Abstract
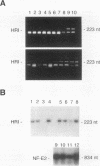
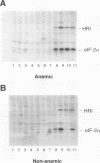
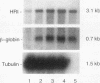
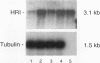
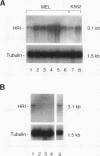
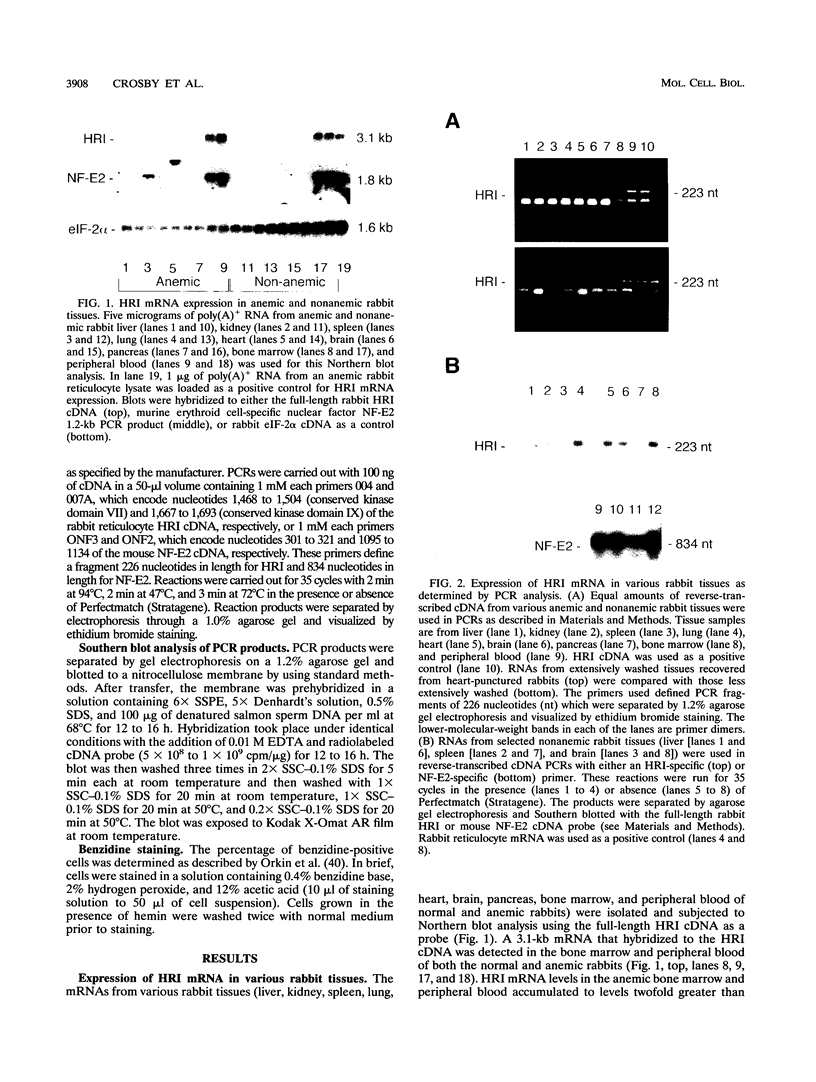
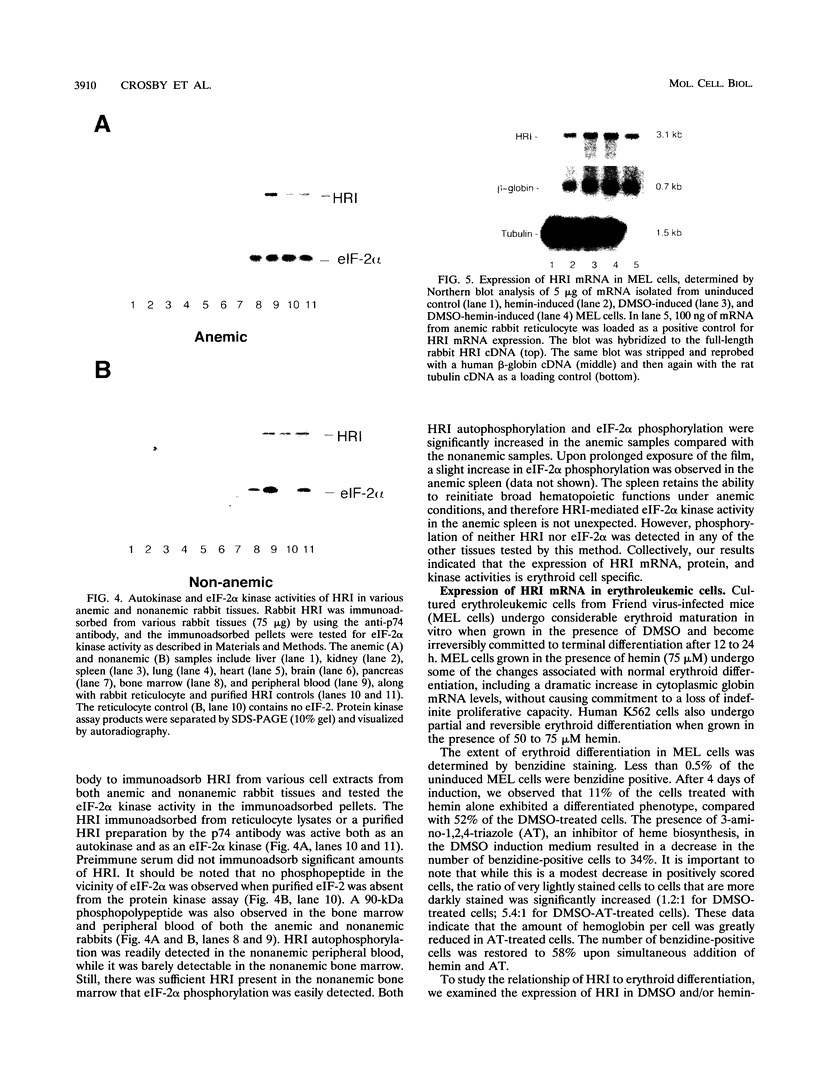
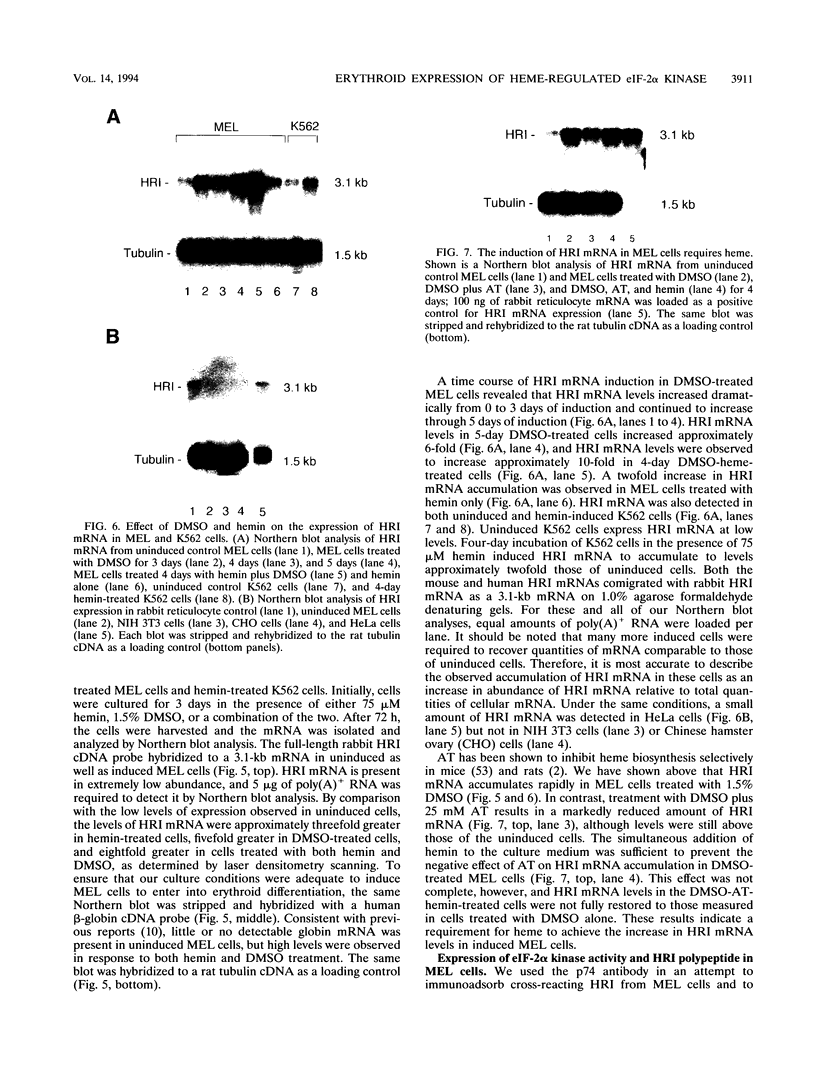
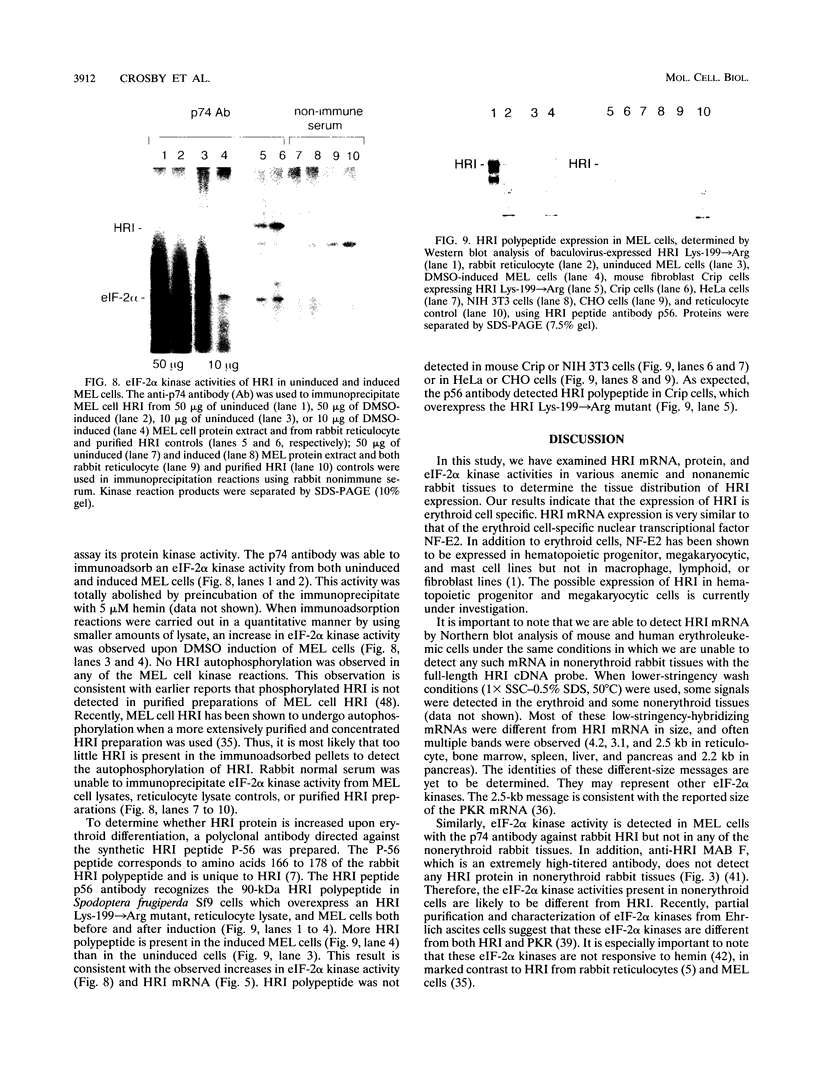
The role of heme-regulated eIF-2 alpha kinase (HRI) in the regulation of protein synthesis in rabbit reticulocytes is well documented. Inhibitors of protein synthesis with properties similar to those of HRI have been described in some nonerythroid cell types, but it has not yet been determined whether these eIF-2 alpha kinase activities are mediated by HRI or one or more as yet uncharacterized kinases. We have studied the expression of mRNA, polypeptide, and kinase activities of HRI in various tissues from both nonanemic and anemic rabbits. Our results indicate that HRI is expressed in an erythroid cell-specific manner. HRI is present in the bone marrow and peripheral blood of both nonanemic and anemic rabbits but not in any of the other tissues tested. HRI mRNA is present at low levels in uninduced mouse erythroleukemic (MEL) cells and human K562 cells and accumulates to higher levels upon induction. The accumulation of HRI mRNA in differentiating MEL cells is dependent upon the presence of heme. The addition of 3-amino-1,2,4-triazole (AT), an inhibitor of heme biosynthesis, to the induction medium markedly reduced HRI mRNA accumulation. Simultaneous addition of hemin and AT to the dimethyl sulfoxide induction medium largely prevented the inhibition of HRI mRNA induction by AT. These findings indicate that HRI is expressed in an erythroid cell-specific manner and that the major physiologic role of HRI is in adjusting the synthesis of globins to the availability of heme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Baron J., Tephly T. R. Effect of 3-amino-1,2,4-triazole on the stimulation of hepatic microsomal heme synthesis and induction of hepatic microsomal oxidases produced by phenobarbital. Mol Pharmacol. 1969 Jan;5(1):10–20. [PubMed] [Google Scholar]
- Bonanou-Tzedaki S. A., Smith K. E., Sheeran B. A., Arnstein H. R. Reduced formation of initiation complexes between Met-tRNAf and 40-S ribosomal subunits in rabbit reticulocyte lysates incubated at elevated temperatures. Activity of the Met-tRNAf binding factor. Eur J Biochem. 1978 Mar 15;84(2):601–610. doi: 10.1111/j.1432-1033.1978.tb12203.x. [DOI] [PubMed] [Google Scholar]
- Chen J. J., Pal J. K., Petryshyn R., Kuo I., Yang J. M., Throop M. S., Gehrke L., London I. M. Amino acid microsequencing of internal tryptic peptides of heme-regulated eukaryotic initiation factor 2 alpha subunit kinase: homology to protein kinases. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):315–319. doi: 10.1073/pnas.88.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Throop M. S., Gehrke L., Kuo I., Pal J. K., Brodsky M., London I. M. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Yang J. M., Petryshyn R., Kosower N., London I. M. Disulfide bond formation in the regulation of eIF-2 alpha kinase by heme. J Biol Chem. 1989 Jun 5;264(16):9559–9564. [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Henshaw E. C., London I. M. Characterization of a macromolecular inhibitor of polypeptide chain initiation from Ehrlich ascites tumor cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):768–775. doi: 10.1016/s0006-291x(76)80105-2. [DOI] [PubMed] [Google Scholar]
- Dabney B. J., Beaudet A. L. Increase in globin chains and globin mRNA in erythroleukemia cells in response to hemin. Arch Biochem Biophys. 1977 Feb;179(1):106–112. doi: 10.1016/0003-9861(77)90092-3. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Activation of hemin-regulated initiation factor-2 kinase in heat-shocked HeLa cells. J Biol Chem. 1986 Jan 5;261(1):338–342. [PubMed] [Google Scholar]
- Delaunay J., Ranu R. S., Levin D. H., Ernst V., London I. M. Characterization of a rat liver factor that inhibits initiation of protein synthesis in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2264–2268. doi: 10.1073/pnas.74.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. In situ phosphorylation of the alpha subunit of eukaryotic initiation factor 2 in reticulocyte lysates inhibited by heme deficiency, double-stranded RNA, oxidized glutathione, or the heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 May;76(5):2118–2122. doi: 10.1073/pnas.76.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R., London I. M. Relationship between phosphorylation and activity of heme-regulated eukaryotic initiation factor 2 alpha kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):866–870. doi: 10.1073/pnas.78.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis by hemin: factors influencing formation of an inhibitor of globin chain initiation in reticulocyte lysates. Biochim Biophys Acta. 1972 Dec 6;287(2):340–352. doi: 10.1016/0005-2787(72)90383-8. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hurst R., Schatz J. R., Matts R. L. Inhibition of rabbit reticulocyte lysate protein synthesis by heavy metal ions involves the phosphorylation of the alpha-subunit of the eukaryotic initiation factor 2. J Biol Chem. 1987 Nov 25;262(33):15939–15945. [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Mechanism of the inhibition of protein synthesis by vasopressin in rat liver. J Biol Chem. 1990 Oct 5;265(28):16794–16798. [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Kosower E. M. Glutathione. 8. The effects of glutathione disulfide on initiation of protein synthesis. Biochim Biophys Acta. 1972 Jul 31;272(4):623–637. [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop J. T., Timko M. P. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993 Jan 22;259(5094):522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Schlessinger J. Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Mol Cell Biol. 1991 Jul;11(7):3756–3761. doi: 10.1128/mcb.11.7.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Stanley E. R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991 Feb;10(2):277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D., Bernstein A. The role of heme in the regulation of the late program of Friend cell erythroid differentiation. J Cell Physiol. 1979 Sep;100(3):467–479. doi: 10.1002/jcp.1041000310. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969 Apr 10;35(1):79–85. doi: 10.1016/0006-291x(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Mellor H., Price N. T., Oldfield S., Sarre T. F., Proud C. G. Purification and characterisation of an initiation-factor-2 kinase from uninduced mouse erythroleukaemia cells. Eur J Biochem. 1993 Feb 1;211(3):529–538. doi: 10.1111/j.1432-1033.1993.tb17579.x. [DOI] [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990 Jun;4(6):993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Nikkilä H., Gitlin J. D., Muller-Eberhard U. Rat hemopexin. Molecular cloning, primary structural characterization, and analysis of gene expression. Biochemistry. 1991 Jan 22;30(3):823–829. doi: 10.1021/bi00217a036. [DOI] [PubMed] [Google Scholar]
- Olmsted E. A., O'Brien L., Henshaw E. C., Panniers R. Purification and characterization of eukaryotic initiation factor (eIF)-2 alpha kinases from Ehrlich ascites tumor cells. J Biol Chem. 1993 Jun 15;268(17):12552–12559. [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal J. K., Chen J. J., London I. M. Tissue distribution and immunoreactivity of heme-regulated eIF-2 alpha kinase determined by monoclonal antibodies. Biochemistry. 1991 Mar 5;30(9):2555–2562. doi: 10.1021/bi00223a037. [DOI] [PubMed] [Google Scholar]
- Petryshyn R., Rosa F., Fagard R., Levin D., London I. M. Control of protein synthesis in human reticulocytes by heme-regulated and double-stranded RNA dependent eIF-2 alpha kinases. Biochem Biophys Res Commun. 1984 Mar 30;119(3):891–899. doi: 10.1016/0006-291x(84)90857-x. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Kim K. S., Kogan S., Guarente L. Functional dissection and sequence of yeast HAP1 activator. Cell. 1989 Jan 27;56(2):291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- Prostko C. R., Brostrom M. A., Malara E. M., Brostrom C. O. Phosphorylation of eukaryotic initiation factor (eIF) 2 alpha and inhibition of eIF-2B in GH3 pituitary cells by perturbants of early protein processing that induce GRP78. J Biol Chem. 1992 Aug 25;267(24):16751–16754. [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarre T. F., Hermann M., Bader M. Differential effect of hemin-controlled eIF-2 alpha kinases from mouse erythroleukemia cells on protein synthesis. Eur J Biochem. 1989 Jul 15;183(1):137–143. doi: 10.1111/j.1432-1033.1989.tb14905.x. [DOI] [PubMed] [Google Scholar]
- Sarre T. F. Presence of haemin-controlled eIF-2 alpha kinases in both undifferentiated and differentiating mouse erythroleukaemia cells. Biochem J. 1989 Sep 1;262(2):569–574. doi: 10.1042/bj2620569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorsone K. A., Panniers R., Rowlands A. G., Henshaw E. C. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J Biol Chem. 1987 Oct 25;262(30):14538–14543. [PubMed] [Google Scholar]
- Sorrentino B., Ney P., Bodine D., Nienhius A. W. A 46 base pair enhancer sequence within the locus activating region is required for induced expression of the gamma-globin gene during erythroid differentiation. Nucleic Acids Res. 1990 May 11;18(9):2721–2731. doi: 10.1093/nar/18.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSCHUDY D. P., COLLINS A. Effect of 3-amino-1, 2, 4-triazole on delta-amino-levulinic acid dehydrase activity. Science. 1957 Jul 26;126(3265):168–168. doi: 10.1126/science.126.3265.168. [DOI] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Doble B. W., Mulvey M. R., Sorby P. A., Loewen P. C. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J Bacteriol. 1988 Sep;170(9):4415–4419. doi: 10.1128/jb.170.9.4415-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Yang J. M., London I. M., Chen J. J. Effects of hemin and porphyrin compounds on intersubunit disulfide formation of heme-regulated eIF-2 alpha kinase and the regulation of protein synthesis in reticulocyte lysates. J Biol Chem. 1992 Oct 5;267(28):20519–20524. [PubMed] [Google Scholar]