Abstract
Objective
We supposed that it will be a promising strategy to "prevent" multidrug resistance (MDR) instead of "reversing" it. This study was designed to investigate the potency of curcumin to prevent the acquired drug resistance induced by adriamycin (ADM) in native K562 cells.
Methods
K562 cells were pretreated with curcumin or 0.5% DMSO for 24 h and then were co-incubated with ADM. P-glycoprotein (P-gp) and mdr1 mRNA levels were analyzed separately by flow cytometry and quantitative real-time RT-PCR. The intracellular Rh-123 accumulation was also detected by flow cytometer. Finally, we performed a MTT assay to determine the ADM-induced cytotoxicity with or without pretreatment of curcumin.
Results
P-gp and mdr1 mRNA expressions were elevated in the ADM alone group. While in the curcumin pretreated groups, the induced P-gp and mdr1 mRNA levels gradually decreased with increasing curcumin concentrations, and the Rh-123 accumulation level was almost recovered close to the control group's. Finally, the MTT colorimetric assay verified the enhanced effect of curcumin on ADM-induced cytotoxicity.
Conclusion
Our present study suggested that curcumin exhibits the novel ability to prevent the up-regulation of P-gp and its mRNA induced by ADM. The prevention capacity is also functionally associated with the elevated intracellular drug accumulation and parallel enhanced ADM cytotoxicity. We revealed a novel function of curcumin as a potential drug resistance preventor.
Key words: Curcumin, Drug resistance, P-glycoprotein, K562 cell
INTRODUCTION
Multidrug resistance (MDR) constitutes one of the major obstacles to successful treatment of cancer. Occurrence of acquired MDR was closely related to the up-regulation of the mdr1 mRNA and its encoded protein, P-glycoprotein (P-gp). P-gp is a transmembrane protein and functions as an ATP-dependent drug transporter which pumps intracellular drugs out of the cells[1-3]. To elevate drug accumulation in cancer cells with overexpressed P-gp, this transporter protein was supposed to be suppressed to increase the efficiency of chemoagents. The effective way to reverse cancer drug resistance is inhibiting the transport function and expression of P-gp.
Previously, P-gp inhibitors or chemosensitizing agents have usually been studied as “reverse MDR agents” to down-regulate the expression or inhibit the activity of this drug transporter in an effort to improve the effectiveness of chemotherapy drugs[4-7]. However, it’s really hard to reverse when drug resistance is formed because the cancer cells have already expressed high level of P-gp and accompanied with many up-stream and down-stream activated targets through cell signaling pathways[8,9]. Therefore, it is promising and important to explore the strategy for preventing or blocking the occurrence of acquired MDR ahead of reversing MDR in the present and future studies[10,11].
In our previous studies concerning “MDR reversal”, we have successfully demonstrated that several naturally occurring compounds such as honokiol, schisandrin A and schisandrin B, exhibit the capacity of reversing P-gp associated MDR[7,12]. Moreover, in the recent research of screening naturally occurring compounds with potential efficacies in preventing drug resistance, we found that one kind of phenolic coloring compounds, curcumin, extracted from the rhizomes of Curcuma longa Linn., displayed the potency of preventing P-gp-associated drug resistance. So in the present study, we turned our research strategy from “reversing” to “preventing” drug resistance in native cancer cells prior to the resistance is formed. Our results revealed a novel function of curcumin as a potential drug resistance preventor.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
Human leukemia native cell line K562 was maintained in RPMI 1640 containing 10% fetal bovine serum (FBS). No antibiotics were included in the culture medium. Cells were cultured at 37°C in a 5% CO2 humidified atmosphere.
Reagents
Curcumin was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) with purity of >99%. RPMI 1640 and FBS were purchased from GIBCO-BRL; Rh-123, MTT[3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] and adriamycin (ADM) were purchased from the Sigma Chemical Co.; primer pairs and probe were purchased from Sangon Co. (Shanghai, China); Trizol reagent and RT-PCR kit were purchased from Invitrogene; FITC-conjugated mouse anti-human monoclonal antibody was purchased from BD Bioscienses.
Cell Viability Assay and ADM Cytotoxicity Assay
Cells were seeded into 96-well plates. For the cell viability assay, cells were incubated with serial concen- trations of curcumin for 3 or 4 days. And for the ADM cytotoxicity assay, cells were pretreated with or without 5 μmmol/L curcumin for 24 h, followed by addition of a series concentrations of ADM, and then incubated for 72 h.
After incubation, 20 μl of MTT (5 mg/ml in PBS) was added to each well and the cells were incubated for 4 h. After aspiration of the culture medium, the resulting formazan was dissolved with 150 μl of DMSO. The plates were placed on a shaker, shaken for 5 min and read immediately at 570 nm with a model ELX800 Micro Plate Reader (Bio-Tek Instruments, Inc.).
RT-PCR Determination of mdr1 mRNA
The mdr1 mRNA level was analyzed by quantitative real-time RT-PCR. K562 cells were seeded into 24-well plates at a density of 0.5×105/well. Then the cells were pretreated with 1 μmol/L, 5 μmol/L curcumin or 5 μmol/L cyclosporin A (CsA) (combined group) or 0.5% DMSO (control and ADM alone groups) for 24 h and then were co-incubated with 40 ng/ml ADM for 48 h (combined group and ADM alone group).
Total RNA was isolated from K562 cells by Trizol reagent (Invitrogene) as described by the manufacturer. Total RNA was reverse-transcribed in a reaction mixture containing oligo (dT), deoxynucleoside triphosphate (dNTP), and Moloney murine leukemia virus reverse transcriptase (Promega) as described by the standard methods[13].
The sequence information of primer pairs and TaqMan probe for mdr1 mRNA was described previously[14]. Primers and TaqMan probe for GAPDH were purchased from PE Applied Biosystems (TaqMan GAPDH Control Reagent Kit).
Quantitative real-time PCR was performed in the ABI-prism 7700 sequence detector (PE Applied Biosystems, Foster City, CA). In brief, mdr1 forward primer 5' AGA- AAGCGAAGCAGTGGTTCA 3' and mdr1 reverse primer 5' CGAACTGTAGACAAACGATGAGCTA 3' were used to amplify a 90-bp fragment from the mdr1 cDNA that was detected by the FAM-labeled TaqMan probe 5' TGG- TCCGACCTTTTCTGGCCTTATCCA 3'. The reaction was performed in triplicate for each RT product. Samples were heated for 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C and 1 min at 60°C. The fluorescent signal was determined using Sequence DetectorTM software (PE Applied Biosystems), giving the threshold cycle number (CT) at which PCR amplification reached a significant threshold. Then the △CT value is defined as the difference in CT values for the mdr1 and GAPDH mRNA. Accordingly, △CT = (mdr1 mRNA CT) – (GAPDH mRNA CT). And the relative mdr1 mRNA expression level was presented as 2–△CT. Thus the mRNA expression levels of mdr1 are expressed as concentrations relative to GAPDH mRNA.
Flow Cytometry Analysis of P-gp Expression
For determination of P-gp expression level, K562 cells were seeded into 6-well plates at a density of 2×105/well. K562 cells were pretreated with 1 or 5 μmol/L curcumin or 5 μmol/L cyclosporin A for 24 h and then were co-incubated with 40 ng/ml ADM for 72 h. While in the control and ADM alone groups, K562 cells were only pretreated with 0.5% DMSO for 24 h. Then cells were incubated with FITC-conjugated mouse anti-human monoclonal antibody (1:200 diluted) at 4°C for 1 h, then washed twice with ice-cold PBS and the level of fluorescent staining was analyzed using a Beckman EPICS Flow Cytometry (Coulter Electronics, Hialeah, FL).
Rh-123 Accumulation Assay
In order to analyze the Rh-123 accumulation, K562 cells were firstly seeded into 6-well plates at a density of 2×105/well. Then the cells were pretreated with 1μmol/L or 5 μmol/L curcumin for 24 h and then were co-incubated with 40 ng/ml ADM for another 48 h. While in the control and ADM alone group, K562 cells were only pretreated with 0.5% DMSO for 24 h. After the pretreatment, cells were incubated with 1 μg/ml of Rh-123 in the dark at 37°C in 5% CO2. After 120 min, cells were washed twice with ice-cold PBS. Then the cells were determined quantitatively using a Beckman EPICS Flow Cytometry (Coulter Electronics, Hialeah, FL) equipped with a 488 nm argon laser. The green fluorescence of Rh123 was measured by a 530 nm band-pass filter.
Statistical Analysis
All experiments were repeated at least three times and the values are given as the x±s. Statistical analysis was done with the SPSS 11.0 Statistical Analysis software. The values of mdr1 mRNA, P-gp expression levels and Rh-123 accumulation levels were calculated and compared between curcumin combined groups and ADM alone group, and the differences between means of compared groups were determined using the one-way ANVOA. P<0.05 was considered statistically significant and groups with a common letter are not significantly different.
RESULTS
Effect of Curcumin on the Growth of K562 Cells
The results showed that curcumin did not affect native K562 cell viability at doses ranging from 1 to 5 μmol/L for 3 and 4 days. While at doses ranging from 10 to 30 μmol/L, the cell viability gradually decreased with increasing curcumin concentrations (Figure 1). Since curcumin below 5 μmol/L had virtually no inhibitory effect on K562 cell growth, the concentrations of curcumin for further study were kept below 5 μmol/L.
Figure 1.
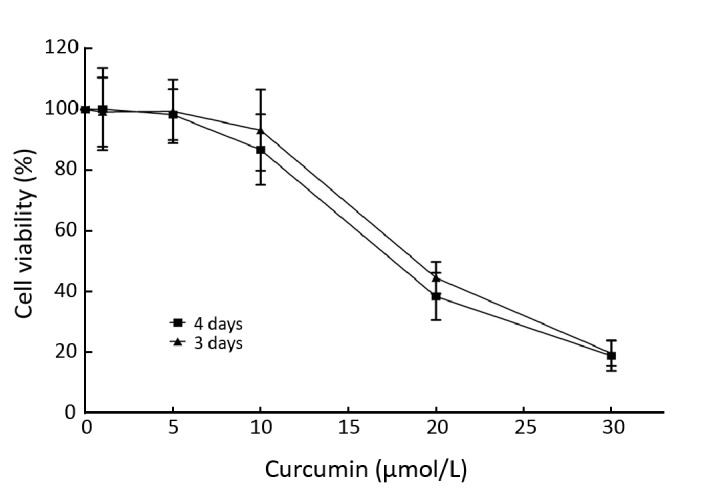
Effect of curcumin on the growth of K562 cells. K562 cells were cultured with curcumin at 1, 5, 10, 20, 30 μmol/L for 3–4 days and the viability of K562 cells was determined by a MTT colorimetric assay. The results are presented as x̄±s from three independent experiments.
Prevention Effect of Curcumin on mdr1 mRNA Expression Induced by ADM in K562 Cells
Real-time RT-PCR was performed to prove if curcumin is able to prevent the induction of mdr1 mRNA caused by exposure to ADM. As shown in Figure 2, mdr1 expression was obviously elevated in the ADM alone group. While in the combined treatment groups, the induced mdr1 expression decreased with increasing curcumin con- centrations. This tendency indicated that curcumin could inhibit ADM-induced mdr1 over-expression in a dose- dependent manner.
Figure 2.
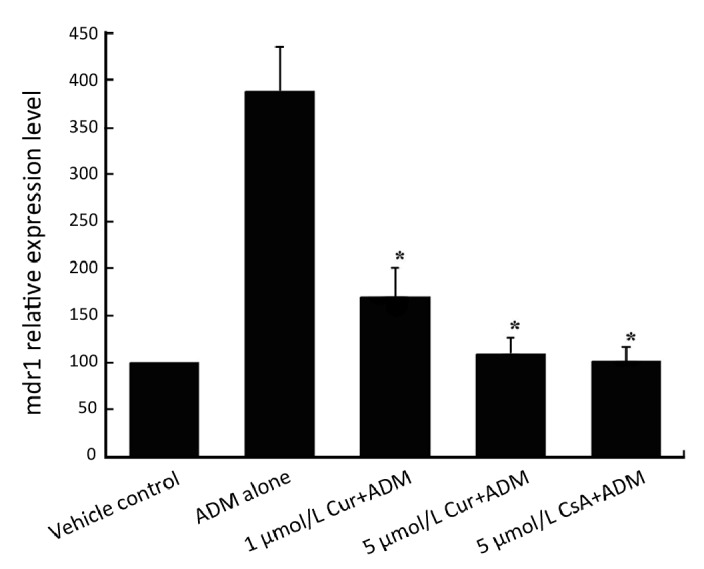
Dose-dependent prevention effect of pretreated curcumin on mdr1 mRNA expression induced by ADM in K562 cells. Cells were pretreated with 1 μmol/L, 5 μmol/L curcumin or 5 μmol/L CsA (combined group) or 0.5% DMSO (control and ADM alone groups) for 24 h and then were co-incubated with 40 ng/ml ADM for 48 h (combined group and ADM alone group). After the above procedures, cells in 5 groups were then determined for mdr1 mRNA expression by RT-PCR. Data are shown as x̄±s of three independent experiments. *Significantly different from the ADM alone group (P<0.05).
After statistical analysis, we found that there were significant statistical differences between the ADM alone group and two curcumin-combined treatment groups (P<0.05), between the ADM alone group and CsA-ombined treatment group (P<0.05). Contrarily, no statistical significance could be seen between 5 μmol/L curcumin- combined treatment group and the control group (P>0.05). Also, no significance could be seen between 5 μmol/L curcumin and 5 μmol/L CsA-combined treatment groups (P>0.05). Taken together, these results showed that curcumin could prevent the elevated mdr1 expression induced by ADM, and the prevention effect of 5 μmol/L curcumin was similar to 5 μmol/L CsA, the positive control in our study.
Prevention Effect of Curcumin on P-gp Expression Induced by ADM
In order to prove whether the prevention effect at mRNA level is correlated to changes in P-gp expression level, FCM analysis for P-gp expression was then performed. As shown in Figure 3A and Figure 3B, just like the previous RT-PCR results, a rapid up-regulation of P-gp expression level was also observed after the exposure of K562 cells to ADM alone. While in the combined treatment groups, 1 or 5 μmol/L curcumin exhibited the capacity of partly preventing the ADM-induced P-gp expression, and the prevention effect of 5 μmol/L curcumin was similar to the same concentration of CsA. Similar to previous RT-PCR results, the prevention capacity of curcumin was also dose- dependent.
Figure 3.
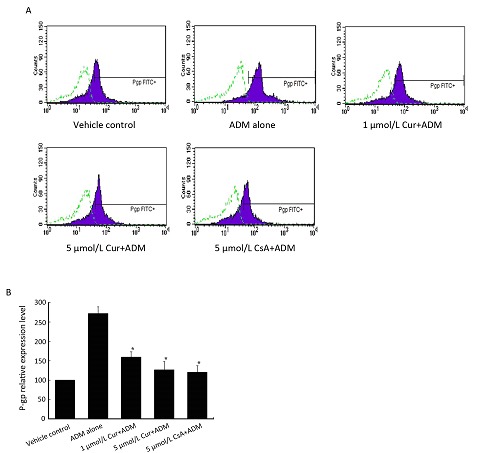
Dose-dependent prevention effect of pretreated curcumin on P-gp expression induced by ADM. K562 cells were pretreated with 1 μmol/L, 5 μmol/L curcumin or 5 μmol/L CsA (combined group) or 0.5% DMSO (control and ADM alone groups) for 24 h and then were co-incubated with 40 ng/ml ADM for 72 h (combined group and ADM alone group). A: P-gp expression levels in 5 groups were determined by flow cytometry and the non-specific fluorescent labeling was corrected by the isotype control. The x-axis represents the fluorescent intensity associated with P-gp; dotted line, isotype control; solid line, FITC-conjugated mouse anti-human monoclonal antibody against P-gp. B: Summary of the above flow cytometry results. Data are shown as x̄±s of three independent experiments. *Significantly different from the ADM alone group (P<0.05).
Further statistical analysis showed a significant difference between the ADM alone group and two curcumin-combined treatment groups (P<0.05). And no statistical significance could be seen between 5 μmol/L curcumin-combined treatment group and the control group (P>0.05). Also, no significance could be seen between 5 μmol/L curcumin and 5 μmol/L CsA-combined treatment group (P>0.05). These results indicated that short-time exposure of native K562 cells to ADM resulted in not only elevated mdr1 expression (Figure 2) but also increased P-gp expression (Figure 3), while curcumin could obviously block these processes in a dose-dependent manner.
Effect of Curcumin on Intracellular Rh-123 Accumulation in K562 Cells
Rh-123 is a substrate of P-gp and can be used as an indicator for testing the P-gp functional activities. After incubation with ADM with or without the pretreatment of curcumin, cells were then further incubated with Rh-123 for 120 min, and the intracellular fluorescence intensity was monitored by a flow cytometer.
As shown in Figure 4, intracellular Rh-123 fluorescence was reduced in native K562 cells treated with ADM alone perhaps due to the up-regulation of P-gp pump, but was significantly induced (increasing 60.7%; P<0.05 versus cells treated with ADM alone) by combined treatment with 5 μmol/L curcumin. These results indicated that ADM alone treatment reduced intracellular drug accumulation and resulted in induced cell chemoresistance, while combined curcumin pre-treatment recovered drug accumulation level close to the control group’s and parallel restored cell sensitivity to drug.
Figure 4.
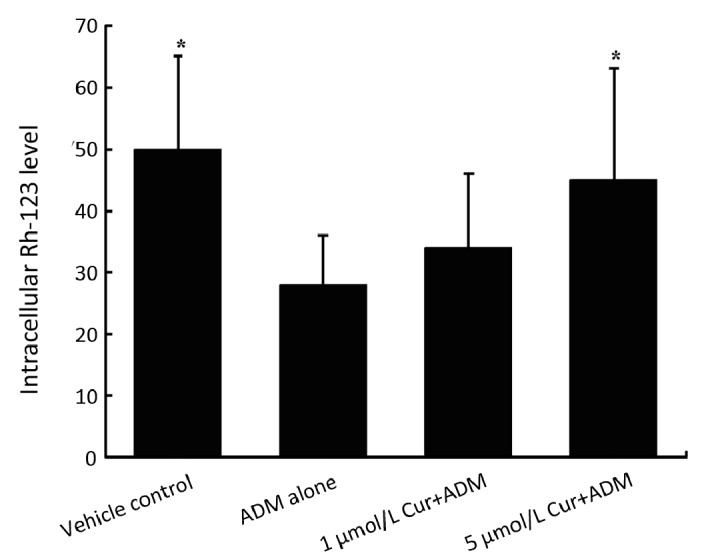
Effect of curcumin on intracellular Rh-123 accumulation in K562 cells. Cells were pretreated with 1 μmol/L or 5 μmol/L curcumin (combined group) or 0.5% DMSO (control and ADM alone groups) for 24 h and then were co-incubated with 40 ng/ml ADM for another 48 h (combined group and ADM alone group). After the above treatments, cells in 3 groups were then tested for a 120-min intracellular Rh-123 accumulation as described in methods and materials. Data are shown as x̄±s of three independent experiments. *Significantly different from the ADM alone group (P<0.05).
Effect of Curcumin on the Sensitivities of Native K562 Cells Towards ADM
The data showed above suggested that curcumin is capable to prevent the up-regulation of P-gp and its mRNA induced by ADM. The prevention capacity is also functionally associated with the increased intracellular drug accumulation. In order to verify the in vitro effect of curcumin on ADM-induced cytotoxicity, we performed a MTT colorimetric assay to determine the ADM-induced toxicity toward K562 cells with or without pretreatment of curcumin.
As shown in Figure 5, pretreatment of K562 cells with curcumin markedly increased the sensitivity of native cancer cells toward ADM. In detail, the calculated IC50 of ADM for K562 cells with pretreatment of curcumin was 61.8 ng/ml, decreased almost 1/3 compared with that (88.7 ng/ml) for K562 without pretreatment of curcumin. Taken the above RT-PCR and FCM results together, we supposed that the capacity of curcumin to prevent the up-regulation of P-gp and its mRNA induced by ADM is responsible for the enhanced ADM toxicity toward K562 cells.
Figure 5.
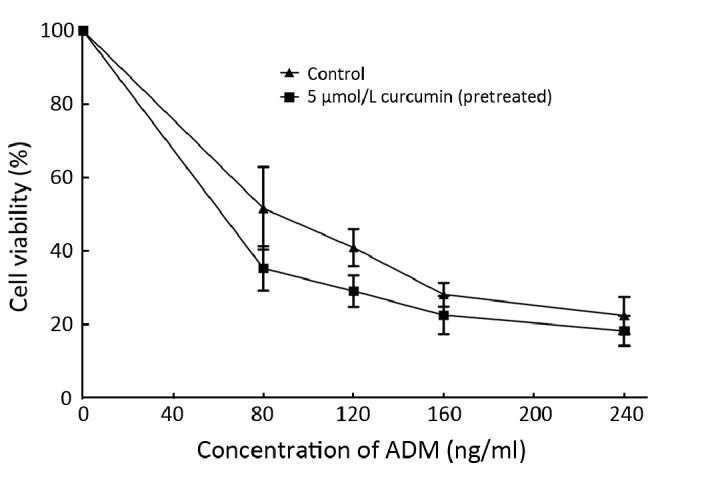
Effect of curcumin on the sensitivities of native K562 cells toward ADM. Cells were pretreated with or without 5 μmol/L curcumin for 24 h, followed by addition of a series concentrations of ADM, and then incubated for 72 h. The viability of K562 cells was determined by a MTT colorimetric assay. Data are shown as x̄±s of three independent experiments.
DISCUSSION
Occurrence of acquired MDR was closely related to the up-regulation of mdr1 mRNA and its encoded protein, P-gp. The effective way to reverse MDR is inhibiting the transport function and expression of P-gp. At present, most anti-cancer MDR research only studied the drug-resistant cancer cells and targeted the “reversing effect” of some new promising chemicals[15]. Many compounds have been previously reported the potency to down-regulate P-gp expression in MDR cancer cells, including the immuno- suppressor such as CsA and PSC 833[4], the calcium channel blockers such as verapamil[5], and some naturally occurring compounds such as curcumin and tetrandrine[6, 16].
But once drug resistance is formed, it’s really hard to reverse because the cancer cells have already expressed high level of P-gp[17] and accompanied with many up-stream and down-stream activated targets through cell signaling pathways. Therefore it’s a promising strategy to “prevent” drug-resistance in cancer cells prior to the resistance is formed. Recently, some of the above P-gp down-regulators, such as CsA and tetrandrine, have also been proved the ability to prevent the induction of P-gp expression[11, 18], which is caused by the short-term exposure of native cancer cells to ADM. So in the present study, we turned our research strategy from “reversing” to “preventing” drug resistance in native cancer cells prior to the resistance is formed, and introduced a new member in the class of compounds with activities to prevent the induced drug resistance.
In the present study, we chose K562 cell line as the model for observing drug-resistance preventing effect. Because the K562 cell line expresses very low levels of mdr1 mRNA and P-gp, it appears to be a useful model for studying clinical drug resistance and therefore preventing effect [11, 19]. It was previously reported[18] that CsA and PSC 833 have the capacity to prevent the induction of P-gp expression, which is caused by the short-term exposure of native cancer cells to ADM. So we chose CsA as positive control in this study.
As above demonstrated, a rapid up-regulation of mdr1 mRNA expression level was observed after the exposure of K562 cells to ADM. The up-regulation of mdr1 mRNA expression was associated with an increase in P-gp level and decreased Rh-123 accumulation. Moreover, we found curcumin can prevent the up-regulation of mdr1 mRNA and P-gp expression levels induced by ADM, and the prevention effect of curcumin was dose dependent. Consistently, the functional activity of P-gp was also inhibited, which led to elevated intracellular Rh-123 accumulation and increased sensitivity of native K562 cells toward ADM in curcumin pre-treatment groups. In our study, the drug-resistance preventing effect of curcumin is similar to CsA, but in clinical context, curcumin may be much safer than CsA because it is a naturally occurring edible compound and is low-toxic to normal human cells[20,21]. While contrarily, CsA is restricted in clinical use for its severe side effects such as immunotoxicity and renal toxicity [22].
Curcumin has been discovered to combat the cancer cells apparently through multiple mechanisms[23,24]. Previous studies have demonstrated that curcumin has enormous anticancer and chemosensitization abilities: Giladi, et al.[25] indicated that curcumin potentiates the pro-apoptotic effects of sulindac sulfone in colorectal cancer cells, and Su CC, et al.[26] demonstrated curcumin induced cytotoxicity, cell cycle arrest and apoptosis in human colon cancer cells. Some studies concerning cancer cell metastasis[27,28] showed that curcumin can inhibit invasion and migration of human colon cancer cells and human lung adenocarcinoma cells. Curcumin has also been shown to inhibit tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB (NF-kB) pathway[29]. In addition, investigators also found that curcumin modulated the expression of drug-resistance protein such as P-gp[6,30] and MRP1[31,32]. In a word, curcumin was remembered as a chemosensitization or reversal agent before but not something for a drug resistance preventor.
According to the previous MDR research, many compounds have the ability to reverse MDR. But not all of them have the potency to prevent chemoresistance. We previously reported honokiol, a naturally occurring compound, can reverse MDR by down-regulating P-gp expression[7], but we found it cannot act as a chemoresistance preventor in the further research work (data not shown). Fortunately, our present study proved curcumin, another naturally occurring MDR reversal agent, has a novel function of preventing chemoresistance. This result suggests that curcumin can work as a dual drug resistance inhibitor, not only in MDR cancer cells but also in native cancer cells.
The mechanism underlying the preventing effect of curcumin on drug resistance is still uncertain, but may be correlated to the NF-κB expression level and its DNA- binding activity, through which the expression of downstream gene mdr1 is regulated[11,27]. The exact role of NF-κB pathway and its potential correlation to curcumin in the chemoresistance preventing model are under study in our research group.
In summary, our study reveals a novel function of curcumin as a potential drug resistance preventor. We suppose that in the clinical context, the administration of drug resistance preventors such as curcumin in early course of chemotherapy is possible to prevent the induction of P-gp, which maybe result in MDR.
REFERENCES
- 1.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 1983; 221: 1285-8 [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993; 62:385-427 [DOI] [PubMed] [Google Scholar]
- 3.Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992; 258:1650-4 [DOI] [PubMed] [Google Scholar]
- 4.Egashira M, Kawamata N, Sugimoto K.P-Glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of p-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood 1999; 93:599-606 [PubMed] [Google Scholar]
- 5.Tsuruo T, Iida H, Tsukagoshi S, et al. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 1981; 41:1967-72 [PubMed] [Google Scholar]
- 6.Limtrakul P, Anuchapreeda S, Buddhasukh D.Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 2004; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Lu Q, Hu X.Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett 2006; 243:274-80 [DOI] [PubMed] [Google Scholar]
- 8.Sakaeda T, Nakamura T, Hirai M, et al. MDR1 Up-regulated by apoptotic stimuli suppresses apoptotic signaling. Pharm Res 2002; 19:1323-9 [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 2006; 580:998-1009 [DOI] [PubMed] [Google Scholar]
- 10.Zatelli MC, Luchin A, Tagliati F, et al. Cyclooxygenase-2 inhibitors prevent the development of chemoresistance phenotype in a breast cancer cell line by inhibiting glycoprotein p-170 expression. Endocr Relat Cancer 2007; 14:1029-38 [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Xu W, Chen Q, et al. Tetrandrine prevents acquired drug resistance of K562 cells through inhibition of mdr1 gene transcription. J Cancer Res Clin Oncol 2010; 136:659-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Q, Lu Q, Zhang K, et al. Dibenzocyclooctadiene lingnans: a class of novel inhibitors of P-glycoprotein. Cancer Chemother Pharmacol 2006; 58:99-106 [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory, 1989. [Google Scholar]
- 14.Schiedlmeier B, Kühlcke K, Eckert HG, et al. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood 2000; 95: 1237-48 [PubMed] [Google Scholar]
- 15.Li DQ, Pan LH, Shao ZM. Reversal effects of mifepristone on multidrug resistance(MDR) in drug-resistant breast cancer cell line MCF7/ADR in vitro and in vivo. Chin J Cancer Res 2004; 16:93-8 [Google Scholar]
- 16.Fu L, Liang Y, Deng L, et al. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol 2004; 53:349-56 [DOI] [PubMed] [Google Scholar]
- 17.Fu JX, Wang W, Cen JN, et al. Retroviral mediated efficient transfer and expression of multiple drug resistance gene to human leukemia cells. Chin J Cancer Res 2000; 12:120-4 [Google Scholar]
- 18.Hu XF, Slater A, Wall DM, et al. Cyclosporin A and PSC 833 prevent up-regulation of MDR1 expression by anthracyclines in a human multidrug-resistant cell line. Clin Cancer Res 1996; 2:713-20 [PubMed] [Google Scholar]
- 19.Yague E, Armesilla AL, Harrison G, et al. P-glycoprotein (MDR1) expression in leukemia cells is regulated at two distinct steps, mRNA stabilization and translational initiation. J Biol Chem 2003; 278: 10344-52 [DOI] [PubMed] [Google Scholar]
- 20.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005; 223:181-90 [DOI] [PubMed] [Google Scholar]
- 21.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 2005; 41:1955-68 [DOI] [PubMed] [Google Scholar]
- 22.Richter-Reichhelm HB, Schulte AE. Results of a cyclosporin A ring study. Toxicology 1998; 129:91-4 [DOI] [PubMed] [Google Scholar]
- 23.Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett 2008; 267:133-64 [DOI] [PubMed] [Google Scholar]
- 24.Wilken R, Veena MS, Wang MB, et al. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011; 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giladi N, Kazanov D, Shpitz B, et al. Curcumin potentiates the pro-apoptotic effects of sulindac sulfone in colorectal cancer. Expert Opin Investig Drugs 2010; 19(Suppl 1):S117-24 [DOI] [PubMed] [Google Scholar]
- 26.Su CC, Lin JG, Li TM, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res 2006; 26(6B):4379-89 [PubMed] [Google Scholar]
- 27.Su CC, Chen GW, Lin JG, et al. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B /p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res 2006; 26(2A):1281-8 [PubMed] [Google Scholar]
- 28.Chen HW, Lee JY, Huang JY, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res 2008; 68:7428-38 [DOI] [PubMed] [Google Scholar]
- 29.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res 2007; 13:3423-30 [DOI] [PubMed] [Google Scholar]
- 30.Anuchapreeda S, Leechanachai P, Smith MM, et al. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol 2002; 64: 573-82 [DOI] [PubMed] [Google Scholar]
- 31.Chearwae W, Wu CP, Chu HY, et al. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother Pharmacol 2006; 57: 376-88 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Revalde JL, Reid G, et al. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol 2011; 68: 603-10 [DOI] [PubMed] [Google Scholar]


