Abstract
Objective
To analyze the outcomes of patients who received TKI immediately after the first-line without progression as maintenance treatment (immediate group) vs. those received delayed treatment upon disease progression as second-line therapy (delayed group).
Methods
The study included 159 no-small-cell lung cancer (NSCLC) patients who received gefitinib or erlotinib as maintenance treatment in the immediate group (85 patients) or as second-line therapy in the delayed group (74 patients). The primary end point was progression-free survival (PFS). EGFR mutation status was detected using denaturing high-performance liquid chromatography (DHPLC).
Results
PFS was 17.3 and 16.4 months in the immediate and delayed groups, respectively (hazard ratio [HR], 0.99; 95% Confidence Interval [CI]: 0.69-1.42; P=0.947). In a subgroup analysis that included only patients with EGFR mutation, however, PFS was significantly longer in the immediate group than in the delayed group (HR, 0.48; 95% CI: 0.27-0.85; P=0.012). In patients with wild type EGFR, the risk for disease progression was comparable between the two groups (HR, 1.23; 95% CI: 0.61-2.51; P=0.564). No significant difference was demonstrated between the immediate and delayed group in terms of the overall survival (OS) (26.1 months vs. 21.6 months, respectively; HR=0.53; 95% CI: 0.27 to 1.06; P=0.072). There was also no difference in the incidence of adverse events between the two groups.
Conclusions
EGFR TKI maintenance improves PFS in patients with EGFR mutation. Prospectively designed clinical studies that compare TKI immediate vs. delayed treatment after first-line chemotherapy upon disease progression are needed.
Key words: EGFR tyrosine kinase inhibitor, Maintenance therapy, Non-small-cell lung cancer
INTRODUCTION
Non–small-cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide[1]. Most of patients were in advanced stage when diagnosed. Four to six cycles of platinum-based chemotherapy are recommend as standard first-line therapy[2]. For treatment subsequent to disease control, the standard practice is to initiate second-line therapy only upon disease progression, using either the same drugs as in the initial treatment or other agents that are not cross-resistant with the initial drugs[3].
Theoretically, maintenance therapy immediately after achieving disease control has several advantages[3]. First, the early use of non-cross-resistant regimens may delay the occurrence of eventual resistance[4]. Second, the tumor burden is low at the time of the treatment[5]. A numbers of randomized clinical trials have demonstrated improvement in progression-free survival (PFS) or time to progression (TTP) patients with advanced NSCLC receiving immediate maintenance therapy[5-9,10-12]. However, prolonged overall survival (OS) was only observed in two trials with pemetrexed (the JMEN study) and erlotinib (the SATURN study). Further perplexing the issue, only a small portion of patients in the placebo arms received pemetrexed/erlotinib as post-study therapy in the two trials.
The epithelial growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs; e.g., gefitinib or erlotinib) have been recommended as the first-line therapy for patients with EGFR mutations[2]. In comparison to classic cytotoxic agents, EGFR-TKIs are highly selective and have much more favorable toxicity profile, and hence could improve quality of life (QoL). As a result, EGFR-TKIs represent an attractive choice for maintenance therapy in patients with advanced NSCLC.
In this retrospective study, we compared the efficacy and safety of maintenance EGFR TKI maintenance therapy, implemented immediately after achieving disease control with first-line chemotherapy vs. delayed treatment at the time of disease progression. Data analysis was stratified based on EGFR mutation status.
MATERIAL AND METHODS
Patients and Treatment
Between November 2005 and November 2009, a total of 568 consecutive patients with histologically or cytologically confirmed advanced-stage NSCLC (stage IIIB with pleural effusion or stage IV) were treated with oral gefitinib or erlotinib at the Beijing Cancer Hospital or General Hospital of Navy, Beijing, China. All patients selected for the current study met the following criteria: 1. Platinum-based chemotherapy was used as first line therapy; 2. Patient must completed no less than 2 cycles of first-line chemotherapy; 3. Patient must attain disease control (DC) defined as complete response (CR), partial response (PR) or stable disease (SD) according to RECIST criteria. 4. EGFR TKI treatment started either within one month after the first-line chemotherapy and without evidence of disease progression (referred to as “immediate group”), or upon confirmation of progressive disease (PD) according to RECIST criteria (referred to as “delayed group”).
All patients gave consents to the standard therapy indicated for their illness. The retrospective review of the clinical data was approved by the Institutional Review Board of the Beijing Cancer Hospital.
Study Endpoints and Assessment
The primary endpoint of the study is PFS. The secondary endpoints included objective response rate (ORR), OS and adverse events. Baseline tumor measurement (obtained with CT or MRI) was available for all subjects. Treatment responses were assessed every two cycles during the initial first-line chemotherapy and every 6-8 weeks during the period of EGFR TKI treatment using the Response Evaluation Criteria In Solid Tumors (RECIST)[13]. Imaging data were reviewed by an independent radiologist. PFS was defined as the period from the beginning of first-line chemotherapy to cessation of gefitinib/erlotinib due to PD, or death from any cause. OS was defined as the period from the beginning of first-line chemotherapy until death from any cause. The responses in the TKI treatment phase were evaluated relative to the tumor status at the beginning of TKI use. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (version 3.0).
Detection of EGFR Mutation
Specimen collection, DNA extraction and denaturing high-performance liquid chromatography (DHPLC) were performed as previously described[14]. To confirm mutations identified by DHPLC, the PCR products used for DHPLC were sequenced bi-directionally using a Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA). The reactions were carried out in an automated DNA analyzer (ABI Prism 377; Applied Biosystems).
Statistical Analysis
The PFS and OS were estimated by the Kaplan-Meier method and were analyzed by using the the log-rank test. In addition, a Cox proportional hazards regression model was used to caculate hazard ratios. The models included effects for treatment groups. The 95%CI for median time was caculated by using the method of Brookmeyer and Crowley.Categorical valuables were compared using χ2 test. All statistical comparisons were considered statistically significant with a P value of less than 0.05 (two sided). A SPSS software for PC (version 13.0 for windows; SPSS Inc., IL, USA) was used for all statistical analysis.
RESULTS
Patient Characteristics
A total of 159 patients fulfilled the selection criteria: 85 in the immediate group and 74 in the delayed group. In the immediate group, majority of patients (67/85, 79%) were placed under EGFR-TKI treatment within 2 weeks after the completion of first-line chemotherapy. More patients were female in the immediate group (61.2% versus 43.2% in the delayed group, P=0.024; Table 1). Higher percentage of patients in the immediate group received less than 4 cycles of first-line chemotherapy (median: 3 cycles versus 5 cycles in the delayed group, P<0.001) and. Otherwise, the two groups did not differ in demographics and baseline characteristics. For both groups, adencarcinoma was the most common type. Tissue samples for EGFR mutation were available in 116 out of 159 patients (immediate group: 66; delayed group: 50).
Table 1. Baseline characteristics and demographic distribution in overall population.
| Characteristics | Immediate group (n=85) | Delayed group (n=74) | P valuesa |
|---|---|---|---|
| Age (years) | 59(36-80) | 61(34-81) | |
| Sex | 0.024 | ||
| Male | 33(38.8%) | 42(56.8%) | |
| Female | 52((61.2%) | 32(43.2%) | |
| Smoking history | 0.394 | ||
| Neverb | 68(80.0%) | 55(74.3%) | |
| Ever | 17(20.0%) | 19(25.7%) | |
| WHO performance statusc | 0.723 | ||
| 0 or 1 | 54(63.5%) | 49(66.2%) | |
| 2 | 31(36.5%) | 25(33.8%) | |
| Tumor histology | 0.873 | ||
| Adenocarcinomad | 72(84.7%) | 62(83.8%) | |
| Non-adenocarcinoma | 13(15.3%) | 12(16.2%) | |
| Disease stage at entry | 0.554 | ||
| JIIB with pleural effusion | 29(34.1%) | 22(29.7%) | |
| JV | 56(65.9%) | 52(70.3%) | |
| First-line agents combined with platinum | 0.891 | ||
| Gemcitabine | 43(50.6%) | 35(47.3%) | |
| Novelbine | 28(32.9%) | 25(33.8%) | |
| Taxel | 14(16.5%) | 14(18.9%) | |
| Cycle numbers of previous chemotherapy | <0.001 | ||
| 2 or 3 | 38(44.7%) | 12(16.2%) | |
| 4 to 6 | 47(55.3%) | 62(83.8%) | |
| EGFR mutation statuse | 0.777 | ||
| Mutation type | 40(60.6%) | 29(58.0%) | |
| Wide type | 26(39.4%) | 21(42%) |
a: The statistic was analyzed by pearson Chi-square tests. b: Never smokers were defined as patients who had smoked <100 cigarettes in their lifetime. c: The World Health Organization (WHO) performance status measures level of activity and is assessed on a scale of 0 to 4, with lower numbers indicating a higher degree of activity. The time point assessing WHO performance status in our study was that at the beginning of gefitinib or erlotinib. d: The subgroup of adenocarcinoma also included bronchoalveolar carcinoma. e: Sixty-six and 50 patients had records of EGFR mutation status in immediate group and delayed group respectively.
The main cause leading to discontinuation of the first-line chemotherapy was adverse events. The most common cause of death was disease progression (156/159), followed by infection (2 cases) and acute cardiac infarction (1 case). The median duration of the follow-up was 20.3 months (range: 4.4-50.9 months).
Adverse Events
The most common adverse events were rash and diarrhea (mostly grade 1 or 2) that dissipated spontaneously during the treatment (Table 2). Other adverse events included anorexia, nausea/vomiting, fatigue and elevated aminotransferase level, but were relatively uncommon. Five patients (5.9%) in the immediate group and three (4.1%) in the delayed group developed grade 3/4 toxicity, and either discontinued TKI or reduced the dosage. No hematological toxicity or adverse-event related death was recorded. One patient (1.2%) in the immediate group developed grade 3 hepatic damage; gefitinib was adjusted to 250 mg every two days until recovery. The toxicity profile did not differ between the two EGFR TKIs.
Table 2. Adverse events in the EGFR-TKI phase.
| Adverse eventsa | Immediate group (n=85) |
Delayed group (n=74) |
||
|---|---|---|---|---|
| All adverse events |
CTC grade 3 or 4b |
All adverse events |
CTC grade 3 or 4b |
|
| Number (percent) | Number (percent) | |||
| Rash | 47(55.2) | 3(3.5) | 41(55.4) | 2(2.7) |
| Diarrhea | 39(45.9) | 1(1.2) | 32(43.2) | 1(1.4) |
| Anorexia | 15(17.6) | 0 | 11(14.9) | 0 |
| Fatigue | 17(20.0) | 0 | 13(17.6) | 0 |
| Elevated aminotransferase | 1(1.2) | 1(1.2) | 2(2.7) | 0 |
| Hematologic toxicityc | 0 | 0 | 0 | 0 |
a: Calculations were based on the total 159 patients. The Common Terminology Criteria (CTC) grade is defined on the basis of the national Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. b: No death occurred due to TKI related adverse events. c: No Hematologic toxicity was considered to be associated with TKI therapy.
Efficacy Comparation between Immediate and Delayed Groups
ORR did not differ between the immediate and delayed groups (43.5% vs. 44.6%, P=0.893). Tumor response rate was also similar between the two groups regardless of mutation status (62.6% vs. 69.0%, P=0.578 for patients with EGFR mutation; 11.5% vs. 9.5%, P=0.824 for patients without mutation).
Disease progression (assessed up to November 1, 2009) did not differ between the two groups (63/85, 74.1% in the immediate group vs. 57/74, 76.0% in the delayed group; Figure 1A). The median PFS was 17.3 and 16.4 months in the immediate and delayed groups, respectively. The hazard ratio [HR] was 0.99 (95% confidence interval: 0.69-1.42; P=0.947). In an effort to reduce the influence of the unbalanced cycle number during the first-line chemotherapy, we also calculated PFS from the completion of first-line treatment to TKI treatment failure. The results of such analysis also failed to show any difference between the two groups (14.2 vs. 14.3 months, log rank test P=0.857). In patients completing only 4 cycles during first-line chemotherapy (immediate group: 30, 35.3%, and delayed group: 30, 40.5%), disease progression seemed to be lower in the immediate group but a statistically the difference was not significant (HR=0.67; 95% CI: 0.36-1.23; P=0.194; Figure 1C).
Figure 1.
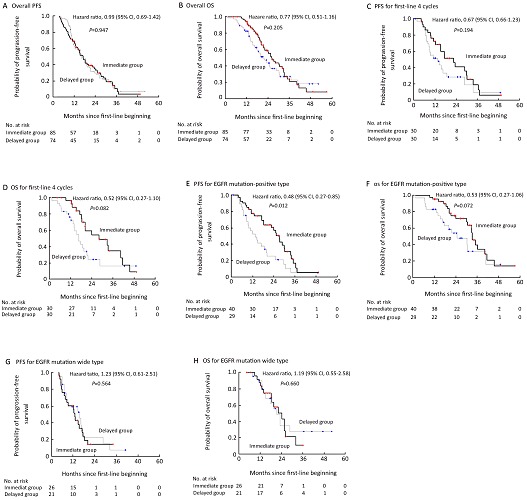
Kaplan-Meier curves for progression-free survival (PFS) and overall survival(OD). Kaplan-Meier curves for progression-free survival are shown for the overall population (Panel A) and patients who received only 4 cycles of first-line chemotherapy (Panel C), patients who were positive for the EGFR mutation (Panel E), and patients with EGFR mutation wide type (Panel G). Kaplan-Meier curves for overall survivaol are shown for the overall population (Panel B) and patients who reeived ony 4 cycles of first-line chemotherapy (Panel D), patients who were positive for the EGFR utation (Panel F), and patients with EGFR mutation wide type (Panel H). Hazard ratios were calculated with the use of a Cox proportional-hazards model, with sex smoking history (never smoker or ever smoker), and histology (adeocarvinoma or non-adenocarcinoma) as covariates.
Similar to PFS, there was no significant difference in terms of OS between the two groups either in the overall analysis (26.1 months in the maintenance group vs. 21.6 months in the delayed group; HR=0.77; 95% CI: 0.51-1.16; P=0.205; Figure 1B), or in the subgroup of patients receiving only 4 cycles during first-line chemotherapy (HR=0.52; 95% CI: 0.27-1.10; P=0.082; Figure 1D).
The Influence of EGFR Mutation Status
Incidence of EGFR mutation (deletion in exon 19 and missense mutation at exon 21) was balanced between the two groups (40/66, 60.6% in the immediate group, and 29/50, 58.0% in the delayed group). For PFS, there was a significant interaction between treatment and EGFR mutation. PFS was significantly longer in patients with EGFR mutation in the immediate group (HR=0.48; 95% CI: 0.27-0.85; P=0.012; Figure 1E), and there was no difference in terms of OS between the two groups with EGFR mutation (HR=0.53; 95% CI: 0.27 to 1.06; P=0.072; Figure 1F). But in patients without EGFR mutation, neither PFS (HR=1.23; 95% CI: 0.61-2.51; P=0.564; Figure 1G) nor OS (HR=1.19; 95% CI: 0.55-2.58; P=0.660; Figure 1H) differed between the immediate and delayed groups.
Stratification based upon other clinical characteristics, including gender smoking history, and histology (adenocarcinoma and non-adenocarcinoma), did not affect the results (Table 3).
Table 3. Clinical outcome by subgroups: EGFR mutation status, tumor histology, sex and smoking history.
| Subgroup | Progression-free survival (months)a |
Overall survival (months)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Immediate |
Delayed |
PFS for Immediate Vs. delyayed |
P |
Immediate |
Delayed |
OS for Immediate Vs. delyayed |
P |
||
| Median | Median | HR(95%CI)b | P | Median | Median | HR(95%CI)b | P | ||
| EGFR mutation positivec | 27.2 | 13.5 | 0.48 | 0.012 | 33.2 | 24.9 | 0.53 | 0.072 | |
| (0.27-0.85) | (0.27-1.06) | ||||||||
| EGFR mutation negative | 13.0 | 15.6 | 1.23 | 0.564 | 24.3 | 21.3 | 1.19 | 0.660 | |
| (0.61-2.51) | (0.55-2.58) | ||||||||
| Adenocarcinomad | 17.3 | 12.0 | 0.73 | 0.115 | 26.1 | 20.2 | 0.81 | 0.331 | |
| (0.49-1.08) | (0.52-1.25) | ||||||||
| Non-adenocarcinoma | 18.3 | 24.8 | 1.83 | 0.530 | 30.2 | 24.9 | 0.48 | 0.571 | |
| (0.28-12.11) | (0.04-6.25) | ||||||||
| Female | 15.4 | 16.6 | 0.97 | 0.905 | 24.0 | 23.3 | 1.02 | 0.955 | |
| (0.57-1.66) | (0.57-1.81) | ||||||||
| Male | 18.3 | 14.2 | 0.87 | 0.125 | 25.2 | 23.8 | 0.95 | 0.871 | |
| (0.35-1.26) | (0.56-1.45) | ||||||||
| Never smokere | 17.5 | 14.7 | 0.76 | 0.189 | 26.3 | 21.9 | 0.76 | 0.231 | |
| (0.50-1.15) | (0.48-1.19) | ||||||||
| Ever smoker | 12.9 | 11.6 | 0.59 | 0.252 | 26.1 | 19.9 | 0.63 | 0.406 | |
| (0.24-1.45) | (0.21-1.87) | ||||||||
a: The statistical analysis was based upon the entire sample of 159 subjects; b: Hazard ratio was calculated against the delayed group using a Cox proportional-hazards model, with sex, smoking history, and histology as covariantes; c: EGFR mutation included exon 19 deletion and exon 21 L858R only; d: Also included bronchoalveolar carcinoma; e: <100 cigarettes in lifetime.
DISCUSSION
EGFR TKIs (e.g., gefitinib and erlotinib) are the currently second-line therapy for advanced NSCLC[15,16,17]. An increasing number of randomized clinical trials suggested that maintenance therapy with TKIs immediately after first-line therapy in NSCLC patients achieving disease control is a promising strategy[8,18]. The results from the current study suggested immediate TKI maintenance is potentially superior to delayed therapy in NSCLC patients with EGFR mutation.
Both PFS and OS were prolonged by immediate TKI maintenance and delayed TKI treatment. Although the differences between the two groups were not statistically significant, there was a trend towards longer PFS and OS in the immediate group. Female NSCLC patients seem to have better prognosis than males[19,20]. The gender composition was significantly different between the two groups in our study. However, a stratified analysis failed to show a significant difference in either PFS or OS between the two groups. Responses to chemotherapy typically occur within the first two to four cycles[21]. Therefore, only subjects who completed no less than 2 cycles of the first-line chemotherapy were included in our study. Despite of such effort, patients in the immediate group received less cycles of initial chemotherapy than the delayed group. In order to minimize the potential influence of such a bias, we took two measures. First, we defined the survival time from the end of initial treatment to disease progression so that the starting point was identical in the two groups. We also conducted a separate analysis for patients who received only four cycles of first-line chemotherapy. Such an analysis failed to reach statistical significance, but suggested a trend towards decreased risk of progression/death in the immediate group compared with delayed group.
EGFR mutation is a powerful predictor for treatment response to EGFR-TKIs[22-27]. In the BR.21 study[28], patients harboring EGFR mutation had a 23% reduction in risk of death with erlotinib versus placebo (HR, 0.77). In SATURN study[29], patients with EGFR mutation who received immediate erlotinib maintenance versus placebo as maintenance had a remarkable reduction of progression risk (91%, HR, 0.09), suggesting that the patients with EGFR mutation could benefit from immediate TKI treatment. Our study showed that patients with EGFR mutation could benefit from immediate TKI maintenance treatment. A possible explanation underlying this phenomenon is the smaller tumor burden in patients who receive two treatment regimens without significant delay vs. those who received two regimens with a substantial interval.
In summary, immediate maintenance treatment with EGFR-TKIs after achieving disease control improves PFS (and potentially OS as well) in patients with EGFR mutation. This finding encourages prospectively designed clinical studies that compare immediate TKI maintenance with delayed treatment.
Acknowledgements
We thank Dr. Ning Wang, for conducting imaging evaluation and Yan Zhang for contribution in statistical analysis. We also thank Dr. Liang Li from Peking University Third Hospital for the support in the study.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43-66 [DOI] [PubMed] [Google Scholar]
- 2. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- 3.Mok TS, Ramalingam SS. Maintenance therapy in non-small-cell lung cancer: a new treatment paradigm. Cancer 2009; 115: 5143-54 [DOI] [PubMed] [Google Scholar]
- 4.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979; 63: 1727-33 [PubMed] [Google Scholar]
- 5.Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006; 52: 155-63 [DOI] [PubMed] [Google Scholar]
- 6.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J Clin Oncol 2009; 27: 591-8 [DOI] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009, 374:1432-40 [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Ciuleanu T, Stelmakh l, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010, 11: 521-9 [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355: 2542-50 [DOI] [PubMed] [Google Scholar]
- 10.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil [J] J Clin Oncol. 2009; 27: 1227-34 [DOI] [PubMed] [Google Scholar]
- 11.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 201021: 1804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009; 373: 1525-31 [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205-16 [DOI] [PubMed] [Google Scholar]
- 14.Bai H, Mao L, Wang SH, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small cell lung cancer. J Clin Oncol 2009; 27: 2653-9 [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology: Non-small cell lung cancer. http://www.nccn.org/ professionals/default.asp
- 16.Kim ES, Hirsh V, Mok TS, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008; 372: 1809-18 [DOI] [PubMed] [Google Scholar]
- 17.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med 2005; 353: 123-32 [DOI] [PubMed] [Google Scholar]
- 18.Hida T, Okamoto I, Kashii T, et al. Randomized phase III study of platinum-doublet chemotherapy followed by gefitinib versus continued platinum-doublet chemotherapy in patients with advanced non-small cell lung cancer: Results of West Japan Thoracic Oncology Arm trial (WJTOG 0203). J Clin Oncol 2008; 26: (suppl; Abstr LBA8012). [DOI] [PubMed]
- 19.Patel JD. Lung cancer in women. J Clin Oncol 2005; 23: 3212-8 [DOI] [PubMed] [Google Scholar]
- 20.Båtevik R, Grong K, Segadal L, et al. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung cancer 2005; 47: 173-81 [DOI] [PubMed] [Google Scholar]
- 21.Socinski MA, Morris DE, Masters GA, et al. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest 2003; 123: 226S-43S [DOI] [PubMed] [Google Scholar]
- 22.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129-39 [DOI] [PubMed] [Google Scholar]
- 23.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497-500 [DOI] [PubMed] [Google Scholar]
- 24.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci 2004; 101: 13306-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosell R, Moran T, Queralt C, et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N Engl J Med 2009; 361: 958-67 [DOI] [PubMed] [Google Scholar]
- 26.Rosell R, Viterib S, Molina MA, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancer [J] Curr Opin Oncol 2010; 22: 112-20 [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or Carboplatin- Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med 2009; 361: 947-57 [DOI] [PubMed] [Google Scholar]
- 28.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in Lung Cancer-Molecular and Clinical Predictors of Outcome. N Engl J Med 2005; 353: 133-44 [DOI] [PubMed] [Google Scholar]
- 29.Brugger W, Kim JH, Hansen O, et al. Molecular markers and clinical outcome with erlotinib: results from the phase III placebo-controlled SATURN study of maintenance therapy for advanced NSCLC. 2009 13th World Conference on Lung Cancer, Abstract, B9.1. [Google Scholar]


