Abstract
Objective
Estradiol (E2) plays an important role in the development of breast cancer. In postmenopausal women, the estrogen can be synthesized via aromatase (CYP19) pathway and steroid-sulfatase (STS) pathway in peripheral tissues, when the production in ovary has ceased. The objective of our study was to explore the effects of Shu-Gan-Liang-Xue Decoction (SGLXD) on the expressions of CYP19 and STS in estrogen receptor positive breast cancer MCF-7 and T47D cells.
Methods
The effects of SGLXD on the cell viability of MCF-7 and T47D were analyzed by MTT assay. By quantitative real-time RT-PCR and Western blot, we evaluated the mRNA and protein expressions of CYP19 and STS in MCF-7 and T47D cells after SGLXD treatment.
Results
By MTT assay, the cell viability rates of MCF-7 and T47D were significantly inhibited by SGLXD in a dose-dependent manner, the IC50 values were 40.07 mg/ml for MCF-7 cells and 25.62 mg/ml for T47D cells, respectively. As evidenced by real-time PCR and Western blot, the high concentrations of SGLXD significantly down-regulated the expressions of CYP19 and STS both in the transcript level and the protein level.
Conclusion
The results suggest that SGLXD is a potential dual aromatase-sulfatase inhibitor by simultaneously down-regulating the expressions of CYP19 and STS in MCF-7 and T47D cells.
Key words: Shu-Gan-Liang-Xue Decoction (SGLXD), Aromatase (CYP19), Steroid-sulfatase (STS), Breast cancer
INTRODUCTION
Breast cancer is one of the most common malignancies among women worldwide[1, 2]. About one-third of all breast cancer and two-thirds of postmenopausal breast cancer are hormone dependent, contain estrogen receptors and require estrogen for tumor growth[3-5]. It has been accepted that estradiol (E2) which is the most potent endogenous estrogen, participates in either the initiation or promotion stage of breast cancer[6, 7]. Ovary is the main source of estradiol in premenopausal women, but in postmenopausal women, the main site of estradiol production moves to peripheral tissues[8]. Compared with premenopausal women, the circulating estrogen in plasma of the postmenopausal decreased about 80%-90%, due to the loss of ovarian function[9]. However, it is reported that there are only minor differences for the estrogen level in breast tissue[10].
The human breast tissue has all important enzymes necessary to the synthesis, conversion and storage of estrogens[10]. The estradiol can be synthesized either in normal or tumor breast tissue, and this local estrogen production can be involved in the development of breast cancer[9]. Aromatase (CYP19) pathway and steroid-sulfatase (STS) pathway are the two major routes involved in the synthesis of estradiol in these tissues[11, 12]. In the production of estradiol, CYP19 catalyzes the final rate-limiting reaction[7,13], and is in charge of the conversion of androstenedione to estrone and testosterone to estradiol, respectively[14,15], while STS is responsible for the formation of active steroids from systemic precursors, such as estrone sulfate and dehydroepiandrosterone sulfate (DHEAS)[12,16-18]. Both of CYP19 and STS contribute to maintaining the high level of estradiol in breast, and then irritate the growth and survival of hormone-dependent breast cancer.
Shu-Gan-Liang-Xue Decoction (SGLXD), a clinical experienced prescription of Prof. Ping-ping Li (Peking University School of Oncology), has been used for decades to treat breast cancer and alleviate the side effects caused by tamoxifen, such as hot-flushes[19, 20]. SGLXD and each of the component herbs do not manifest estrogenic activity, and MCF-7 cell viability could be inhibited by SGLXD at high concentrations[21]. SGLXD could act as a selective estrogen enzyme modulator (SEEM) agent by down-regulating the expression of STS in the transcript level and enzymatic activity[22]. In order to study more about the effects of SGLXD on the enzymes involved in the synthesis of estradiol, we performed the present experiment.
MATERIALS AND METHODS
Chemicals and Antibodies
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium- bromide (MTT) was obtained from Sigma–Aldrich (St. Louis, MO, USA). Rabbit polyclonal antibody to steroid sulfatase (ab62219), rabbit polyclonal antibody to aromatase (ab71263), rabbit polyclonal antibody to actin (loading control) (ab1801), and goat polyclonal antibody to rabbit IgG-HRP (ab6721) were purchased from Abcam (Abcam, Hong Kong).
Sources of Component Herbs and Preparation of SGLXD
The component herbs of SGLXD used as follows: Lithospermum (20 g), Cortex Moutan (15 g), Fructus Schisandrae (15 g), Radix Paeoniae Alba (15 g), Radix Bupleuri (10 g), and Radix Curcumae (10 g). All of them were purchased from Bai-Ta-Si Drugstore in Beijing, and authenticated by herbalists of the drugstore. The authenticated voucher specimens are available in our department. SGLXD was extracted by a routine method which is used in our laboratory as previous reports[21,22]. The SGLXD extract was diluted with culture mediums containing 10% fetal bovine serum (FBS, Gibco, Australia) to final concentrations (10, 20, 30, 40, 50 mg/ml for MCF-7 and 5, 10, 15, 20, 25 mg/ml for T47D, respectively).
Cell Lines and Culture
Human mammary epithelial carcinoma cell line MCF-7 (ATCC No. HTB-22) was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and grown in Dulbecco's modified eagle medium (DMEM, Bioroc, Beijing). Human breast carcinoma cell line T47D was kindly supplied by Prof. Yong-feng Shang (School of Basic Medical Sciences, Pecking University) and grown in RPMI-1640 (Bioroc, Beijing). Both of the mediums were supplemented with 10% FBS and contained 1 mmol/L non-essential amino acids, 0.1 mmol/L sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Cell Viability Assay
Colorimetric MTT assay was used to observe cell viability[23]. The density of MCF-7 cells was modulated to 5.0×104 cells/ml, while T47D cells were modulated to 1×105 cells/ml. Cells were seeded in 96-well plates (100 μl/well). After culturing for 24 h in a 37°C humidified incubator (5% CO2), MCF-7 cells and T47D cells were incubated in complete medium in the absence (negative control) and presence of various concentrations of SGLXD for 24h, respectively. The supernatant was discarded after the treatment, and 100 μl of MTT solution (5 mg/ml) was added to each well and incubated for 4 h. After the MTT solution was extracted, 100 μl of dimethyl sulfoxide (DMSO) was added to each well for coloration. The plates were shaken powerfully to ensure complete solubilization for 15 min at room temperature, the optic-metric density (OD) was read on a microplate reader Model 680 (Bio-Rad, Hercules, CA, USA) at single wavelength of 570 nm. Each group included six parallel wells and performed in three independent experiments. Negative control was considered as the baseline (100%) for the analysis. The following formula was used: cell viability rate (%) = OD of the experimental samples/OD of the control × 100%. The 50% inhibitory concentrations of cells (IC50) were calculated using probit- regression analysis with software SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA).
RNA Isolation and Reverse Transcription
MCF-7 cells and T47D cells were cultured and treated with various concentrations of SGLXD (10, 20, 40 mg/ml for MCF-7 and 5, 15, 25 mg/ml for T47D, respectively). After 24h, total RNA was extracted from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The concentration, purity and integrity of RNA samples were determined by UV absorbance (260 nm, 280 nm) and electrophoresis. The TransScript Two Step RT-PCR Super Mix kit (TransGen Boitech, Beijing, China) was used for the synthesis of first-strand cDNA through generating a 20 μl reaction mixture which contains 1 μg of RNA, and setting it at 42°C for 30 min, followed by heating at 80°C for 10 min, according to the manufacturer’s protocol. RT products were stored at -20°C.
Analysis of CYP19 and STS mRNA by Quantitative Real-time RT-PCR
Oligonucleotide primers for CYP19, STS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed through the program Primer Premier 5 (Premier, Palo Alto, CA, USA) and synthesized by Augct (Augct Biotechnology, Beijing, China). The PCR specific primers were 5’-AGC CAT CCT CGT TAC ACT-3’ (forward) and 5’-TCA CCG ACT ATT TCT CCC-3’ (reverse) for CYP19, 5’-ACA GCG GAA CAC TGA GAC-3’ (forward) and 5’-TGT GAA GTA GAT GAG GGT AT-3’ (reverse) for STS[21], 5’- GAC CCC TTC ATT GAC CTC AAC-3’ (forward) and 5’-CTT CTC CAT GGT GGT GAA GA-3’ (reverse) for GAPDH. In quantitative real-time RT-PCR, we used Power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA). PCR reactions were set up as described in the manual, and performed in ABI 7500 Fast Sequence Detection System (Applied Biosystems, Foster City, CA, USA). A typical 20 μl reaction volume contained 1 μl cDNA, 0.5 μl forward primer, 0.5 μl reverse primer, 10 μl mix and 8 μl ddH2O. PCR was carried out at 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and then 72°C for 32 s. All samples were amplified in triplicate and each experiment was performed three times to achieve reproducibility. The mean value of the replicates was expressed as the threshold cycle (CT). The fold change was determined by the 2-ΔΔCT method[24].
Western Blot Analysis
The treated cells were lysed and the protein concentrations were determined by a BCA protein assay (Pierce, Rockford, IL, USA). The proteins 30 μg were separated by 12% SDS-PAGE, and then transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk for 2 h at room temperature, and then overnight at 4°C with the primary antibodies. The next day the membranes were incubated with the secondary antibody for 1 h. An ECL Detection Kit (Millipore, Billerica, MA, USA) provided the chemiluminescence substrate for horseradish peroxidase (HRP), and the targeted proteins were visualized by autoradiography. Optical density was measured by using the software ImageJ 1.43 (National Institutes of Health, MD). Relative intensity of STS and CYP19 proteins were calculated.
Statistical Analysis
Results were expressed as x̄±s. Statistical significant differences between groups in MTT assay were analyzed by one-way ANOVA. For real-time PCR and western blot results, a two-tailed independent-samples t-test was used to compare data from two groups. All data were processed with statistical analysis software SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). For this research, statistical significant level was P<0.05.
Results
Inhibitory Effects of SGLXD on MCF-7 and T47D Cells
The cell viability rates of MCF-7 and T47D were significantly inhibited by SGLXD in a dose-dependent manner, compared with negative control (P<0.05). The cell viability rates were 86.85%, 76.35%, 64.89%, 57.38%, 31.57% in MCF-7 cells with 10, 20, 30, 40, 50 mg/ml SGLXD treatment, while 95.35%, 93.03%, 84.48%, 61.82%, 44.46% in T47D cells with 5, 10, 15, 20, 25 mg/ml SGLXD treatment (Figure 1). IC50 values were 40.07 mg/ml for MCF-7 cells and 25.62 mg/ml for T47D cells, respectively.
Figure 1.
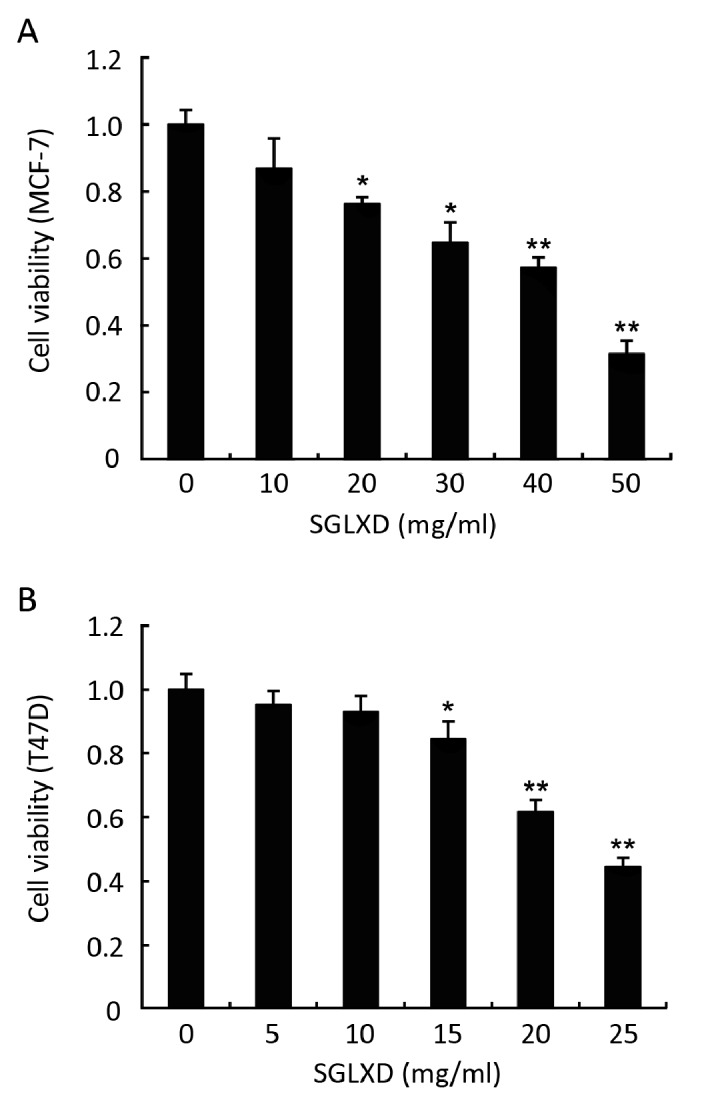
Inhibitory effects of various concentrations of SGLXD on cell proliferation of MCF-7 (A) and T47D (B) by MTT assay. MTT assays were performed after culturing cells in the presence of various concentrations of SGLXD for 24 h. Data of one representative experiment are expressed as a percentage of cell viability relative to negative control group that is normalized to 100%. Data are shown as x̄±s. *P<0.05; **P<0.001 compared with negative control group.
SGLXD Down-regulates mRNA Expressions of CYP19 and STS
The effects of SGLXD on the mRNA expressions of CYP19 and STS were evaluated by relative quantitative real-time RT-PCR. SGLXD-treated MCF-7 cells showed a concentration-dependent decrease in CYP19 and STS mRNA expressions when compared with the negative control. At 10 mg/ml, SGLXD decreased the mRNA expressions of CYP19 and STS in MCF-7 cells by 11.18% and 26.08%, while at 40 mg/ml, the levels of CYP19 and STS mRNA were decreased by 82.91% and 89.05%, respectively (Figure 2A). However, in T47D cells, the levels of CYP19 and STS mRNA were increased by 5, 15 mg/ml SGLXD at first, but when the concentration reached 25 mg/ml, a sudden drop was presented in the mRNA expression, decreased by 21.53% for CYP19 and 98.73% for STS, respectively (Figure 2B).
Figure 2.
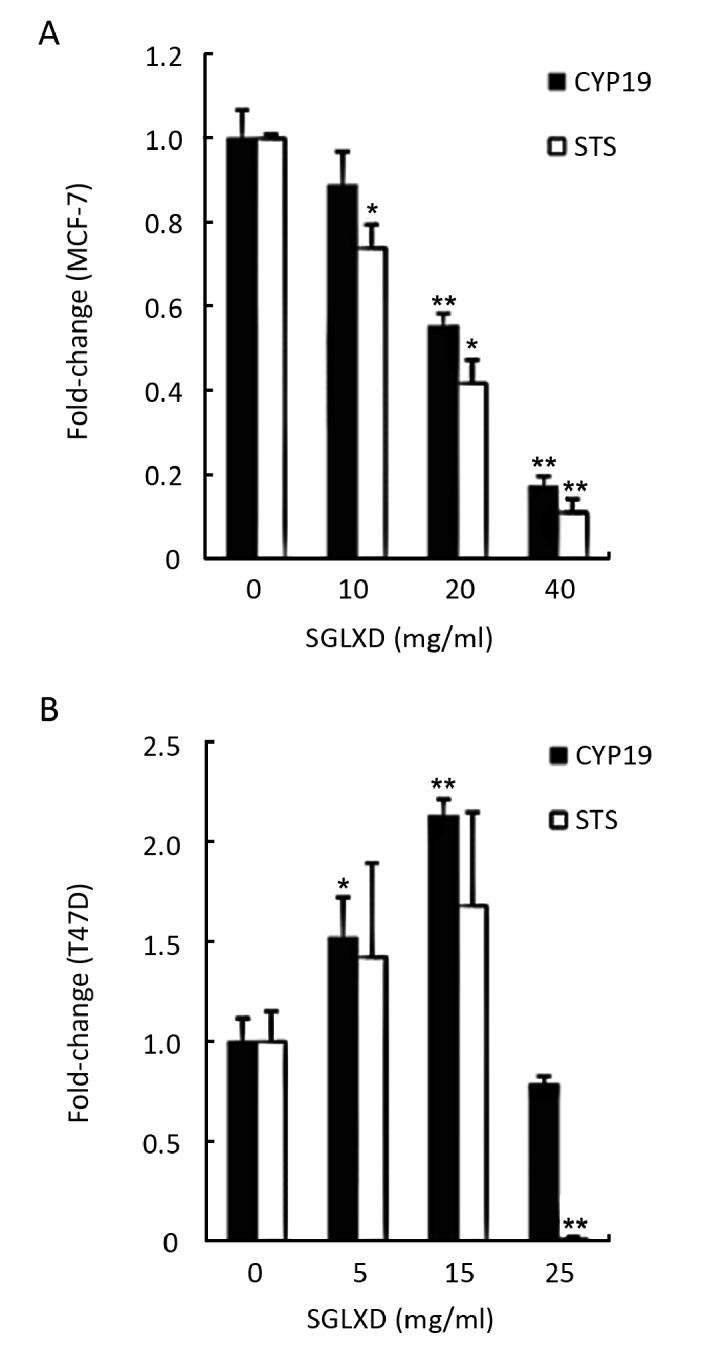
Fold-changes of CYP19 mRNA and STS mRNA in MCF-7 (A) and T47D (B) cells after treated with various concentrations of SGLXD (10, 20, 40 mg/ml for MCF-7 and 5, 15, 25 mg/ml for T47D) for 24h, measured by real-time PCR and calculated by 2-ΔΔCT method. Data were shown as x̄±s. *P<0.05; **P<0.001 compared with negative control group.
Inhibitory Effects of SGLXD on CYP19 and STS Proteins
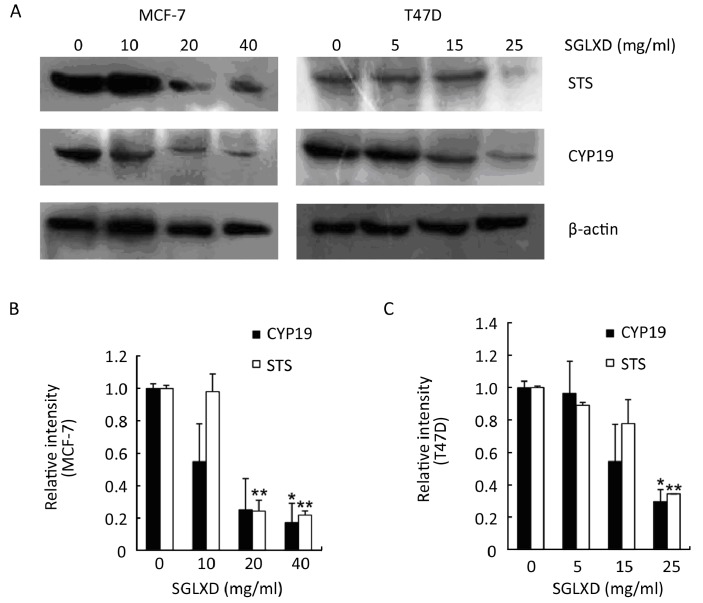
The protein expressions of CYP19 and STS in MCF-7 and T47D cells were inhibited by SGLXD in a dose- dependent manner. There were visible changes of the protein expressions of CYP19 and STS in MCF-7 cells treated with 20 mg/ml SGLXD, and a further decline was observed at 40 mg/ml (Figure 3A). The protein expressions of CYP19 and STS were declined by 45.12% and 2.05% at 10 mg/ml group, and when the concentration reached 40 mg/ml, they were declined by 82.77% and 78.28%, respectively (Figure 3B). In T47D cells, the expression of CYP19 was significantly suppressed by SGLXD in a concentration-dependent manner, while the expression of STS was not changed significantly at lower doses, but there was a sudden decline at 25 mg/ml (Figure 3A). The protein expressions of CYP19 and STS were declined by 4.47% and 8.05% at 5 mg/ml group, and they were declined by 70.44% and 65.63% at 25 mg/ml, respectively (Figure 3C). In the two cell lines, β-actin was not altered significantly after treated with various concentrations of SGLXD (Figure 3A).
Figure 3.
Images of protein expressions of STS, CYP19 and β-actin in MCF-7 and T47D cells (A) which were treated with different concentrations of SGLXD for 24h. Relative intensity of CYP19 and STS proteins in MCF-7 (B) and T47D (C) cells is presented, negative control group is considered as 1. Data are shown as x̄±s. *P<0.05; **P<0.001 compared with negative control group.
DISCUSSION
In breast tumor, inactive steroids in plasma could be locally converted to the bioactive estrogens[25]. Compared with younger premenopausal women, postmenopausal women are more prone to develop breast cancer, which suggesting that local estradiol production in breast tissue plays a critical role in the proliferation of breast cancer cells[25,26]. In peripheral tissues, estradiol is synthesized mainly through the aromatase or the steroid sulfatase pathway[9,11]. Our previous experiment confirmed that SGLXD dose-dependently inhibited the mRNA level and enzymatic activity of STS in MCF-7 cells[22]. However, there are some uncertain questions deserve further research. Such as, could SGLXD affect the protein expression of STS? Or whether SGLXD inhibits the expression of CYP19? Does SGLXD act in other cell lines as same as it in MCF-7 cells? Based on these questions, we performed the present study, and the results were as following: (I) the cell viability of MCF-7 and T47D were significantly inhibited by SGLXD in a dose-dependent manner. (II) SGLXD markedly down- regulated the mRNA and protein expression of CYP19 in MCF-7 and T47D cells at higher concentrations. (III) The STS mRNA and protein in MCF-7 and T47D cells were significantly decreased by higher concentrations of SGLXD.
Aromatase is a particularly striking drug target in the treatment of hormone-dependent breast tumors, and its activity in or near the tumor tissue is greater than that of the normal breast tissue[27-29]. Reports demonstrated that aromatase inhibitors (AIs) are an effective new class of agents and show greater benefit than anti-estrogens[30]. In our study, the cell viability rates of MCF-7 and T47D were significantly inhibited by SGLXD in a dose-dependent manner (Figure 1). Meanwhile, we found that SGLXD down-regulated the expression of CYP19 mRNA in MCF-7 cells dose-dependently (Figure 2A). However, in T47D cells, the expression of CYP19 mRNA was increased at 5 and 15 mg/ml and then decreased significantly at 25 mg/ml (Figure 2B). Although the effect of SGLXD on CYP19 mRNA was different with the lower concentration treatments, both of the expressions of CYP19 mRNA in these two cell lines were inhibited by SGLXD at higher concentrations. The western blot results also showed that the protein expression of CYP19 was significantly inhibited by SGLXD in MCF-7 and T47D cells dose-dependently (Figure 3). The results suggested that SGLXD can inhibit the growth of estrogen receptor positive breast cancer cells through down- regulating the expression of CYP19.
The STS pathway is another major source of estradiol in breast cancer tissue and the activity of STS is increased in breast tumors, suggesting that STS inhibitors are potential therapeutic agents for the treatment of breast cancer[31, 32]. It is expected that STS inhibitors could block the local synthesis of estradiol, and then decrease the active steroid levels[3]. Many potential STS inhibitors were investigated in vitro, such as 2-difluoromethyloestrone 3-O-sulphamate[33] and BENZOMATE[34]. However, none of them have been approved for clinical use due to the trials being under the way. Our findings demonstrated that SGLXD decreased the mRNA and protein expressions of STS in MCF-7 cells dose- dependently (Figure 2 and 3). In T47D cells, the effect of SGLXD on the mRNA expression of STS likes that of CYP19, increased at lower concentrations, and then decreased at 25 mg/ml (Figure 2B). The protein level of STS was without obvious change at lower concentrations (5, 15 mg/ml), but it was significantly inhibited by 25 mg/ml SGLXD (Figure 3C). Our findings showed that SGLXD down-regulated the mRNA and protein expressions of STS, and the inhibition effect was correlated with the concentration of SGLXD.
Our previous experiment demonstrated the anti-tumor effect of SGLXD on human breast cancer bearing nude mice, and the effect of serum estradiol level reduction[35]. The present study showed that SGLXD inhibited the expressions of CYP19 and STS in transcript and protein level at higher concentrations, suggesting that may be the possible anti-tumor mechanisms of SGLXD. Next, we will confirm the inhibition effect of SGLXD on the expressions of CYP19 and STS on xenograft mouse models.
Although it is well established about the role of aromatase inhibitors in the treatment of hormone- dependent breast cancer, the side effects such as bone loss and abnormal lipid metabolism more or less restrain them from serving as long-term medicament[26,36]. Unlike aromatase inhibitors, SGLXD did not manifest any side effects, moreover, it could increase the osteoblast cell viability (unpublished data). In addition, SGLXD and each of the component herbs were without estrogenic activity as demonstrated by dual-luciferase reporter assay[21], suggesting SGLXD is secure for hormone-dependent breast cancer.
Some reports showed that STS inhibitor combined with aromatase inhibitor enhanced the response of hormone- dependent breast cancer to the hormonal therapy[33,37]. A series of dual aromatase–sulfatase inhibitors (DASIs) were developed based on the aromatase inhibitor YM511, in order to explore the potential advantage of dual inhibition by a single agent[38]. Our findings revealed that SGLXD simultaneously inhibited the expressions of CYP19 and STS in estrogen receptor positive breast cancer MCF-7 and T47D cells at higher concentrations, providing new evidence for the treatment of hormone-dependent breast cancer.
In conclusion, SGLXD inhibited the cell viability of MCF-7 and T47D in a dose-dependent manner and significantly down-regulated the mRNA and protein expressions of CYP19 and STS in MCF-7 and T47D cells at high concentrations. Therefore, it provides new evidence for traditional Chinese medicine formula SGLXD on the treatment of hormone-dependent breast cancer.
REFERENCES
- 1.Kwong A, Cheung PS, Wong AY, et al. The acceptance and feasibility of breast cancer screening in the East. Breast 2008; 17:42-50 [DOI] [PubMed] [Google Scholar]
- 2.Tang NL, Choy KW, Pang CP, et al. Prevalence of breast cancer predisposition gene mutations in Chinese women and guidelines for genetic testing. Clin Chim Acta 2001; 313:179-85 [DOI] [PubMed] [Google Scholar]
- 3.Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol 2003; 86:501-7 [DOI] [PubMed] [Google Scholar]
- 4.Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol 2002; 80:203-12 [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Itoh T, Wu K, et al. Transcriptional regulation of aromatase expression in human breast tissue. J Steroid Biochem Mol Biol 2002; 83:93-9 [DOI] [PubMed] [Google Scholar]
- 6.Ishida H, Sato N, Hosogi J, et al. Inactivation of recombinant human steroid sulfatase by KW-2581. J Steroid Biochem Mol Biol 2008; 108: 17-22 [DOI] [PubMed] [Google Scholar]
- 7.Chan MY, Huang H, Leung LK. 2, 3, 7, 8-Tetrachlorodibenzo-para-dioxin increases aromatase (CYP19) mRNA stability in MCF-7 cells. Mol Cell Endocrinol 2010; 317:8-13 [DOI] [PubMed] [Google Scholar]
- 8.Jansson A.17Beta-hydroxysteroid dehydrogenase enzymes and breast cancer. J Steroid Biochem Mol Biol 2009; 114:64-7 [DOI] [PubMed] [Google Scholar]
- 9.Shields-Botella J, Chetrite G, Meschi S, et al. Effect of nomegestrol acetate on estrogen biosynthesis and transformation in MCF-7 and T47-D breast cancer cells. J Steroid Biochem Mol Biol 2005; 93:1-13 [DOI] [PubMed] [Google Scholar]
- 10.Geisler J.Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol 2003; 86:245-53 [DOI] [PubMed] [Google Scholar]
- 11.Smuc T, Rizner TL. Expression of 17beta-hydroxysteroid dehydro- genases and other estrogen-metabolizing enzymes in different cancer cell lines. Chem Biol Interact 2009; 178:228-33 [DOI] [PubMed] [Google Scholar]
- 12.Nakata T, Takashima S, Shiotsu Y, et al. Role of steroid sulfatase in local formation of estrogen in post-menopausal breast cancer patients. J Steroid Biochem Mol Biol 2003; 86:455-60 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ye L, Leung LK. A positive feedback pathway of estrogen biosynthesis in breast cancer cells is contained by resveratrol. Toxicology 2008; 248:130-5 [DOI] [PubMed] [Google Scholar]
- 14.Thyagarajan B, Brott M, Mink P, et al. CYP1B1 and CYP19 gene polymorphisms and breast cancer incidence: no association in the ARIC study. Cancer Lett 2004; 207:183-9 [DOI] [PubMed] [Google Scholar]
- 15.Kagawa N, Hori H, Waterman MR, et al. Characterization of stable human aromatase expressed in E. coli. Steroids 2004; 69:235-43 [DOI] [PubMed] [Google Scholar]
- 16.Billich A, Nussbaumer P, Lehr P.Stimulation of MCF-7 breast cancer cell proliferation by estrone sulfate and dehydroepiandrosterone sulfate: inhibition by novel non-steroidal steroid sulfatase inhibitors. J Steroid Biochem Mol Biol 2000; 73:225-35 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Miki Y, Nakata T, et al. Steroid sulfatase and estrogen sulfotransferase in normal human tissue and breast carcinoma. J Steroid Biochem Mol Biol 2003; 86(3-5):449-54 [DOI] [PubMed] [Google Scholar]
- 18.Stengel C, Newman SP, Day JM, et al. Effects of mutations and glycosylations on STS activity: a site-directed mutagenesis study. Mol Cell Endocrinol 2008; 283(1-2):76-82 [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Xue D, Gao F.Effect of shugan liangxue compound for relieving hot flashes in breast cancer patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009; 29:30-3 [PubMed] [Google Scholar]
- 20.Li PP. Effect of traditional Chinese medicine in improving hot flashes symptom of breast cancer. Chin J Integrat Med 2004; 10:166-7 [Google Scholar]
- 21.Zhang Y, Li PP. Evaluation of estrogenic potential of Shu-Gan-Liang-Xue Decoction by dual-luciferase reporter based bioluminescent measurements in vitro. J Ethnopharmacol 2009; 126:345-9 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Li PP. Shu-Gan-Liang-Xue Decoction, a Chinese herbal formula, down-regulates the expression of steroid sulfatase genes in human breast carcinoma MCF-7 cells. J Ethnopharmacol 2010; 127:620-4 [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2):55-63 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods 2001; 25:402-8 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Moriya T, Ishida T, et al. Intracrine mechanism of estrogen synthesis in breast cancer. Biomed Pharmacother 2003; 57:460-2 [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Reierstad S, Lu M, et al. Regulation of breast cancer-associated aromatase promoters. Cancer Lett 2009; 273:15-27 [DOI] [PubMed] [Google Scholar]
- 27.Knower KC, To SQ, Simpson ER, et al. Epigenetic mechanisms regulating CYP19 transcription in human breast adipose fibroblasts. Mol Cell Endocrinol 2010; 321:123-30 [DOI] [PubMed] [Google Scholar]
- 28.Brueggemeier RW, Su B, Darby MV, et al. Selective regulation of aromatase expression for drug discovery. J Steroid Biochem Mol Biol 2010; 118(4-5):207-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggiolini M, Bonofiglio D, Pezzi V, et al. Aromatase overexpression enhances the stimulatory effects of adrenal androgens on MCF7 breast cancer cells. Mol Cell Endocrinol 2002; 193(1-2):13-8 [DOI] [PubMed] [Google Scholar]
- 30.Brodie A, Njar V, Macedo LF, et al. The Coffey Lecture: steroidogenic enzyme inhibitors and hormone dependent cancer. Urol Oncol 2009; 27:53-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiner EP, Wolff B, Winiski AP, et al. 6-(2-adamantan-2-ylidene- hydroxybenzoxazole)-O-sulfamate: a potent non-steroidal irreversible inhibitor of human steroid sulfatase. Bioorg Med Chem Lett 2003; 13: 4313-6 [DOI] [PubMed] [Google Scholar]
- 32.Billich A, Meingassner JG, Nussbaumer P, et al. 6-[2-(adamantylidene)-hydroxybenzoxazole]-O-sulfamate, a steroid sulfatase inhibitor for the treatment of androgen- and estrogen-dependent diseases. J Steroid Biochem Mol Biol 2004; 92(1-2):29-37 [DOI] [PubMed] [Google Scholar]
- 33.Reed JE, Woo LW, Robinson JJ, et al. 2-difluoromethyloestrone 3-O- sulphamate, a highly potent steroid sulphatase inhibitor. Biochem Biophys Res Commun 2004; 317:169-75 [DOI] [PubMed] [Google Scholar]
- 34.Hejaz HA, Woo LW, Purohit A, et al. Synthesis, in vitro and in vivo activity of benzophenone-based inhibitors of steroid sulfatase [J] Bioorg Med Chem 2004; 12:2759-72 [DOI] [PubMed] [Google Scholar]
- 35.Wu CX, Li PP. Anti-tumor Effect of Shu-Gan-Liang-Xue Decoction Combined with Tamoxifen on Estrogen-dependent Breast Cancer. Chin J Exp Trad Med Formul (in Chinese)2008; 14:31-3 [Google Scholar]
- 36.Castellano S, Stefancich G, Ragno R, et al. CYP19 (aromatase): exploring the scaffold flexibility for novel selective inhibitors. Bioorg Med Chem 2008; 16:8349-58 [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Mickens J, Gao M, et al. Design and synthesis of carbon-11-labeled dual aromatase-steroid sulfatase inhibitors as new potential PET agents for imaging of aromatase and steroid sulfatase expression in breast cancer. Steroids 2009; 74:896-905 [DOI] [PubMed] [Google Scholar]
- 38.Wood PM, Woo LW, Humphreys A, et al. A letrozole-based dual aromatase-sulphatase inhibitor with in vivo activity. J Steroid Biochem Mol Biol 2005; 94(1-3):123-30 [DOI] [PubMed] [Google Scholar]