being the worst in OS (HR=2.32, 95% CI 1.62 to 3.32, P<0.001) (Table 4). We then stratified the patients with lymph node status, among patients with lymph node positive disease, patients with luminal B, HER2+, or triple-negative subtype had a worse DFS and OS than did patients with luminal A in this subgroup in the multivariate analysis, with HER2+ subgroup being the worst one in DFS (HR=1.80, 95% CI 1.11 to 2.91, P=0.017) and luminal B being the worst one in OS (HR=2.27, 95% CI 1.50 to 3.45, P<0.001) (Table 4). On the other hand, among the patients with lymph node negative disease, patients with triple-negative had the worse DFS (HR=2.21, 95% CI 1.43 to 3.41, P<0.001) and OS (HR=2.24, 95% CI 1.18 to 4.25, P=0.013) when compared with patients with luminal A in the multivariate analysis (Table 4), no significant difference in DFS and OS between HER2+ subtype and luminal A subtype was observed; patients with luminal B subtype had no significant difference in DFS but had the worst OS (HR=2.75, 95% CI 1.33 to 5.67, P=0.006) when compared with patients with luminal A (Table 4).
Table 4. Multivariate analysis of disease-free survival and overall survival among the four breast cancer subtypes according to lymph node status.
| Subtypes | Disease-free survival |
Disease-free survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Total | ||||||
| Luminal A | 1.00 | (ref.) | 1.00 | (ref.) | ||
| Luminal B | 1.54 | 1.11-2.13 | 0.009 | 2.32 | 1.62-3.32 | <0.001 |
| HER2 | 1.70 | 1.13-2.58 | 0.012 | 2.01 | 1.26-3.21 | 0.003 |
| Triple-negative | 1.79 | 1.36-2.37 | <0.001 | 1.64 | 1.18-2.28 | 0.004 |
| Lymph node positive | ||||||
| Luminal A | 1.00 | (ref.) | 1.00 | (ref.) | ||
| Luminal B | 1.65 | 1.13-2.41 | 0.009 | 2.27 | 1.50-3.45 | <0.001 |
| HER2 | 1.80 | 1.11-2.91 | 0.017 | 2.09 | 1.23-3.55 | 0.012 |
| Triple-negative | 1.45 | 1.03-2.05 | 0.032 | 1.49 | 0.99-2.22 | 0.052 |
| Lymph node negative | ||||||
| Luminal A | 1.00 | (ref) | 1.00 | (ref.) | ||
| Luminal B | 1.35 | 0.72-2.54 | 0.355 | 2.75 | 1.33-5.67 | 0.006 |
| HER2 | 1.21 | 0.55-2.66 | 0.644 | 1.50 | 0.52-4.35 | 0.452 |
| Triple-negative | 2.21 | 1.43-3.41 | <0.001 | 2.24 | 1.18-4.25 | 0.013 |
HR: hazard ratios; CI: confidence interval.
DISCUSSION
In this cohort of 1538 Chinese women with operable primary breast cancer, we found patients with luminal B, HER2, or triple-negative subtype had a worse survival than did patients with luminal A in the entire study population of 1538 patients. Luminal A subtype, which comprised 62.1% of the current cohort, had a best survival compared with other subtypes, this finding is consistent with most previous studies[4,5,7,8].
We further analyzed the associations between the subtypes and survival according to lymph nodes status. The frequencies of lymph node metastasis were not significantly different between the four subtypes in the current study, the positive lymph node rate was around 40% among the four subtypes. Other studies showed that patients with HER2+ subtype and triple-negative are more likely to be lymph node positive disease[11-13], we did not find such associations in the current study, possibly due to different study populations or samples selected.
In patients with lymph node positive disease, patients with luminal B, HER2+, or triple-negative subtype had a worse survival when compared with patients with luminal A. Furthermore, the prognostic role of HER2+ or luminal B subtypes was even stronger than that of triple-negative subtype in this subgroup. In contrast, in patients with lymph node negative disease, no significant difference in DFS was observed between patients with luminal B or HER2+ subtype and patients with luminal A, whereas patients with triple-negative had a worse survival compared with patients with luminal A. Interestingly, triple-negative was associated with poor survival in both lymph node positive or negative disease, indicating that the prognosis of triple-negative is independent of lymph node status. Previous studies suggested that the poor survival of the triple-negative subtype may be influenced by hematogenous rather than lymphatic metastasis patterns[5,14].
In the current study, we found the worse survival of luminal B and HER2+ subtype was only restricted in patients with lymph node positive disease but not in patients with lymph node negative disease. The common feature of luminal B and HER2+ subtype is HER2 positive in the tumors, the association between the HER2 amplification or overexpression and poor survival is well documented in node-positive breast cancer[15,16], but the association in node negative breast cancer is controversial[17], many studies[18,19] indicated that HER2 amplification or overexpression is not associated with clinical outcome in node-negative breast cancer, the reasons for lack of prognostic significance of HER2 in node-negative patients are still unknown. Our present results indicated that the prognostic role of luminal B and HER2+ subtypes was strongly influenced by lymph nodes status.
In the present study, although the majority of node- positive patients with luminal B or HER2+ subtype received adjuvant chemotherapy and/or endocrine therapy, the prognosis of these patients was still poor, no patients in this cohort received trastuzumab treatment, therefore, this finding may have potential clinical implication, node-positive patients with luminal B or HER2+ subtype may suggest to receive trastuzumab treatment in addition of adjuvant chemotherapy and/or endocrine therapy.
In conclusion, our present study suggests that luminal A subtype is associated with a favorable survival as compared with other subtypes in the entire study population; patients with triple-negative has a worse survival in both node-positive and node-negative disease; whereas luminal B and HER2+ are associated with a worse disease-free survival only restricted in node-positive patients but this is not the case in node-negative patients. Nevertheless, the interpretation of our present findings should be caution, other independent studies to confirm the present findings are warranted.
REFERENCES
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature 2000; 406: 747-52 [DOI] [PubMed] [Google Scholar]
- 2.processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 2008; 14: 5158-65 [DOI] [PubMed] [Google Scholar]
- 3.Correa Geyer F, Reis-Filho JS. Microarray-based gene expression profiling as a clinical tool for breast cancer management: are we there yet? Int J Surg Pathol, 2009; 17: 285-302 [DOI] [PubMed] [Google Scholar]
- 4.Onitilo AA, Engel JM, Greenlee RT, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009; 7: 4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295: 2492-502 [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13: 2329-34 [DOI] [PubMed] [Google Scholar]
- 7.Parise CA, Bauer KR, Brown MM, et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J 2009; 15: 593-602 [DOI] [PubMed] [Google Scholar]
- 8.Zaha DC, Lazar E, Lazureanu C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Rom J Morphol Embryol 2010; 51: 85-9 [PubMed] [Google Scholar]
- 9.Masood S.Prognostic/predictive factors in breast cancer. Clin Lab Med 2005; 25: 809-25, viii. [DOI] [PubMed] [Google Scholar]
- 10.Thorpe SM. Steroid receptors in breast cancer: sources of inter-laboratory variation in dextran-charcoal assays. Breast Cancer Res Treat 1987; 9: 175-89 [DOI] [PubMed] [Google Scholar]
- 11.Ihemelandu CU, Leffall LD, Jr, Dewitty RL, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res 2007; 143: 109-18 [DOI] [PubMed] [Google Scholar]
- 12.Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 2009; 16: 2705-10 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008; 26: 2373-8 [DOI] [PubMed] [Google Scholar]
- 14.Foulkes WD, Brunet JS, Stefansson IM, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/ glomeruloid-microvascular-proliferation+) phenotype of BRCA1- related breast cancer. Cancer Res 2004; 64: 830-5 [DOI] [PubMed] [Google Scholar]
- 15.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res 1990; 50: 4332-7 [PubMed] [Google Scholar]
- 16.Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992; 10: 1049-56 [DOI] [PubMed] [Google Scholar]
- 17.Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989; 7: 1120-8 [DOI] [PubMed] [Google Scholar]
- 18.Agrup M, Stal O, Olsen K, et al. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat 2000; 63: 23-9 [DOI] [PubMed] [Google Scholar]
- 19.Mirza AN, Mirza NQ, Vlastos G, et al. Prognostic factors in node-negative breast cancer: a review of studies with sample size more than 200 and follow-up more than 5 years. Ann Surg 2002; 235: 10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]


