Abstract
Objective
Programmed cell death 5 (PDCD5) is an apoptosis related gene and plays an important role in the pathogenesis and development of cancer. Whether PDCD5 is present in peripheral blood serum has not been reported. The aim of this study is to determine the contents of PDCD5 protein in peripheral blood serum of cancer patients, as well as normal subjects.
Methods
ELISA was used to detect the serum PDCD5 concentrations in 100 normal persons, 83 patients with breast cancer, 74 patients with gastrointestinal tract cancer and 41 patients with lung cancer. The results were statistically analyzed and discussed.
Results
PDCD5 could be detected in peripheral blood serum in both normal subjects and cancer patients. The serum PDCD5 contents in normal persons ranged from 3.8 to 6.1 ng/ml with a median of 4.70±0.68 ng/ml. For cancer patients the PDCD5 levels were 4.59±0.90, 4.79±1.14 and 10.43±22.34 ng/ml for breast cancer, gastrointestinal cancer and lung cancer patients respectively. There was no statistically significant difference between the serum PDCD5 concentrations of normal persons and cancer patients.
Conclusion
PDCD5 is present in peripheral blood. The PDCD5 levels in cancer patients are not statistically different from that of normal persons, though decreased expression of PDCD5 in malignant tissues has been found.
Key words: PDCD5, Apoptosis, Cancer patients, ELISA
INTRODUCTION
Many studies have demonstrated that apoptosis is closely related with the pathogenesis of cancers. It is therefore important to study the variations of apoptosis- regulating factors in cancer patients. TF-1 apoptosis-related gene 19 (TFAR19), cloned from TF-1 cells undergoing apoptosis, was first reported by Liu, et al. in Peking University Center for Human Disease Genomics in 1999[1], then designated as programmed cell death 5 (PDCD5) by International Human Gene Nomination Committee (GenBank accession number: AF014955). It was cloned as a gene whose expression was up-regulated during the apoptotic process of TF-1 cells induced by cytokine withdrawal using a cDNA representational differences analysis (cDNA-RDA) method. The amino acid sequence of PDCD5 is quite conserved among eukaryotic species, which indicates that PDCD5 may have important biological functions across species. PDCD5 is widely expressed in a variety of tissues with its mRNA expression in fetal tissues being significantly lower than that observed in adult tissues. The expression of PDCD5 protein in cells undergoing apoptosis is significantly increased and the appearance of PDCD5 in the nuclei of apoptotic cells1precedes the externalization of phosphatidylserine and fragmentation of chromosomal DNA[2].
Previous studies suggested that PDCD5 could inhibit the growth of some tumor cells (cervical cancer, ovarian cancer, hepatocellular cancer, renal clear cell carcinoma, etc.) by accelerating apoptosis and cooperating with radiotherapy and chemotherapy[3-7]. Also, the decreased expression of PDCD5 has been reported in various human tumors, such as breast cancer[8], hepatocellular carcinoma[9], cervical cancer[10], gastric cancer[11], lung cancer[12], acute and chronic myelogenous leukemia[13-15], astrocytic gliomas[16] and prostate cancer[17]. Recently, an ELISA method for detecting soluble PDCD5 protein was established by scientists in Peking University Center for Human Disease Genomics and it was found that soluble PDCD5 could be detected in normal human peripheral blood (personal communication). Furthermore, increased serum PDCD5 was detected in patients with rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, hepatitis and type A influenza (personal communication). In the light of these progresses and findings, we asked if there were changes of PDCD5 contents in peripheral blood serum of cancer patients and set out to determine the PDCD5 levels in peripheral blood serum from cancer patients.
MATERIALS AND METHODS
Samples
Peripheral blood samples were collected from 100 normal subjects (45 males and 55 females with a median age of 49.5 years, range 25-75), 83 patients with breast cancer (median age 51.8 years, range 32-79), 74 patients with gastrointestinal tract cancer (51 males and 23 females with a median age of 60.1 years, range 32-77) and 41 patients with lung cancer (27males and 14 females with a median age of 62.1 years, range 40-87). None of the patients had received chemotherapeutic, radiotherapeutic or surgical treatment before the sample collection. The diagnoses of the patients were all proved by biopsy or pathological examination after surgical excision. The 74 patients with gastrointestinal tract cancer included 35 gastric and 39 colorectal cancer patients. Serum was separated from the blood sample and stored at -20°C for later PDCD5 detection.
Reagents
The ELISA kits for PDCD5 detection were kindly provided by Professor Yingyu Chen at Peking University Center for Human Disease Genomics.
Determination of Serum PDCD5
Serum samples were properly diluted with phosphate buffered saline (PBS) containing 10 mg/ml bovine serum albumin (BSA) and 0.1 ml of the diluted serum was added in duplicate into wells of ELISA plates which had been coated with mouse monoclonal antibody against human PDCD5. After 1h incubation at 37°C in a humid chamber, the plates were washed with Immunowash (BioRAD, Model 1575). Then properly diluted HRP-rabbit-anti-human PDCD5 IgG was added to each well and the plates were incubated at 37°C in a humid chamber for 1 h. After a final washing with the washer, 3,3’,5,5’-tetramethyl benzidine (TMB) was added to develop the color, and absorbance was measured using a microplate reader (BioRAD Model 680) at wave length of 450 nm.
Statistical analysis
The concentrations of serum PDCD5 were expressed as x̄±sx̄. In normal persons, the serum PDCD5 levels of male and female subjects were compared. For cancer patients, the serum PDCD5 levels in breast cancer, gastrointestinal tract cancer and lung cancer patients were separately compared with normal persons. Within gastrointestinal tract cancer group, the difference between gastric cancer and colorectal cancer patients was also compared. Statistical analysis was performed on Excel 2003 (Microsoft Corp. USA) and all the comparisons of serum PDCD5 levels were analyzed with student’s t-test. Differences were considered significant at P<0.05.
RESULTS
PDCD5 Detection in Normal Subjects
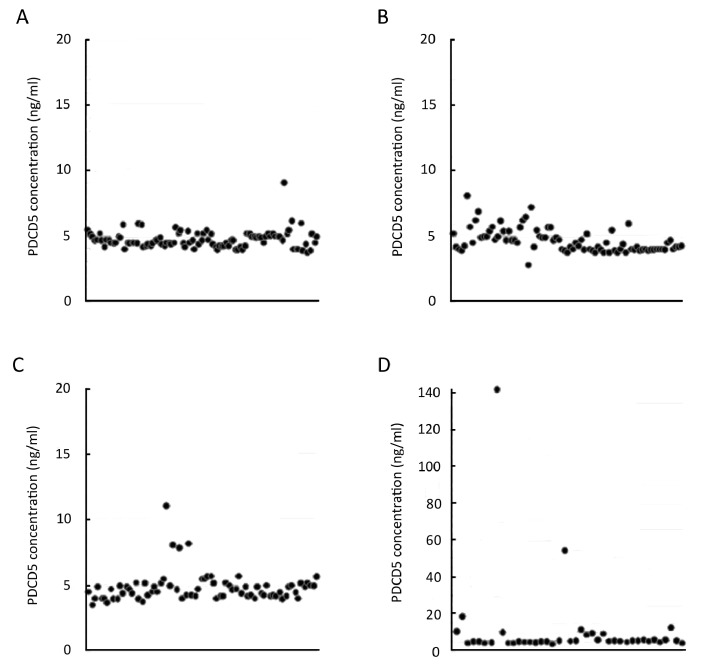
Using the ELISA kit provided by Peking University Center for Human Disease Genomics, PDCD5 could be detected in all the normal serum samples. The serum concentration of PDCD5 ranged from 3.8 to 6.1 ng/ml with a median of 4.70±0.68 ng/ml (Table 1 and Figure 1A). There was no significant difference between the serum PDCD5 contents in male (4.59 ng/ml) and female (4.78 ng/ml) subjects.
Table 1. Serum PDCD5 concentrations in normal persons and cancer patients.
| Subjects | n | Serum PDCD5 (ng/ml) |
|---|---|---|
| Normal | 100 | 4.70±0.68 |
| Breast cancer | 83 | 4.59±0.90 |
| Gastrointestinal cancer | 74 | 4.79±1.14 |
| Lung cancer | 41 | 10.43±22.43 |
Figure 1.
Serum PDCD5 concentrations in A: normal subjects; B: Breast cancer patients; C: Gastrointestinal tract cancer patients; D: and lung cancer patients. Vertical axis represents the PDCD5 concentration (ng/ml).
PDCD5 Detection in Breast Cancer Patients
The serum PDCD5 contents in 83 breast cancer patients ranged from 3.8 to 8.0 ng/ml with a median of 4.59±0.90 ng/ml (Table 1 and Figure 1B). There was no statistical significance compared with the serum PDCD5 level in normal persons (P=0.39).
PDCD5 Detection in Patients with Gastrointestinal Tract Cancer
The serum PDCD5 contents in 74 patients with gastrointestinal tract cancer ranged from 3.5 to 11.0 ng/ml with a median of 4.79±1.14 ng/ml (Table 1 and Figure 1C). There was no statistical significance compared with the serum PDCD5 level in normal persons (P=0.50). The gastric and colorectal cancer patients had similar serum PDCD5 contents (4.87 versus 4.71 ng/ml).
PDCD5 detection in Patients with Lung Cancer
The serum PDCD5 contents in 41 lung cancer patients ranged from 3.6 to 141.3 ng/ml with a median of 10.43±22.43 ng/ml (Table 1 and Figure 1D). Though the median PDCD5 concentration in lung cancer patients was higher, there was still no statistical significance compared with the serum PDCD5 level in normal persons (P=0.11). There were two patients with very high serum PDCD5 levels (141.3 and 54 ng/ml respectively). The serum PDCD5 median would be 5.95±2.90 ng/ml if these two data were excluded from the population. There were also four more lung cancer patients who had higher serum PDCD5 (≥10 ng/ml).
DISCUSSION
PDCD5 is an apoptosis-related gene cloned from TF-1 cells undergoing cytokine deprivation-induced apoptosis by a differential cloning strategy, cDNA-RDA method[1]. PDCD5 gene encodes a protein that shares significant homology with the corresponding proteins of species ranging from yeast to mice and the amino acid sequence of PDCD5 is quite conserved among eukaryotic species, which indicates that PDCD5 may have important biological function across species. PDCD5 is widely expressed in a variety of tissues with its mRNA expression in fetal tissues being significantly lower than that observed in adult tissues. The expressed PDCD5 protein has been shown to traverse rapidly from the cytoplasm to the nucleus of cells and distribute uniformly in cells that undergo apoptosis[2]. Functional studies show that the overexpression of PDCD5 facilitates programmed cell death triggered by growth factor withdrawal or serum deprivation in various types of tumor cells. PDCD5 also enhances paraptotic cell death induced by TAJ/TROY, a novel member of the tumor necrosis factor receptor family[18]. Thus PDCD5 plays a role in accelerating apoptosis rather than being an initiating factor in this process.
As an apoptosis related gene, PDCD5 has been proved to be involved in malignancy pathogenesis by increasing evidences. Downregulation of PDCD5 expression has been found in various malignancies, such as breast cancer[8], hepatocellular carcinoma[9], cervical cancer[10], gastric cancer[11], lung cancer[12], acute and chronic myelogenous leukemia[13-15], astrocytic gliomas[16], prostate cancer[17] and papillary thyroid carcinoma (PTC)[3]. Inhibition of PDCD5 expression by siRNA could inhibit cell apoptosis[19], and the sensitivity of HeLa cells to apoptosis induced by etoposide is reduced by in situ electroporation of the anti-PDCD5 monoclonal antibody[20]. Overexpression of PDCD5 facilitates apoptosis triggered by certain stimuli in cancer cell lines such as HeLa and MGC-803[1]. Exogenous PDCD5 delivered by adenovirus-mediated gene transfer in leukemia cells can markedly enhance the sensitivity to topoisomerase II inhibitor idarubicin in vitro and in vivo[21], and even exert cell-killing effect without the use of chemotherapeutic drugs both in vitro and in vivo[22]. Wang et al. further proved that recombinant human PDCD5 protein can markedly increase the susceptibility of tumor cells to cytotoxic drug-induced apoptosis and enhance the therapeutic efficacy in a xenograft leukemia model[23]. All these data indicate that PDCD5 plays an important roll in the pathogenesis and development of cancer. There may be a link between the decreased expression of PDCD5 and the formation of carcinoma and malignant progression.
Recently, scientists at Peking University Center for Human Disease Genomics established an ELISA method which can quantitatively detect the amount of soluble PDCD5 protein in serum and body fluids. They found that PDCD5 protein exists in normal peripheral blood, which has been further proved by Western blot (unpublished data from personal communication). As we already know, PDCD5 protein is normally uniformly distributed in cells and rapidly translocates to the nucleus of cells undergoing apoptosis. So far we do not know if PDCD5 can be expressed on cell membrane, and sequence analysis does not show the existence of leader peptide homology sequence and membrane anchor region within PDCD5. There is no evidence that PDCD5 can be secreted out of cells, thus the origin of serum PDCD5 is a mystery by know. It is possible that cells undergoing apoptosis may release PDCD5 protein which constitutes the detected serum PDCD5. Is the PDCD5 in serum and body fluids functional? From the evidences accumulated by now, PDCD5 seems to exert its function within cells, particularly within nucleus. Nevertheless, Wang et al. reported an interesting experimental result. They found intra-tumor injected recombinant human PDCD5 protein, combined with daunorubicin, could significantly suppress tumor growth in U937 xenograft nude mice. Furthermore, scientists at Peking University Center for Human Disease Genomics found recently that intraperitoneally injected PDCD5 protein could significantly alleviate the onset of encephalomyelitis in a mouse model of experimental allergic encephalomyelitis (unpublished data from personal communication). These data show that exogenous PDCD5 protein is capable of mediating its function in cells, thus suggesting that it is not entirely impossible that serum PDCD5 may have certain function.
Preliminary works found increased serum PDCD5 in patients with rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, hepatitis and type A influenza (unpublished data from personal communication). Though the significance of the elevated serum PDCD5 in those patients is not clear, it may be related to the highly active status of immune system. The importance and decreased expression of PDCD5 in tumor tissues in malignancy prompted us to investigate if there were changes of serum PDCD5 in cancer patients. In this study the serum PDCD5 levels in three kinds of cancer patients (breast, gastrointestinal and lung cancer) showed no statistical difference compared with normal control. Considering the decreased expression of PDCD5 in various cancer tissues, one might expect that the contents of serum PDCD5 may be lower than normal. To us, this result is not totally unexpected, because the origin of serum PDCD5 is not clear by now and a decreased expression of PDCD5 restricted to cancer tissues may not affect the PDCD5 contents in blood serum as disturbance of apoptosis process should be restricted to malignant tissues, not a system disorder. On the other hand, seven cancer patients (1 gastric and 6 lung cancer patients) had higher serum PDCD5 levels (≥10 ng/ml). Due to unpublished data communicated by Dr. Yingyu Chen, increased serum PDCD5 has been found in patients with immunological disturbance or infectious diseases which are usually accompanied by immune system mobilization. However none of these conditions were found in the cancer patients with higher serum PDCD5. There seems to be a trend that lung cancer patients are apt to have higher serum PDCD5 and we do not think that this has a direct link to the malignancy.
To our knowledge, there is no published report on the detection of serum PDCD5. This work reports the detection of serum PDCD5 in normal persons and cancer patients. The significance of serum PDCD5 and its changes in various disease conditions await further investigations.
Acknowledgement
The authors would like to thank Professor Yingyu Chen (Peking University Center for Human Disease Genomics) for providing the PDCD5 ELISA kit and offering their preliminary experimental results.
REFERENCES
- 1.Liu H, Wang Y, Zhang Y, et al. TFAR19, a novel apoptosis related gene cloned from human leukemia cell line TF21, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun 1999; 254:203-10 [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Sun R, Han W, et al. Nuclear translocation of PDCD5 (TFAR19): an early signal for apoptosis? FEBS Lett 2001; 509:191-6 [DOI] [PubMed] [Google Scholar]
- 3.Du Y, Hong TP. A preliminary study on the relationship between TF-1 cell apoptosis-related gene 19 and thyroid tumor. Zhonghua Nei Ke Za Zhi (in Chinese)2003; 42:492-4 [PubMed] [Google Scholar]
- 4.Ji X, Cheung R, Cooper S, et al. Interferon Alfa regulated gene expression in patients initiating interferon treatment for chronic hepatitis C. Hepatology 2003; 37:610-21 [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Cui H, Wei LH, et al. Expression of TFAR19 protein in epithelial ovarian cancer. Chin J Clin Obstet Gynecol 2002; 3:164-7 [Google Scholar]
- 6.Zhang D, Liu ZH, Li KM, et al. RhPDCD5 protein can enhance the apoptosis of SiHa cervical cells induced by IFNγ. Chin J Clin Obstet Gynecol (in Chinese)2004; 5:286-9 [Google Scholar]
- 7.Tan WL, Xiong L, Zheng SB, et al. Relationship between programmed cell death 5 protein expression and prognosis of renal clear cell carcinoma. Nan Fang Yi Ke Da Xue Xue Bao (in Chinese)2006; 26:1316-8 [PubMed] [Google Scholar]
- 8.Hedenfalk I, Duggan D, Chen Y, et al. Gene-Expression Profiles in Hereditary Breast Cancer. N Engl J Med 2001; 344:539-48 [DOI] [PubMed] [Google Scholar]
- 9.Xu XR, Huang J, Xu ZG, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci 2001; 98:15089-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu ZH, Zhang D, Li KM, et al. Expression of PDCD5 in tissues of normal cervix, CIN I–III and cervical Cancer. J Peking Univ (Health Sci) (in Chinese)2004; 36:407-10 [PubMed] [Google Scholar]
- 11.Yang YH, Zhao M, Li WM, et al. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis 2006; 11: 993-1001 [DOI] [PubMed] [Google Scholar]
- 12.Spinola M, Meyer P, Kammerer S, et al. Association of the PDCD5 locus with lung cancer risk and prognosis in smokers. J Clin Oncol 2006; 24:1672-8 [DOI] [PubMed] [Google Scholar]
- 13.Ruan GR, Chen SS, Chang Y, et al. Abnormality expression of a novel apoptosis-promoting molecule TFAR19 (PDCD5) in the bone marrow cells from adult chronic myeloid leukemia. J Peking Univ (Health Sci) (in Chinese)2002; 34:676-9 [Google Scholar]
- 14.Ma X, Ruan G, Wang Y, et al. Two Single-Nucleotide Polymorphisms with Linkage Disequilibrium in the Human Programmed Cell Death 5 Gene 5′ Regulatory Region Affect Promoter Activity and the Susceptibility of Chronic Myelogenous Leukemia in Chinese Population. Clin Cancer Res 2005; 11:8592-99 [DOI] [PubMed] [Google Scholar]
- 15.Ruan GR, Qin YZ, Chen SS, et al. Abnormal expression of the programmed cell death 5 gene in acute and chronic myeloid leukemia. Leuk Res 2006; 30:1159-65 [DOI] [PubMed] [Google Scholar]
- 16.Li H, Wang Q, Gao F, et al. Reduced expression of PDCD5 is associated with high-grade astrocytic gliomas. Oncol Rep 2008; 20:573-9 [PubMed] [Google Scholar]
- 17.Du YJ, Xiong L, Lou Y, et al. Reduced Expression of Programmed Cell Death 5 Protein in Tissue of Human Prostate Cancer. Chin Med Sci J 2009; 24:241-5 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li X, Wang L, et al. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci 2004; 117:1525-32 [DOI] [PubMed] [Google Scholar]
- 19.Chen LN, Wang Y, Ma DL, et al. Short interfering RNA against the PDCD5 attenuates cell apoptosis and caspase-3 activity induced by Bax overexpression. Apoptosis 2006; 11:101-11 [DOI] [PubMed] [Google Scholar]
- 20.Rui M, Chen YY, Zhang YM, et al. Transfer of anti-TFAR19 monoclonal antibody into HeLa cells by in situ electroporation can inhibit the apoptosis. Life Sci 2002; 71:1771-8 [DOI] [PubMed] [Google Scholar]
- 21.Ruan GR, Zhao HS, Chang Y, et al. Adenovirus-mediated PDCD5 gene transfer sensitizes K562 cells to apoptosis induced by idarubicin in vitro and in vivo. Apoptosis 2008; 13:641-8 [DOI] [PubMed] [Google Scholar]
- 22.Xie M, Niu JH, Chang Y, et al. A novel triple-regulated oncolytic adenovirus carrying PDCD5 gene exerts potent antitumor efficacy on common human leukemic cell lines. Apoptosis 2009; 14:1086-94 [DOI] [PubMed] [Google Scholar]
- 23.Wang YF, Shi L, Song QS, et al. Recombinant human PDCD5 protein enhances chemosensitivities of hematologic malignancies. Chin Sci Bullet 2009; 54:3981-9 [Google Scholar]