Abstract
Objective
Src is a protein tyrosine kinase that plays important roles in cancer development, and Src kinase activity has been found to be elevated in several types of cancers. However, the cause of the elevation of Src kinase activity in the majority of human colon carcinomas is still largely unknown. We aim at finding the cause of elevated Src kinase activity in human colon carcinomas.
Methods
We employed normal colon epithelial FHC cells and examined Src activation in human colon carcinoma specimens from 8 patients. Protein expression levels were determined by Western blotting, and the activity of Src kinase by kinase assay.
Results
Actin levels were different between tumor and normal tissues, demonstrating the complexities and inhomogeneities of the tissue samples. Src kinase activities were increased in the majority of the colon carcinomas as compared with normal colon epithelial cells (range 13-29). Src protein levels were reduced in the colon carcinomas. Src Y530 phosphorylation levels were reduced to a higher extent than protein levels in the carcinomas.
Conclusion
The results suggest that Src specific activities were highly increased in human colon carcinomas; phosphorylation at Src Y530 was reduced, contributing to the highly elevated Src specific activity and Src kinase activity.
Key words: Src, Kinase activation, Dephosphorylation, Colon carcinoma
INTRODUCTION
Src is a protein tyrosine kinase that plays important roles in cancer development, and Src kinase activity has been found to be elevated in several types of cancers[1]. In many human colon carcinoma cell lines and tissues, elevated Src kinase activity has been reported[2-4], which also contributes to the tumorigenicity of colon cancer cells[5, 6]. In a small subset of advanced human colon cancer specimens, this elevation of Src activity has been linked to a Src truncating mutation[7]. However, the cause of the elevation of Src kinase activity in the majority of human colon carcinomas is still largely unknown.
Src kinase activity can be regulated both transcriptionally and posttranslationally, by mechanisms including myristoylation[8], phosphorylation at several sites, and allosterically[9]. Recently, we have observed dephosphorylation at Y530 of Src in several colon cancer cell lines[10].
In the current study, we further explored the mechanism of regulation of Src activity in human colon carcinomas. We report herein that Src kinase activity was elevated up to 29 fold in the majority of colon carcinoma specimens; molecular phosphorylation at Y530 of Src was reduced. This suggests that Src was activated in human colon carcinomas via changing its Y530 phosphorylation levels.
MATERIALS AND METHODS
Human colon carcinoma and adjacent normal colon tissues from 8 patients were obtained from Alberta Research Tumor Bank and the quality of cancers was further verified by histopathological examination of the samples. Primary human colon epithelial cells FHC and CCD 841 CoN (ATCC) were cultured in complete growth medium according to ATCC.
Colon tissues and cells were rinsed with PBS and homogenized in RIPA lysis buffer (supplemented with phosphatase and protease inhibitors[10]) on ice (using a Dounce homogenizer for tissues) and clarified by centrifugation. Proteins were quantified using Bradford assay. Alternatively, protein SDS-PAGE gels were stained with Coomassie blue.
PVDF membranes with proteins transferred from SDS-PAGE gels were incubated with the primary antibodies. Anti-Src antibody MAb2-17 was from Quality Biotechnology. Phospho-Src (Y530) antibody was from Cell Signaling. Anti-tubulin (Ab-1) was from Oncogene Sciences. Following incubation with an anti-mouse or anti-rabbit secondary antibody conjugated to horseradish peroxidase, the blots were visualized using ECL reagents from Amersham.
For Src kinase activity, 200 g of cell lysates were incubated with Src antibody and the immune complexes were assayed using Src optimal peptide, as described previously[10].
For statistical analysis, data were presented as x̄±s.
RESULTS AND DISCUSSION
Inhomogeneity in Human Colon Tissues
Previously, Src kinase activity has been reported to be elevated in the majority of colon tumor specimens examined. These results were typically obtained by measuring the ability of Src to autophosphorylate or to phosphorylate enolase or other substrates in normalized amounts of tissue homogenates. However, control proteins such as actin have not been used to monitor the quality of the tissues. We measured actin levels in 20 μg lysate protein of tissue extract from cancer (C) and adjacent normal (N) colon tissues from 8 patients. Actin levels were very different between normal and cancer tissues in most patients (Figure 1). The results revealed the inhomogeneities of the tissue samples. This is consistent with the fact that colon carcinoma predominantly consists of epithelial cells, while normal colon tissue consists of multiple cell types that excludes epithelial cells that gave rise to the carcinoma. The data suggested that when cancer and adjacent normal colon tissues were compared in the past regarding Src activation, different types of cells might have been employed for the comparison. Therefore, Src activation in cancers deserves a new evaluation.
Figure 1.
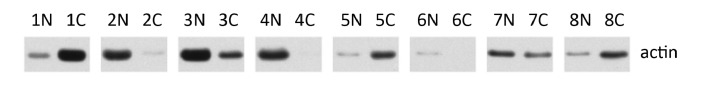
Actin levels in human colon cancer specimens. Protein lysates 20 μg were separated by SDS-PAGE and analyzed by Western blotting with anti-actin antibody. N: Normal colon tissues; C: Colon carcinomas.
Src Activation in Human Colon Carcinomas
To ensure that our comparisons were made to the same type of cells that are present in the tumor material, we used normal colon epithelial primary cells FHC. Src kinase activities (in the same amount of lysate proteins) of 7 of the 8 carcinoma specimens were greatly increased, relative to FHC primary epithelial cells, ranging from 13 to 29 fold (Table 1). We then examined Src protein levels in the above samples (Figure 2A). Src protein levels in two of the carcinoma specimens (C1, C8) were similar to that in FHC, while in the majority of the carcinoma specimens (C2-C7) Src protein levels were decreased. The combined results in Figure 2A and Table 1 indicate that Src specific activity (Src kinase activity per Src molecule) was greatly increased in at least 7 of the carcinoma specimens (unclear for C6). Increased Src specific activity (i.e., Src activation) often leads to more ubiquitin-dependent degradation of Src itself[11]. Therefore the decrease in Src protein levels we have observed in these colon carcinomas may be a result of an increased degradation rate. This Src activation reflects its enhanced phosphorylation efficiency per Src molecule, hence is particularly important when there are a limited number of Src docking sites available at specific subcellular locations for Src to phosphorylate substrates. This agrees with evidence that very often wild-type Src does not induce significant neoplastic transformation when overexpressed in cells, in contrast to activated forms of Src[12-14]. The increase in Src specific activity exceeded 15 fold in most carcinoma specimens, more than that of the transforming Src mutant previously reported[7], suggesting elevated Src specific activity plays an important role in the development of the carcinomas.
Table 1. Src kinase activity in human colon carcinomas.
| Sample | Src kinase activity (fold)* |
|---|---|
| FHC | 1.0 ± 0.1 |
| C1 | 28.5 ± 0.3 |
| C2 | 14.6 ± 0.2 |
| C3 | 17.0 ± 0.6 |
| C4 | 26.8 ± 1.4 |
| C5 | 28.8 ± 2.1 |
| C6 | 0.3 ± 0.0 |
| C7 | 12.5 ± 0.1 |
| C8 | 17.6 ± 0.4 |
* x̄±s, n=2
Figure 2.
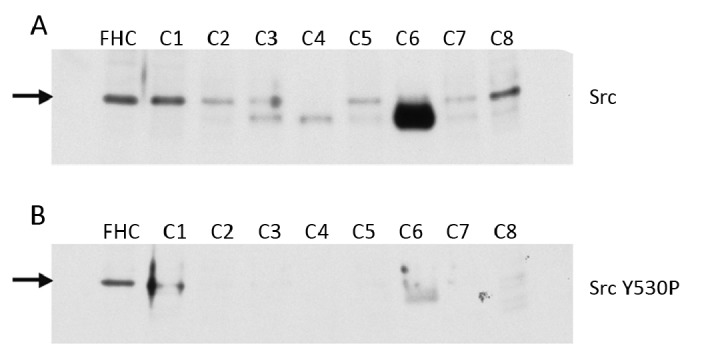
Src in human colon cancer specimens. A: Src protein levels; B: levels of phosphorylation at Src Y530. Protein lysates 20 μg were separated by SDS-PAGE and analyzed by Western blotting with indicated antibodies. C: Colon carcinomas.
Next, we determined the levels of phosphorylation at Src Y530 (Src Y530P) in the samples (Figure 2B). Y530 phosphorylation in the carcinomas was greatly reduced as compared to FHC (Figure 2B). More importantly, in the 7 carcinoma specimens with elevated Src kinase activity (C1-C5, C7-C8), Src Y530P levels were lower than the corresponding Src protein levels in at least 6 of them (unclear for C4) (Figure 2A). This indicated that the phosphorylation at Src Y530 per Src molecule was considerably reduced in these carcinoma specimens. Since Y530 phosphorylation downregualates Src kinase activity, these data suggest that a reduction in the phosphorylation level of Y530 contributed to the elevation in Src specific activity (and Src kinase activity) in most of the colon cancer specimens tested.
Src in Normal Colon Epithelial Cells
CCD 841 CoN, another strain of normal epithelial primary cells of human colon, was also examined in my studies. CCD 841 CoN and FHC have similar Src activity and Src protein level (Figure 3A and 3B), which leads to a similar Src specific activity. Phosphorylation of Src at Y530 was also very similar in both cells (Figure 3B). Therefore, using CCD 841 CoN as a normal cell control for carcinomas would result in similar conclusions regarding Src activation. This result confirmed our analysis, and also suggested that independently-isolated normal colon epithelial cells from different human beings are similar.
Figure 3.
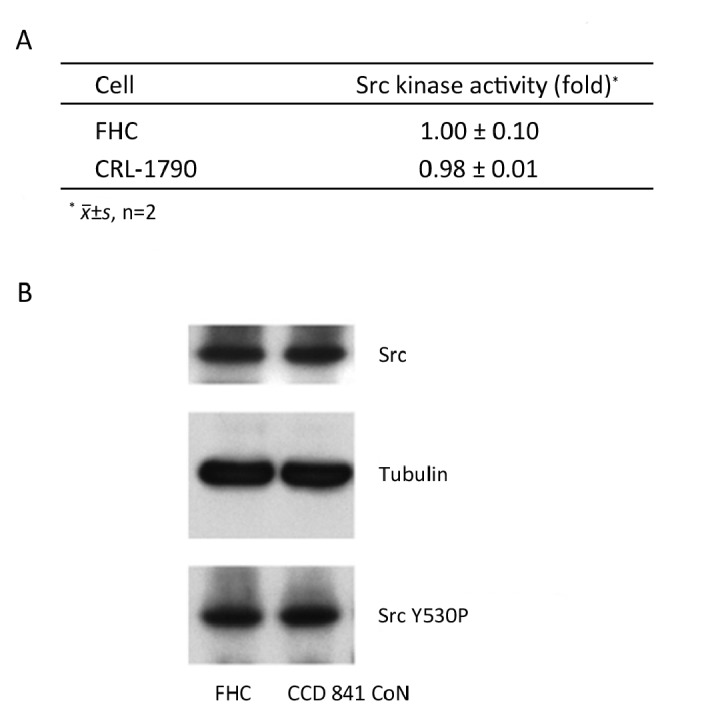
Properties of normal colon epithelial cells. A: Src kinase activity; B: levels of Src and Src Y530 phosphorylation. Src kinase activity was measured in 200 μg of protein lysates immuno- precipitated with an anti-Src antibody. Protein lysates 20 μg were separated by SDS-PAGE and analyzed by Western blotting with indicated antibodies.
In conclusion, Y530 dephosphorylation is likely a general mechanism responsible for activating Src in a high percentage of human colon carcinomas. This is the first general mechanism discovered for Src activation in cancers, and might also be the mechanism in other cancer types besides colon cancer. Drugs targeting Y530 dephosphory- lation are thus potential therapeutic interventions in human colon cancers.
REFERENCES
- 1.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist 2009; 14:667-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolen JB, Veillette A, Schwartz AM, et al. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci USA 1987; 84:2251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright CA, Kamps MP, Meisler AI, et al. pp60c-src activation in human colon carcinoma. J Clin Invest 1989; 83:2025-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talamonti MS, Roh MS, Curley SA, et al. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest 1993; 91:53-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irby R, Mao W, Coppola D, et al. Overexpression of normal c-Src in poorly metastatic human colon cancer cells enhances primary tumor growth but not metastatic potential. Cell Growth Differ 1997; 8: 1287-95 [PubMed] [Google Scholar]
- 6.Staley CA, Parikh NU, Gallick GE. Decreased tumorigenicity of a human colon adenocarcinoma cell line by an antisense expression vector specific for c-Src. Cell Growth Differ 1997; 8:269-74 [PubMed] [Google Scholar]
- 7.Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet 1999; 21:187-90 [DOI] [PubMed] [Google Scholar]
- 8.Patwardhan P, Resh MD. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol Cell Biol 2010; 30: 4094-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene 2000; 19:5620-35 [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res 2007; 67:10129-37 [DOI] [PubMed] [Google Scholar]
- 11.Hakak Y, Martin GS. Ubiquitin-dependent degradation of active Src. Curr Biol 1999; 9:1039-42 [DOI] [PubMed] [Google Scholar]
- 12.Iba H, Takeya T, Cross FR, et al. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci USA 1984; 81:4424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker RC, Varmus HE, Bishop JM. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell 1984; 37:131-9 [DOI] [PubMed] [Google Scholar]
- 14.Shalloway D, Coussens PM, Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A 1984; 81:7071-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


