Abstract
Objective
To examine plasma microRNA-21 (miR-21) level in patients with non-small cell lung cancer (NSCLC) and its potential correlation with chemotherapeutic response.
Methods
77 NSCLC patients and 36 age and sex-matched healthy controls were included. Plasma miR-21 concentration was examined using a quantitative real-time reverse transcription polymerase chain reaction assay (qRT-PCR). Potential correlation between plasma mir-21 concentrations with chemotherapeutic responses was analyzed in 35 patients with advanced NSCLC (stages IIIB and IV).
Results
Plasma miR-21 was significantly higher in NSCLC patients relative to the healthy controls (P<0.0001). As a biomarker, plasma mir-21 had a receiver operating characteristic (ROC) curve area of 0.729 with 61.04% sensitivity and 83.33% specificity. Chemotherapeutic response in the 35 patients with advanced NSCLC (stages IIIB and IV) included partial response (PR) (n=11), stable disease and progression disease (SD+PD) (n=24). The overall response rate (CR+PR) was 31.4%. Plasma miR-21 in patients who achieved PR was significantly lower than those who did not respond (SD+PD) (P=0.0487), and comparable to that of the healthy controls (P=0.2744).
Conclusion
Plasma miR-21 is a good biomarker for NSCLC, and could be used to predict responses to chemotherapy.
Key words: NSCLC, miR-21, Biomarker, Plasma, Chemotherapeutic response
INTRODUCTION
Lung cancer is one of the most common malignant tumors and is also the leading cause of cancer-related death in the world, 80% of which are from non-small cell lung cancer (NSCLC). Five-year survival rate of NSCLC patients is only 15%-20%. The two main reasons for such poor prognosis are paucity in reliable plasma markers for NSCLC and common chemo-resistance in therapy.
MicroRNAs (miRNAs) are 18- to 25-nucleotide, noncoding RNA molecules that regulate the translation of many genes. MiRNAs expression mainly by base-pairing to the 3’-UTR of the target mRNA and play a role in regulation of gene expression[1]. At cellular level, miRNAs play critical roles in differentiation, proliferation, apoptosis, and metabolism and have ultimately been linked to cancer development[2]. It was demonstrated that miRNA is important for development of human cancers including leukemia[3], neuroblastoma[4], pituitary adenoma[5], breast cancer[6], thyroid cancer[7], and many other cancers[8-16]. Over 50% of annotated human miRNA genes are located within regions of cancer-associated genomic regions or fragile sites, and they may have important functions similar to oncogenes or tumor suppressors[3]. Various studies have shown that miRNA may become useful biomarker for cancer diagnosis, therapy and prognosis. Since 1999, tumor-associated RNAs have been detected in serum or plasma from patients suffering from nasopharyngeal, breast, and colon cancers[17-21]. More recently, several studies also suggested that circulating miRNAs existed in serum and plasma[22-29], raising the possibility of using miRNAs as novel non-invasive molecular markers for cancer detection. The miR-21 gene is located at the 10th intron of coding region for TMEM49 at chromosome 17. Multiple studies showed that miR-21 might be one of the most relevant oncogene-like factors among the class of miRNAs[30]. It has been implicated in not only the promotion of tumor growth, proliferation, anti-apoptosis, but also tumor invasion, migration. In addition, it is associated to the response to chemotherapy and prognosis. MiR-21’s targets include PTEN, PDCD4, MTAP, and TGF-ß, all of which regulate cell cycle. Their aberrant expression in various cancers suggests them to be a novel class of oncogenes or tumor suppressor gene. Zhang et al[31] demonstrated that miR-21 post- transcriptionally down-regulated the expression of tumor suppressor PTEN and stimulated growth and invasion of NSCLC. Their data showed that miR-21 was possible to become a potential therapeutic target for NSCLC. In the present study, we assessed the feasibility of using plasma miR-21 as a non-invasive tool to diagnose NSCLC and predict treatment outcome.
MATERIAL AND METHODS
Patients and Plasma Samples
A total of 113 subjects were enrolled in this study, including 77 NSCLC patients ((September 2009-July 2010), 39 patients with tumor resection (stages I, II and IIIA) and 38 without (stages IIIB and IV)), and 36 age- and sex-matched healthy volunteers as controls. NSCLC patients were recruited at Department of Oncology, First Affiliated Hospital of Nanjing Medical University, China. Blood samples were taken before chemotherapy in both operable and non-operable patients. All patients underwent stage classification according to AJCC/UICC guidelines. None of these patients had received adjuvant chemotherapy or radiotherapy before admission. Informed consents were obtained from all enrolled subjects and the local Ethics Committee approved the protocol.
Chemotherapy and Clinical Response Evaluation
Seventy-seven NSCLC patients were routinely treated by cisplatin- or carboplatin-based chemotherapy, and the clinical response was evaluated after 2-3 cycles of chemotherapy. Response to chemotherapy was graded by standard WHO criteria. Patients received chemotherapy every 3-4 weeks.
Selection and Validation of Internal Control of Plasma miRNA
At first, miR-16 was selected as a control based on previous reports[32-34], and was measured using qRT-PCR on a small set of plasma samples (20 NSCLCs and 20 controls). These samples were processed under the exactly same conditions.
Isolation of Human Plasma
For miRNA detection, whole blood samples (5 ml per patient) were collected from subjects via a direct venous puncture into tubes containing EDTA, which were then centrifuged at 2000 × g for 10 min, and the supernatant (plasma) was carefully transferred into an RNase-free tube for RNA extraction. The resultant plasma was aliquoted into Eppendorf tubes and stored at –80°C.
Plasma RNA Extraction
Total RNA was extracted from 400μl plasma using mirVana PARIS Kit (Ambion), and finally eluted into 100μl pre-heated(95°C) Elution Solution according to the manufacturer’s protocol. To allow normalization of sample-to-sample variation in the RNA isolation step, synthetic cel-miR-39 was added to each sample as described by Mitchell et al[22].
MiR-21 Quantification by qRT-PCR
The extracted total RNA was polyadenylated by poly(A) polymerase and then reversely transcribed to cDNA in a final volume of 20μL using miScript Reverse Transcription kit (Qiagen, Germany). Real-time PCR was performed in duplicate measurements using miScript SYBR Green PCR kit (Qiagen, Germany) with the manufacture-provided miScript Universal primer and the miRNA-specific forward primers in the ABI PRISM 7300 Real-time PCR system (Applied Biosystems). The miRNA-specific primer sequences were 5' UAGCAGCACGUAAAUAUUGGCG3' for miR-16; 5' UAGCUUAUCAGACUGAUGUUGA3' for miR-21; 5'UCACCGGGUGUAAAUCAGCUUG3' for cel-miR-39. The miRNA-specific primer sequences were designed based on the miRNA sequences obtained from the miRBase database(http://microrna.sanger.ac.uk). Each amplification reaction was performed in a final volume of 25μl containing 2μl of cDNA, 2.5μl of each primer and 12.5μl of 1×SYBR Green PCR Master mix. The amplify- cation profile was: denaturation at 95°C for 15min, followed by 40 cycles of 94°C for 15s, 57°C for 30s and 70°C for 34s, in which fluorescence was acquired. At the end of the PCR cycles, melting curve analyses were performed as well as electrophoresis of the products on 2% agarose gels in order to validate the specific generation of the expected PCR products. Each sample was tested in duplicates. The expression levels of miR-21 were normalized to miR-16, and were calculated utilizing the 2–△△Ct method, where △△Ct=(CtmiR-21-CtmiR- 16) NSCLC-(CtmiR-21-CtmiR-16) Meannormal△△Ct’=(CtmiR-21- CtmiR-16)pro-chemotherapy-(CtmiR-21-CtmiR-16)post-chemotherapy, and Ct is the threshold cycle to detect fluorescence[35].
Statistical Analysis
The significance of plasma miR-21 levels was determined by the Mann-Whitney test, Wilcoxon test, Independent-Samples t test, the χ2 test or Kruskal-Wallis test where appropriate. The probability of progression- free survival (PFS) as a function of time was determined by the Kaplan-Meier method. Receiver operating characteristic (ROC) curves were established for discriminating NSCLC and controls. All P values are two-sided and less than 0.05 was considered statistically significant. All statistical calculations were performed by GraphPad Prism V4.03 or the SPSS 16.0 software.
RESULTS
Patient Characteristics
A total of 113 participants including 77 NSCLC patients and 36 healthy controls were recruited. Patient characteristics are summarized in Table 1. There were no significant differences of age or gender between NSCLC patients and healthy controls (P=0.0890, Independent- Samples t test, P=0.8420, χ2 test).
Table 1. Characteristics of enrolled subjects.
| Characteristics | NSCLC | Healthy controls | P value |
|---|---|---|---|
| Age | |||
| x̄±s | 59.6±9.1 | 56.4±9.2 | 0.0890 |
| <59 | 37 | ||
| >59 | 42 | 0.7495 | |
| Gender | |||
| Male | 55 | 27 | |
| Female | 22 | 9 | 0.8420 |
| Smoking status | |||
| Smoker | 40 | ||
| Never smoker | 37 | 0.3290 | |
| Histological | |||
| Adenocarcinoma | 44 | ||
| Squamous-cell | 21 | ||
| Large cell | 2 | ||
| Unclassified | 10 | 0.1855 | |
| TNM stage | |||
| I-II | 24 | ||
| III-IV | 53 | 0.2812 | |
| T1-2 | 31 | ||
| T3-4 | 46 | 0.2621 | |
| Lymph node | |||
| N0 | 47 | ||
| N+ | 30 | 0.7140 | |
| Metastasis | |||
| M0 | 46 | ||
| M1 | 31 | 0.7633 | |
| Operation status | |||
| Yes | 39 | ||
| No | 38 | 0.5276 |
The Validation of Internal Control on a Small Set of Plasma Samples
RNU6B and miR-16 have been proposed as the internal normalization control for miRNA quantification. In our experiment, miR-16 was selected as an internal control. We examined the levels of miR-16 by using qRT-PCR in the plasma of 20 controls and of 20 NSCLC patients. Our data showed that no significant difference was observed in terms of Ct values of miR-16 (P=0.2314, Mann-Whitney U test, Figure 1) between controls and NSCLC samples. Therefore, miR-16 was served as an internal control.
Figure 1.
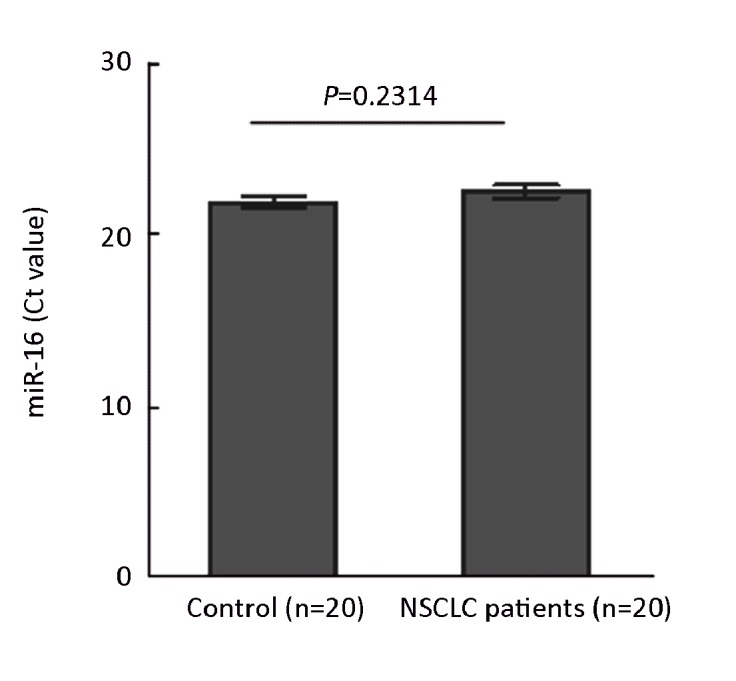
MiR-16 levels were tested as a baseline internal control. Levels of miR-16 in the plasma of 20 controls and 20 NSCLC patients were measured by qRT-PCR.
The Diagnostic Value of miR-21 in Plasma for NSCLC
qRT-PCR analysis indicated that the NSCLC patients had significant higher levels of miR-21 in their plasma than in the controls (P<0.0001, Figure 2A). ROC curve analyses also showed that miR-21 yielded an area of 0.729 under ROC. At the cutoff value of 1.630 for this marker, the sensitivity and the specificity were 61.04% and 83.33%, respectively(95%CI:0.639-0.819, Figure 2B). The odds ratio (OR) for cases with miR-21 >1.630 being associated with NSCLC was 7.833 (95%CI: 2.914-21.060). These results demonstrated that miR-21 levels possessed good diagnostic value for NSCLC patients.
Figure 2.
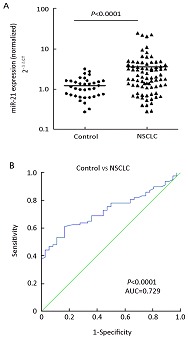
MiR-21 levels in NSCLC patients and healthy controls. A: Scatter plots of plasma levels of miR-21 in healthy subjects (n=36) and NSCLC patients (n=77). Expression levels of the miR-21(Log10 scale at Y-axis) are normalized to miR-16. The line represents the median value. Mann-Whitney U test was used to determine statistical significance. B: Receiver operating characteristics (ROC) curve analysis using plasma miR-21 for discriminating NSCLC.
Relationship between Plasma Level of miR-21 and Clinical Characteristics
We examined the correlation between the expression of miR-21 and clinical parameters. Statistical analysis studies revealed that operable patients of NSCLC still had significantly higher levels of miR-21 in their plasma than the controls(P=0.0019, Figure 3A). No significant association was found between the miR-21 and gender, age, smoking status or history classification (P>0.05). There is no difference between the plasma miR-21 levels of T1–2 patients and those of T3-4 patients, but both of them were significantly higher than controls. The plasma levels of miR-21 in N0 and N+, M0 and M1, or stage I–II and III-IV patients showed the same negative results as above (Figure 3B). So we suggested that there was no clear correlation between miR-21 and TNM staging.
Figure 3.
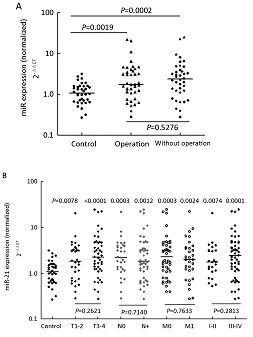
The correlation between the expression of miR-21 and clinical parameters. A: MiR-21 levels were measured in NSCLC patients with or without operation. The line represents the median value. B: MiR-21 levels in patients at different TNM stages were measured. The line represents the median value.
The Relationship between miR-21 Level in Plasma of NSCLC Patients and Effects of Chemotherapy
The concentration of miR-21 was analyzed in paired pre- and post-chemotherapy plasma samples from 2 TNM stage I, 3 with stage II, and 8 stage III patients. The plasma miR-21 levels of these 13 post-operative patients were still higher than those of normal controls (P=0.0002). They were then received 2-3 cycles of platinum-based combination chemotherapy. MiR-21 levels were significantly reduced in post-chemotherapy samples compared with the levels in pre-chemotherapy samples (P=0.0479, Wilcoxon tests). And if compared to controls, chemotherapy seemly resulted in a dramatically decreased plasma miR-21 level (P=0.0002 for pre-, P=0.0250 for post-chemotherapy, Mann-Whitney test; Figure 4). These findings showed that surgery can not completely reduce the level of miR-21 to baseline and there could be a possible correlation between therapeutic effect and the plasma miR-21 level. Another 35 patients (stages IIIB and IV) were evaluated for responses to chemotherapy. Response was graded by standard WHO criteria. After 2-3 cycles of platinum-based chemotherapy, 11 patients acquired partial response (PR), 24 patients acquired stable disease or progression disease (SD+PD) and no patients acquired complete response (CR). The total effective power (CR+PR) is 31.4%. Importantly, the levels of plasma miR-21 in PR samples were lower than that of (SD+PD) samples (P=0.0487, Mann-Whitney test). Furthermore, the plasma miR-21 levels of 11 PR samples were close to those of healthy controls (P=0.2744, Mann-Whitney test; Figure 5). These results indicated that the quantity of miR-21 in plasma could be used as a potential biomarker for predicting platinum-based chemo-sensitivity of NSCLC.
Figure 4.
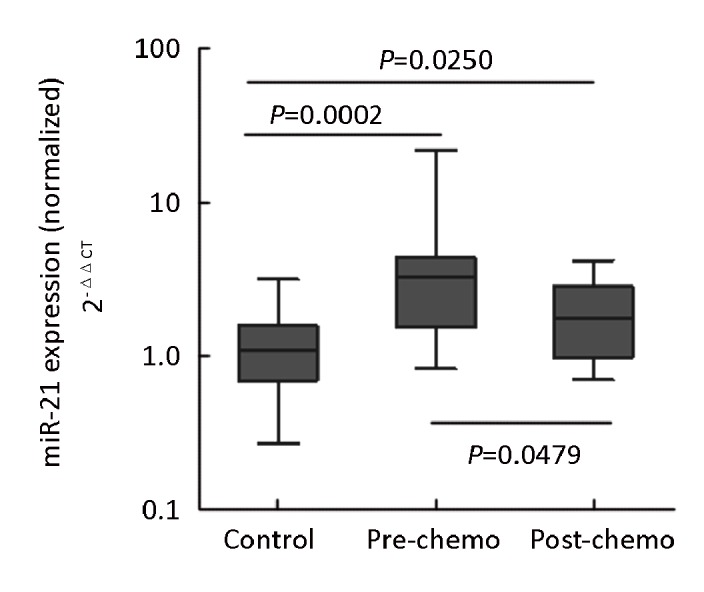
Shows the measurement of plasma miR-21 level in healthy controls and 13 post-operative patients before and after chemotherapy. Expression levels of the miR-21(Log10 scale at Y-axis) are normalized to miR-16. Statistically significant differences were determined using Mann-Whitney U test. pre-chemo: pre-chemotherapy; post-chemo: post-chemotherapy.
Figure 5.
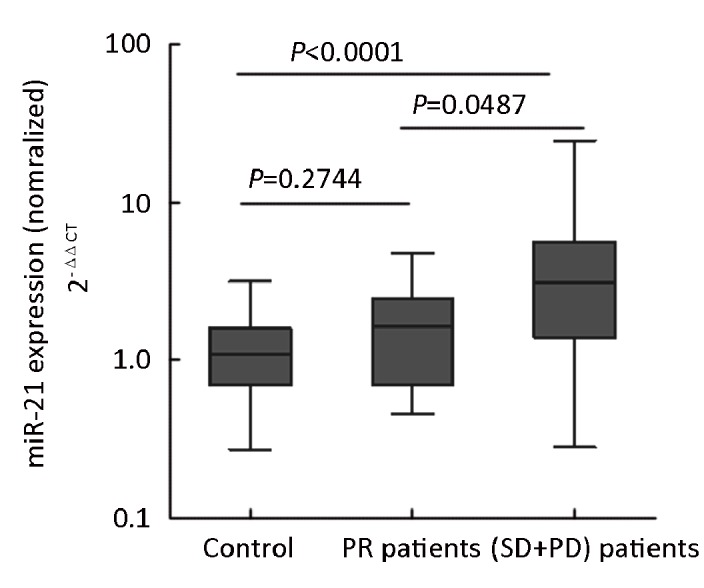
Analysis of the miR-21 plasma levels in PR and (SD+PD) samples. Expression levels of the miR-21(Log10 scale at Y-axis) are normalized to miR-16. Statistically significant differences were determined using Mann-Whitney U test. PR: partial response; SD: stable disease; PD: progression disease
Relationship between MiR-21 Plasma Level and Treatment Outcome in NSCLC Patients
To determine whether the miR-21 level could serve as a prognostic marker of chemotherapy, we reviewed follow-up care for the 77 NSCLC patients. During the follow-up, 4 operable patients and 13 non-operable patients occurred to progress, and 9 of all patients, operable and non-operable, died. At 12 month, analysis indicated that the median time to progression of 17 PD patients was 4.3 months (95% CI, 1.476-7.124).
DISCUSSION
In this study, we found that the plasma levels of miR-21 in NSCLC patients were markedly higher than those in healthy controls. MiR-21 had important diagnostic value for NSCLC and yielded AUC of 0.729 with 61.04% sensitivity and 83.33% specificity (95% CI0.639-0.819). The result demonstrated that plasma miR-21 could be a potential noninvasive biomarker for NSCLC. There was no significant difference in the expression levels of miR-21 when stage I-II patients were compared with stage III-IV patients, indicating that miR-21 remained relatively high level after surgery and miR-21 over-expression is an early event in lung carcinogenesis.
Higher levels of miR-21 expression have been reported in non-smoker NSCLC than smoker ones[36]. Nevertheless, there was no difference between the plasma miR21 levels of smokers and non-smokers in our study. Either way, it has not been confirmed whether this was associated with sample size or ethnics. Based on this we suggested that miR-21 might not be involved in the carcinogenesis of NSCLC in non-smokers which should be further investigated. Among all patients, early stage patients received operation. After surgery, however, plasma miR-21 levels remained higher than controls. This result indicated that surgery can decrease miRNA expression, but not to normal level. Several more recent studies have documented that tumor cells released functional miRNA-enriched microparticles into the blood[37-40]. Nevertheless, the mechanism of plasma miRNAs generation and physiological function are unclear.
The kinetics and metabolism of the plasma miRNAs have not been clearly elucidated, but the plasma miRNAs showed potentials to be new therapeutic targets in treating NSCLC. Our study explored the possible association between miR-21 and clinical response of chemotherapy in NSCLC. We hypothesize that the plasma miR-21 expression level may help in predicting a patient’s response to platinum-based combination chemotherapy, commonly used for the treatment of NSCLC in the clinic and lower levels of miR-21 expression in plasma correlates with better response to platinum-based chemotherapy in NSCLC.
Platinum-based chemotherapy is the mainstream method of treatment against advanced NSCLC and for early stage patients who had high risk factors. However, tumor resistance to chemotherapy is the leading cause of poor treatment outcome. Thus, predictive markers of sensitivity to adjuvant therapy and new therapeutic targets are urgently needed in NSCLC. Recent studies suggest that miR-21 might be potential biomarker and drug targets to predict chemotherapy’s efficiency. Meng et al[41] found that inhibition of miR-21 expression in cholangiocarcinoma cells can increase cell sensitivity to gemcitabine. Si et al[42] reported that suppression of over-expressed miR-21 in MCF-7 cells was associated with increased cell apoptosis and decreased proliferation. Then, Schetter et al[43] first analyzed the correlation between miR-21 expression, 5-FU activity and therapeutic outcomes in colon cancer patients, showing that high miR-21 expression was associated with a poor response to 5-FU-based chemotherapy and poor prognosis. Yan et al[44] showed that overexpression of miR-21 was correlated with disease progression and poor prognosis in breast cancer. Recently, Hwang et al[45] investigated whether miR-21 can predict clinical outcome in pancreatic ductal adenocarcinoma (PDAC) patients treated with adjuvant therapy. The results suggested that low miR-21 expression was seen by adjuvant treatment in PDAC patients and anti-miR-21 increased anticancer drug activity in vitro. Gao et al[46] indicated that high-level miR-21 in squamous cell lung carcinoma tissue was associated with shortened survival time. On the contrary, Voortman and his colleagues[47] suggested that there was no significant association between the tested microRNAs (miR-21, miR-29b, miR-34a/b/c, miR-155 and let-7a) expression and survival as well as predicted chemotherapy response in resected NSCLC patients. Our results demonstrated that high plasma miR-21 level is associated with poor treatment response in NSCLC patients. Furthermore, the results suggested that tumor resection can not completely return miRNAs level to baseline level and chemotherapy may be a powerful and useful elimination tool. Because patients with advanced lung cancer are difficult to obtain tissue samples, plasma miR-21 may serve as a potential biomarker for diagnosis, therapy assessment and prognosis for NSCLC.
However, some issues should be considered when interpreting the results of this study. First, as the sample size is small, further validations of this marker in large cohorts and independent data set are necessary. Second, it is unable to eliminate other chemotherapy drugs’ effect on miR-21 levels. Third, because the follow-up time is short, the correlation between levels of miR-21 in plasma and prognosis cannot be analyzed. Another limitation is that the molecular mechanism of miR-21 elevation and its role in NSCLC development and progression is currently unclear. Whether plasma miR-21 could be released from the primary tumor tissues remains controversial, thus further studies are warranted.
REFERENCES
- 1.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 2007; 8: 23-36 [DOI] [PubMed] [Google Scholar]
- 2.Sassen S, Miska EA, Caldas C, et al. MicroRNA: implication for cancer. Virchows Arch 2008; 452: 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004; 101: 2999-3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res 2007; 67: 976-83 [DOI] [PubMed] [Google Scholar]
- 5.Bottoni A, Piccin D, Tagliati F, et al. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol 2005; 204: 280-5 [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005; 65: 7065-70 [DOI] [PubMed] [Google Scholar]
- 7.Jazdzewski K, Murray EL, Franssila K, et al. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci USA 2008; 105: 7269-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corney DC, Flesken-Nikitin A, Godwin AK, et al. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 2007; 67: 8433-8 [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005; 435: 834-8 [DOI] [PubMed] [Google Scholar]
- 10.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006; 9: 189-98 [DOI] [PubMed] [Google Scholar]
- 11.Lui WO, Pourmand N, Patterson BK, et al. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007; 67: 6031-43 [DOI] [PubMed] [Google Scholar]
- 12.Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007; 67: 8699-707 [DOI] [PubMed] [Google Scholar]
- 13.Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006; 24: 4677-84 [DOI] [PubMed] [Google Scholar]
- 14.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 2007; 120: 1046-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 2007; 26: 4442-52 [DOI] [PubMed] [Google Scholar]
- 16.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007; 297: 1901-8 [DOI] [PubMed] [Google Scholar]
- 17.Lo KW, Lo YM, Leung SF, et al. Analysis of cell-free Epstein–Barr virus associated RNA in the plasma of patients with nasopharyngeal carcinoma. Clin Chem 1999; 45: 1292-4 [PubMed] [Google Scholar]
- 18.Chen XQ, Bonnefoi H, Pelte MF, et al. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin Cancer Res 2000; 6: 3823-6 [PubMed] [Google Scholar]
- 19.Silva JM, Rodriguez R, Garcia JM, et al. Detection of epithelial tumor RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumor cells. Gut 2002; 50: 530-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng EK, Tsui NB, Lam NY, et al. Presence of filterable and nonfilterable mRNA in the plasma of cancer patients and healthy individuals. Clin Chem. 2002; 48: 1212-7 [PubMed] [Google Scholar]
- 21.Wong SC, Lo SF, Cheung MT, et al. Quantification of plasma beta-catenin mRNA in colorectal cancer and adenoma patients. Clin Cancer Res 2004; 10: 1613-7 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105: 10513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumor-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008; 141: 672-5 [DOI] [PubMed] [Google Scholar]
- 24.Wong TS, Liu XB, Wong BY, et al. Mature miR-184 as Potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res 2008; 14: 2588-92 [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18: 997-1006 [DOI] [PubMed] [Google Scholar]
- 26.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of colorectal cancer patients: A potential marker for colorectal cancer screening. Gut 2009; 58: 1375-81 [DOI] [PubMed] [Google Scholar]
- 27.Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 2010; 102: 1174-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu CJ, Kao SY, Tu HF, et al. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis 2010; 16: 360-4 [DOI] [PubMed] [Google Scholar]
- 29.Ho AS, Huang X, Cao H, et al. Circulating miR-210 as a novel hypoxia marker in pancreatic cancer. Transl Oncol 2010; 3: 109-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259-69 [DOI] [PubMed] [Google Scholar]
- 31.Zhang JG, Wang JJ, Zhao F, et al. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 2010; 411: 846-52 [DOI] [PubMed] [Google Scholar]
- 32.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 2009, 58: 1375-81 [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 2010; 127: 118-26 [DOI] [PubMed] [Google Scholar]
- 34.Liu C-J, Kao S-Y, Tu H-F, et al. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis 2010; 16: 360-4 [DOI] [PubMed] [Google Scholar]
- 35.Gramantieri L, Ferracin M, Fornari F, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 2007; 67: 6092-9 [DOI] [PubMed] [Google Scholar]
- 36.Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA 2009; 106: 12085-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García JM, García V, Peña C, et al. Extracellular plasma RNA from colon cancer patients is confined in a vesicle-like structure and is mRNA-enriched. RNA 2008; 14: 1424-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654-9 [DOI] [PubMed] [Google Scholar]
- 39.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008; 110: 13-21 [DOI] [PubMed] [Google Scholar]
- 40.Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008; 3: e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006; 130: 2113-29 [DOI] [PubMed] [Google Scholar]
- 42.Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene 2007; 26: 2799-803 [DOI] [PubMed] [Google Scholar]
- 43.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 2008; 299: 425-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan LX, Huang XF, Shao Q, et al. MiRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008; 14: 2348-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang JH, Voortman J, Giovannetti E, et al. Identification of MicroRNA-21 as a Biomarker for Chemoresistance and Clinical Outcome Following Adjuvant Therapy in Resectable Pancreatic Cancer. PLoS One 2010; 5: e10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao W, Shen H, Liu L, et al. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol 2011; 137: 557-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voortman J, Goto A, Mendiboure J, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer Res. 2010; 70: 8288-98 [DOI] [PMC free article] [PubMed] [Google Scholar]


