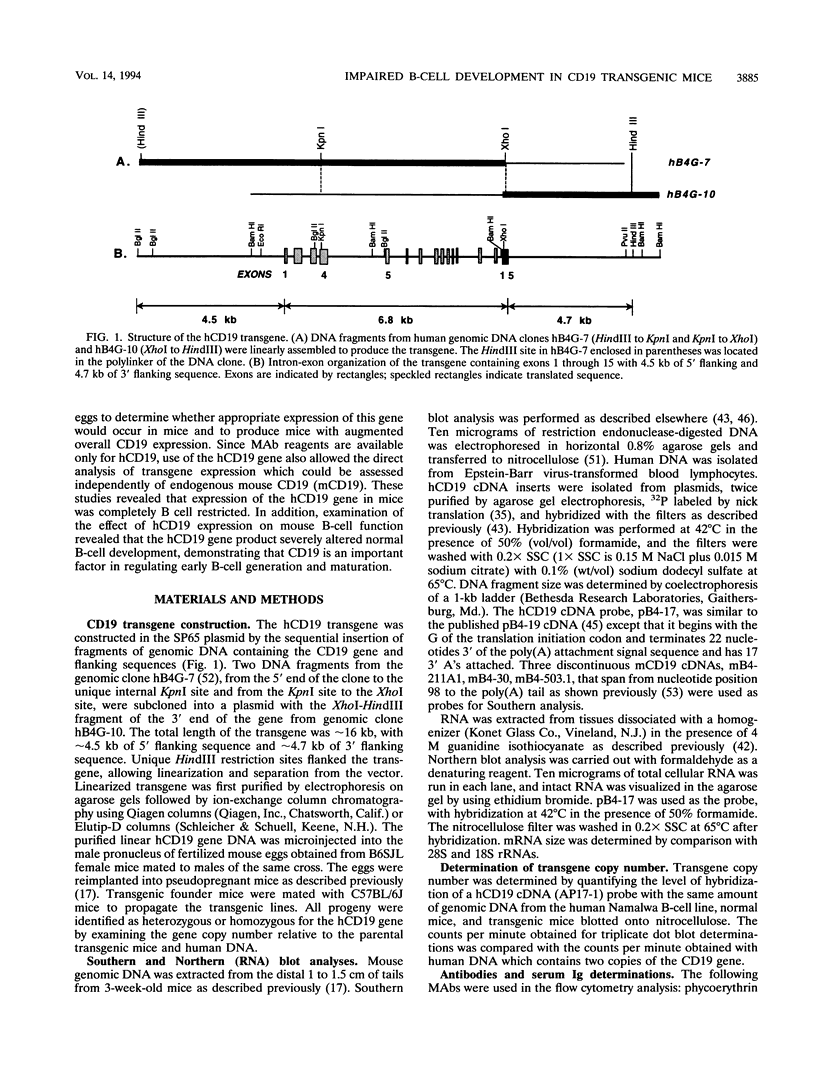
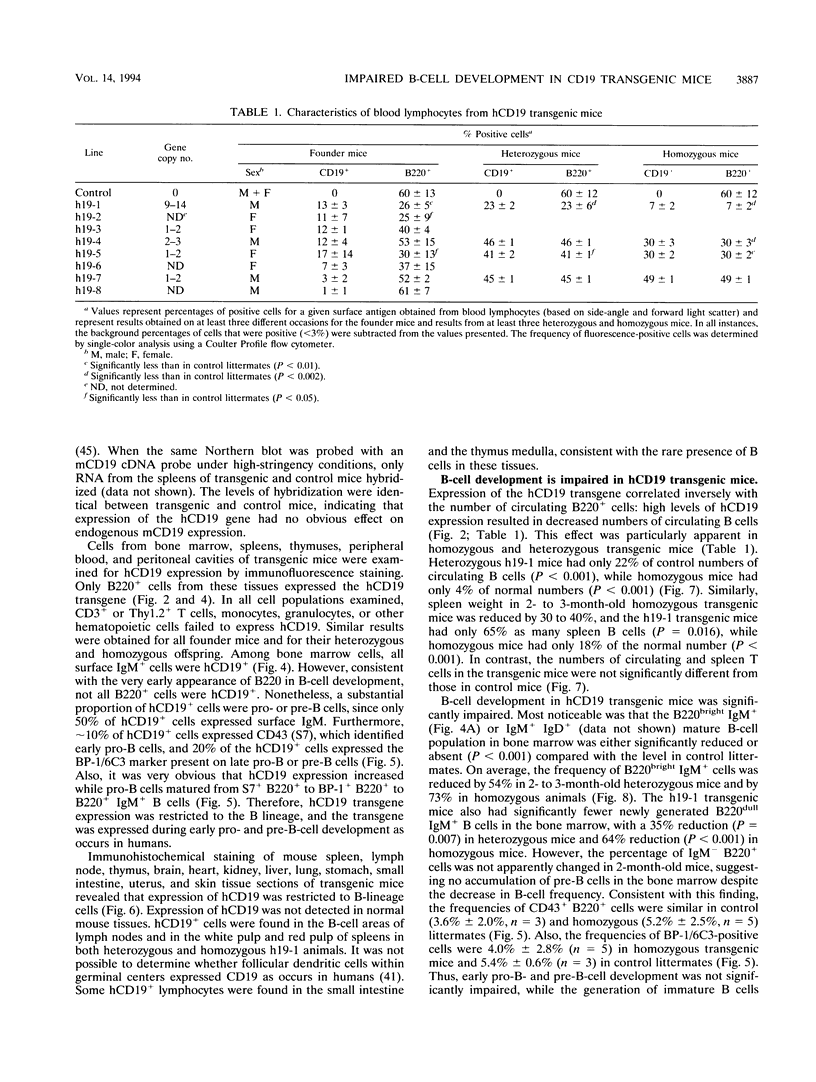
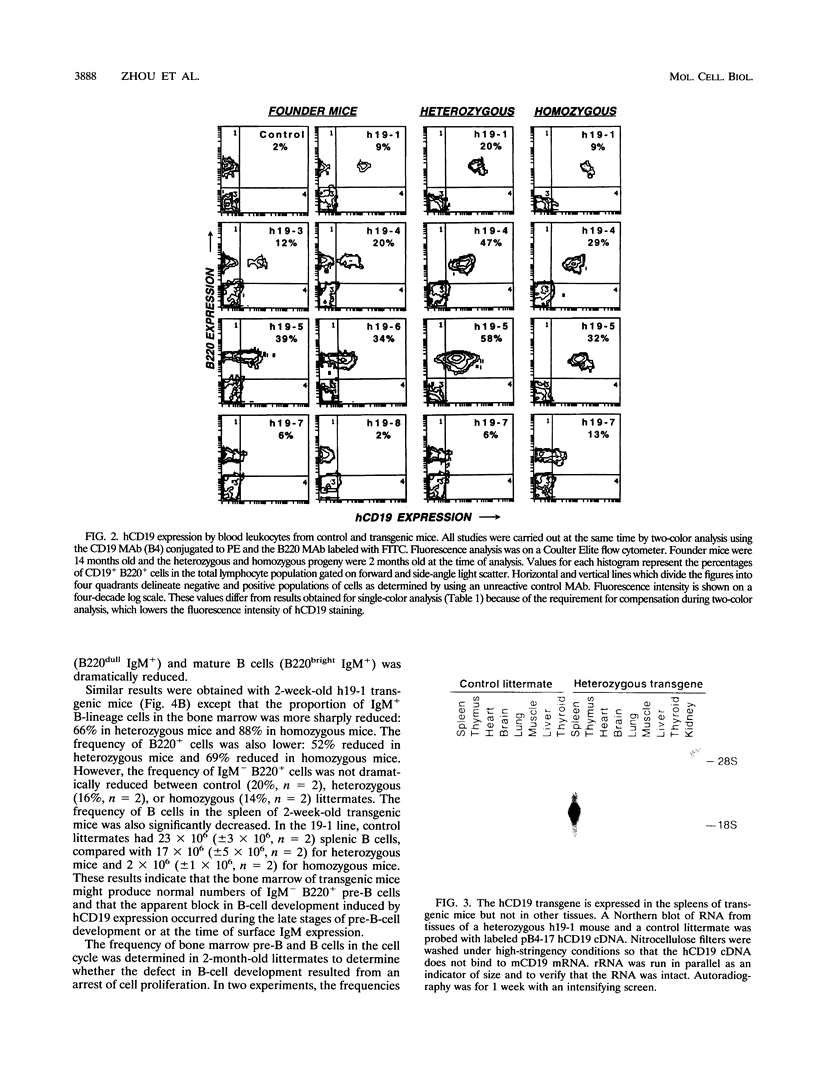
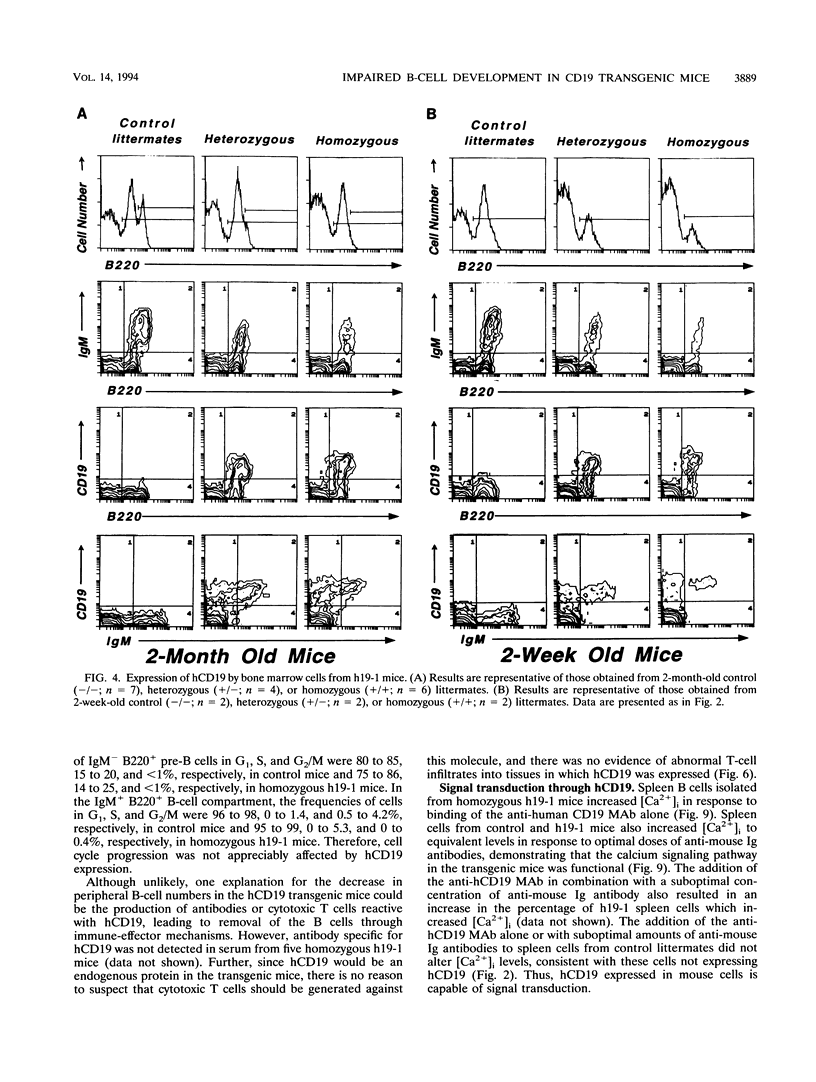
Abstract
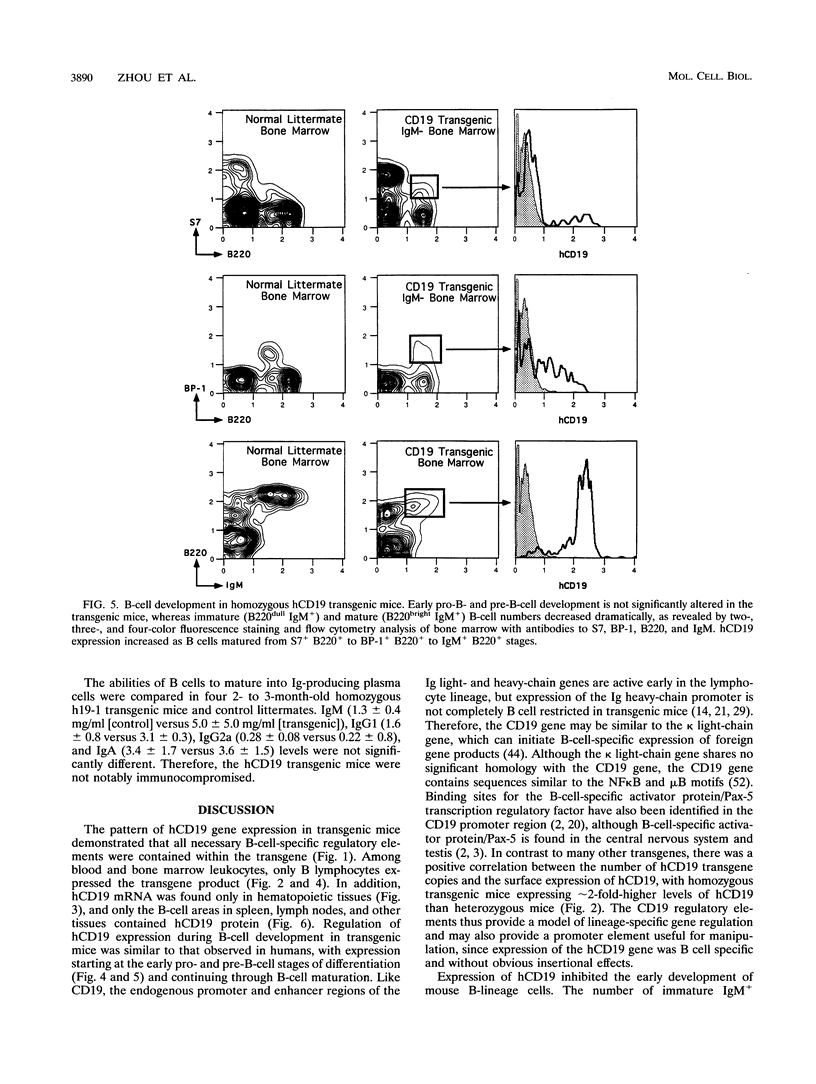
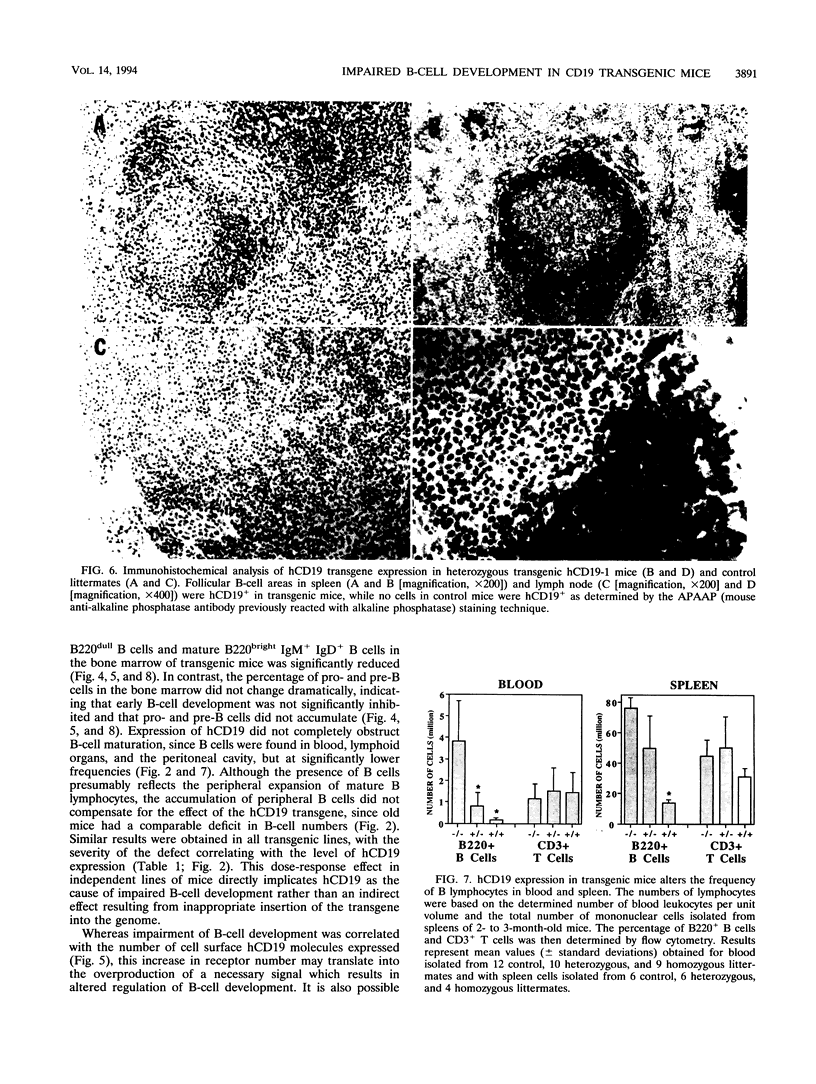
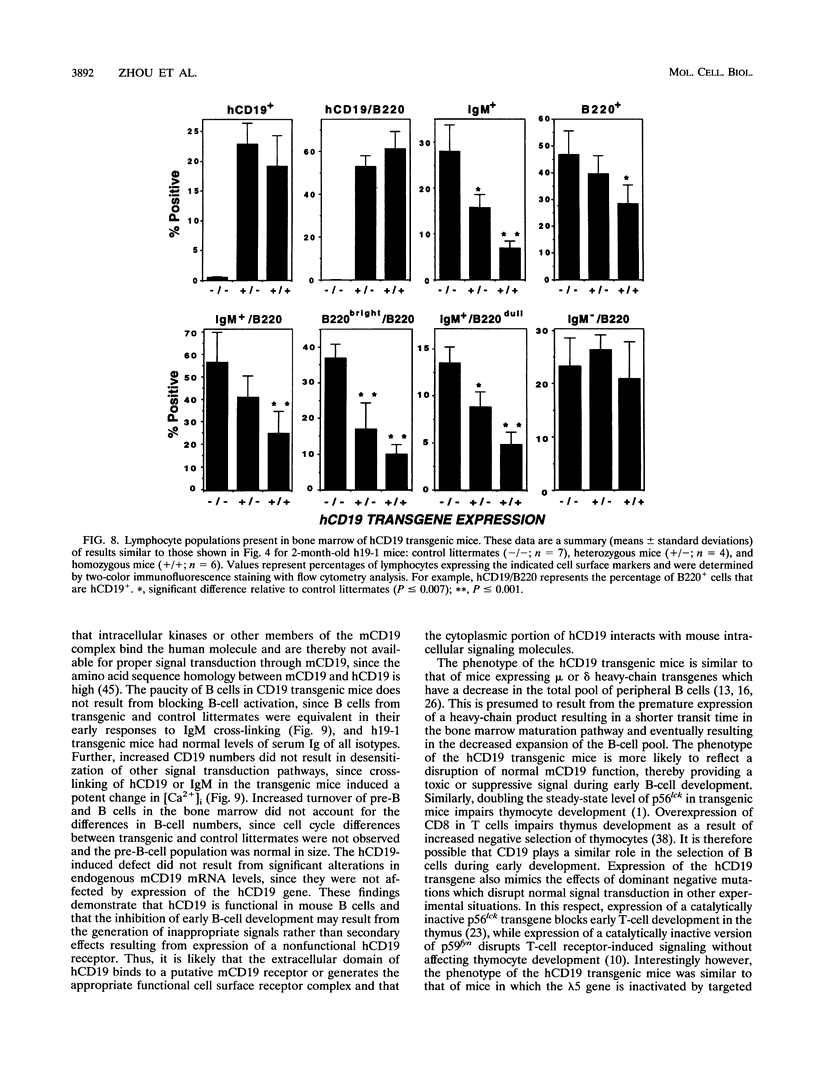
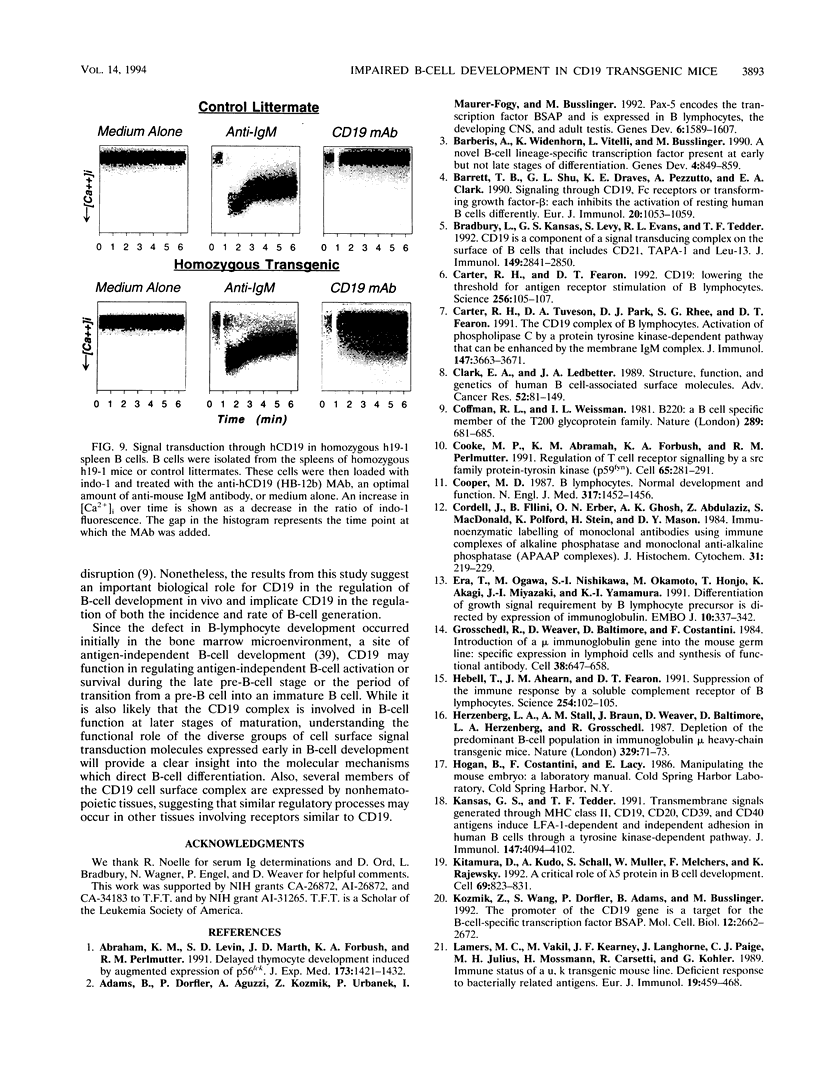
CD19 is a B-cell-specific member of the immunoglobulin superfamily expressed from early pre-B-cell development until plasma cell differentiation. In vitro studies demonstrate that the CD19 signal transduction molecule can serve as a costimulatory molecule for activation through other B-lymphocyte cell surface molecules. However, much remains to be known regarding how CD19 functions in vivo and whether CD19 has different roles at particular stages of B-cell differentiation. Therefore, transgenic mice overexpressing the human CD19 (hCD19) gene were generated to determine whether this transgene would be expressed in a B-lineage-specific fashion and to dissect the in vivo role of CD19 in B-cell development and activation. Expression of the human transgene product was specifically restricted to all B-lineage cells and appeared early in development as occurs with hCD19. In addition, expression of hCD19 severely impaired the development of immature B cells in the bone marrow, with dramatically fewer B cells found in the spleen, peripheral circulation, and peritoneal cavity. The level of hCD19 expressed on the cell surface correlated directly with the severity of the defect in different transgenic lines. These results demonstrate that the hCD19 gene is expressed in a lineage-specific fashion in mice, indicating that the hCD19 gene may be useful for mediating B-lineage-specific expression of other transgene products. In addition, these results indicate an important role for the lineage-specific CD19 molecule during early B-cell development before antigen-dependent activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K. M., Levin S. D., Marth J. D., Forbush K. A., Perlmutter R. M. Delayed thymocyte development induced by augmented expression of p56lck. J Exp Med. 1991 Jun 1;173(6):1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B., Dörfler P., Aguzzi A., Kozmik Z., Urbánek P., Maurer-Fogy I., Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992 Sep;6(9):1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- Barberis A., Widenhorn K., Vitelli L., Busslinger M. A novel B-cell lineage-specific transcription factor present at early but not late stages of differentiation. Genes Dev. 1990 May;4(5):849–859. doi: 10.1101/gad.4.5.849. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Shu G. L., Draves K. E., Pezzutto A., Clark E. A. Signaling through CD19, Fc receptors or transforming growth factor-beta: each inhibits the activation of resting human B cells differently. Eur J Immunol. 1990 May;20(5):1053–1059. doi: 10.1002/eji.1830200516. [DOI] [PubMed] [Google Scholar]
- Bradbury L. E., Kansas G. S., Levy S., Evans R. L., Tedder T. F. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992 Nov 1;149(9):2841–2850. [PubMed] [Google Scholar]
- Carter R. H., Fearon D. T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992 Apr 3;256(5053):105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Carter R. H., Tuveson D. A., Park D. J., Rhee S. G., Fearon D. T. The CD19 complex of B lymphocytes. Activation of phospholipase C by a protein tyrosine kinase-dependent pathway that can be enhanced by the membrane IgM complex. J Immunol. 1991 Dec 1;147(11):3663–3671. [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Structure, function, and genetics of human B cell-associated surface molecules. Adv Cancer Res. 1989;52:81–149. doi: 10.1016/s0065-230x(08)60211-0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Cooper M. D. Current concepts. B lymphocytes. Normal development and function. N Engl J Med. 1987 Dec 3;317(23):1452–1456. doi: 10.1056/NEJM198712033172306. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Era T., Ogawa M., Nishikawa S., Okamoto M., Honjo T., Akagi K., Miyazaki J., Yamamura K. Differentiation of growth signal requirement of B lymphocyte precursor is directed by expression of immunoglobulin. EMBO J. 1991 Feb;10(2):337–342. doi: 10.1002/j.1460-2075.1991.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hebell T., Ahearn J. M., Fearon D. T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991 Oct 4;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Tedder T. F. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-1-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J Immunol. 1991 Dec 15;147(12):4094–4102. [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Wang S., Dörfler P., Adams B., Busslinger M. The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol Cell Biol. 1992 Jun;12(6):2662–2672. doi: 10.1128/mcb.12.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M. C., Vakil M., Kearney J. F., Langhorne J., Paige C. J., Julius M. H., Mossmann H., Carsetti R., Köhler G. Immune status of a mu, kappa transgenic mouse line. Deficient response to bacterially related antigens. Eur J Immunol. 1989 Mar;19(3):459–468. doi: 10.1002/eji.1830190308. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S. D., Anderson S. J., Forbush K. A., Perlmutter R. M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993 Apr;12(4):1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Matsumoto A. K., Kopicky-Burd J., Carter R. H., Tuveson D. A., Tedder T. F., Fearon D. T. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991 Jan 1;173(1):55–64. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Rüther U., Vieira P., Hombach J., Reth M., Rajewsky K. Membrane-bound IgM obstructs B cell development in transgenic mice. Eur J Immunol. 1989 May;19(5):923–928. doi: 10.1002/eji.1830190520. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Anderson K. C., Marti G., Bates M., Park E., Daley J. F., Schlossman S. F. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983 Jul;131(1):244–250. [PubMed] [Google Scholar]
- Nadler L. M., Korsmeyer S. J., Anderson K. C., Boyd A. W., Slaughenhoupt B., Park E., Jensen J., Coral F., Mayer R. J., Sallan S. E. B cell origin of non-T cell acute lymphoblastic leukemia. A model for discrete stages of neoplastic and normal pre-B cell differentiation. J Clin Invest. 1984 Aug;74(2):332–340. doi: 10.1172/JCI111428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods Cell Biol. 1990;33:105–110. doi: 10.1016/s0091-679x(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Bouchard L. S., McMaster B. E. CD19 is functionally and physically associated with surface immunoglobulin. J Exp Med. 1989 Dec 1;170(6):2159–2164. doi: 10.1084/jem.170.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Rabinovitch P. S., Ledbetter J. A., Moldenhauer G., Clark E. A. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J Immunol. 1987 May 1;138(9):2793–2799. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rigley K. P., Callard R. E. Inhibition of B cell proliferation with anti-CD19 monoclonal antibodies: anti-CD19 antibodies do not interfere with early signaling events triggered by anti-IgM or interleukin 4. Eur J Immunol. 1991 Mar;21(3):535–540. doi: 10.1002/eji.1830210302. [DOI] [PubMed] [Google Scholar]
- Rijkers G. T., Griffioen A. W., Zegers B. J., Cambier J. C. Ligation of membrane immunoglobulin leads to inactivation of the signal-transducing ability of membrane immunoglobulin, CD19, CD21, and B-cell gp95. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8766–8770. doi: 10.1073/pnas.87.22.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E. A., Ramsdell F., Kioussis D., Sha W., Loh D., Axel R., Fowlkes B. J. The level of CD8 expression can determine the outcome of thymic selection. Cell. 1992 Jun 26;69(7):1089–1096. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriever F., Freedman A. S., Freeman G., Messner E., Lee G., Daley J., Nadler L. M. Isolated human follicular dendritic cells display a unique antigenic phenotype. J Exp Med. 1989 Jun 1;169(6):2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleasman J. W., Morimoto C., Schlossman S. F., Tedder T. F. The role of functionally distinct helper T lymphocyte subpopulations in the induction of human B cell differentiation. Eur J Immunol. 1990 Jun;20(6):1357–1366. doi: 10.1002/eji.1830200623. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storb U., O'Brien R. L., McMullen M. D., Gollahon K. A., Brinster R. L. High expression of cloned immunoglobulin kappa gene in transgenic mice is restricted to B lymphocytes. Nature. 1984 Jul 19;310(5974):238–241. doi: 10.1038/310238a0. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Isaacs C. M. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol. 1989 Jul 15;143(2):712–717. [PubMed] [Google Scholar]
- Tedder T. F., Klejman G., Schlossman S. F., Saito H. Structure of the gene encoding the human B lymphocyte differentiation antigen CD20 (B1). J Immunol. 1989 Apr 1;142(7):2560–2568. [PubMed] [Google Scholar]
- Tuveson D. A., Carter R. H., Soltoff S. P., Fearon D. T. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993 May 14;260(5110):986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. J., Ord D. C., Hughes A. L., Tedder T. F. Structure and domain organization of the CD19 antigen of human, mouse, and guinea pig B lymphocytes. Conservation of the extensive cytoplasmic domain. J Immunol. 1991 Aug 15;147(4):1424–1432. [PubMed] [Google Scholar]
- Zhou L. J., Ord D. C., Omori S. A., Tedder T. F. Structure of the genes encoding the CD19 antigen of human and mouse B lymphocytes. Immunogenetics. 1992;35(2):102–111. doi: 10.1007/BF00189519. [DOI] [PubMed] [Google Scholar]
- van Noesel C. J., Lankester A. C., van Lier R. A. Dual antigen recognition by B cells. Immunol Today. 1993 Jan;14(1):8–11. doi: 10.1016/0167-5699(93)90316-d. [DOI] [PubMed] [Google Scholar]





