Abstract
Monoaminergic and neuropeptidergic neurons regulate a wide variety of behaviors, such as feeding, sleep/wakefulness behavior, stress response, addiction, and social behavior. These neurons form neural circuits to integrate different modalities of behavioral and environmental factors, such as stress, maternal care, and feeding conditions. One possible mechanism for integrating environmental factors through the monoaminergic and neuropeptidergic neurons is through the epigenetic regulation of gene expression via altered acetylation of histones. Histone deacetylases (HDACs) play an important role in altering behavior in response to environmental factors. Despite increasing attention and the versatile roles of HDACs in a variety of brain functions and disorders, no reports have detailed the localization of the HDACs in the monoaminergic and neuropeptidergic neurons. Here, we examined the expression profile of the HDAC protein family from HDAC1 to HDAC11 in corticotropin-releasing hormone, oxytocin, vasopressin, agouti-related peptide (AgRP), pro-opiomelanocortin (POMC), orexin, histamine, dopamine, serotonin, and noradrenaline neurons. Immunoreactivities for HDAC1,-2,-3,-5,-6,-7,-9, and -11 were very similar among the monoaminergic and neuropeptidergic neurons, while the HDAC4, -8, and -10 immunoreactivities were clearly different among neuronal groups. HDAC10 expression was found in AgRP neurons, POMC neurons, dopamine neurons and noradrenaline neurons but not in other neuronal groups. HDAC8 immunoreactivity was detected in the cytoplasm of almost all histamine neurons with a pericellular pattern but not in other neuropeptidergic and monoaminergic neurons. Thus, the differential expression of HDACs in monoaminergic and neuropeptidergic neurons may be crucial for the maintenance of biological characteristics and may be altered in response to environmental factors.
Introduction
Monoaminergic and neuropeptidergic neurons regulate a wide variety of behaviors, such as feeding, sleep/wakefulness behavior, stress response, addiction, and social behavior. Feeding behavior is mainly regulated by orexigenic neurons containing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and by anorexigenic neurons containing pro-opiomelanocortin (POMC). Both of these types of neurons are localized in the hypothalamic arcuate nucleus (ARC) [1]. Orexin (also known as hypocretin), which was originally identified as an orexigenic neuropeptide, is expressed in the lateral hypothalamic area (LHA) and plays a crucial role in sleep/wakefulness behavior, reward behavior, addiction, and body weight regulation [2]–[4]. Sleep/wakefulness behavior is also regulated by histamine neurons in the tuberomammillary nucleus (TMN), serotonin neurons in the dorsal raphe (DR), and noradrenaline neurons in the locus coeruleus (LC) [2], [5]. Dopamine neurons in the ventral tegmental area (VTA), which is involved in the reward system, also alter feeding behavior, social behavior, and sleep/wakefulness [2], [3], [5], [6]. The hypothalamic paraventricular nucleus (PVN) has neurons that contain corticotropin-releasing hormone (CRH), which constitutes the hypothalamic-pituitary-adrenal axis, a neuroendocrine system that controls stress response [3]. The PVN also contains neurons that produce oxytocin and vasopressin, which are important for social behavior [7].
Environmental factors such as stress, maternal care, and feeding conditions modulate the above-mentioned behaviors [8]–[11]. For instance, stressed animals decrease their food intake compared with non-stressed animals [8]. Male rats that had experienced neonatal maternal deprivation showed a decrease in total sleep time [11], and suppressed social interaction [12]. Recently, increasing reports suggest that the epigenetic regulation of gene expression plays a crucial role in behavioral change in response to environmental factors [13], [14]. One possible mechanism for integrating environmental factors is through the epigenetic gene regulation of monoaminergic and neuropeptidergic neurons.
Acetylation of histones is a dynamic process that regulates gene expression in response to upstream cellular signaling and also regulates multiple behaviors, including addiction, depression, age-related memory impairment, and memory recall [15]–[18]. Histone deacetylases (HDACs) remove an acetyl group from the lysine on the histone in a sequence-specific manner to repress, and in some cases enhance, gene transcription. HDACs also deacetylate nuclear and cytoplasmic proteins, including p53, STAT3, and α-tubulin [19]–[22]. The HDAC family comprises the following classes: class I (HDAC1, -2, -3, and -8), class IIa (HDAC4, -5, -7, and -9), class IIb (HDAC6 and -10), and class IV (HDAC11) [20], [23], [24]. HDAC4 and -5 show a nuclear-cytoplasmic shuttling in an activity-dependent manner in neural cells [25]. Recently, HDAC4 has been localized in the dendritic shaft and spines [26], suggesting that HDACs may work outside the cell body to regulate synaptic activity and dendritic transport. Consistently, overexpression of HDAC2 results in impaired memory formation [27]. In addition to memory formation, HDACs may play important roles in feeding and metabolism [28], [29]. Increasing attention has been given to the versatile roles of HDACs in a variety of brain functions and disorders. However, only a few reports have detailed the localization of HDACs in the brain [26], [29], [30], which prompted us to examine the distribution of the HDAC family in monoaminergic and neuropeptidergic neurons.
In the present study, we examined the subcellular distribution of HDACs in the CRH, oxytocin, vasopressin, orexin, AgRP, POMC, histamine, dopamine, serotonin, and noradrenaline neurons in mice using the immunofluorescence method.
Materials and Methods
Animal
Male C57BL/6J mice (25–30 g, 12–16 week-old; n = 6) were obtained from Charles River Laboratories of Japan (Tokyo, Japan). Mice were provided food and water ad libitum, maintained on a 12-hour light/dark cycle (lights on, 0800–2000 h), and housed under controlled temperature (25±1°C) and humidity conditions. All procedures were approved by the Institutional Animal Care and Use Committee of Toho University (Approved protocol ID #12-52-81).
Antibodies
The primary antibodies used in the current study are summarized in Table 1. As markers of the oxytocin and vasopressin neurons, we used antibodies for neurophysin I and copeptin, respectively. Neurophysin I is a peptide derived from an oxytocin precursor and is a marker for oxytocin neurons. Copeptin, a peptide derived from a vasopressin precursor, co-localizes with vasopressin and not oxytocin [31]. An antibody for tyrosine hydroxylase was used as a marker of dopamine and noradrenaline neurons. The secondary antibodies used for immunofluorescent visualization were Alexa 555-conjugated anti-goat IgG antibody (1∶400, A21432, Invitrogen, CA, USA), Alexa 555-conjugated anti-guinea pig IgG antibody (1∶400, A21435, Invitrogen), or Alexa 555-conjugated anti-mouse IgG antibody (1∶400, A31570, Invitrogen), and Alexa 488-conjugated anti-rabbit IgG antibody (1∶400, A21206, Invitrogen).
Table 1. Primary Antibodies Used.
| Neuronal group marker | Antigen | Antibody characterization or immunogen | Manufacturer, species antibodywas raised in, mono- vs. polyclonal,catalog or lot number | Dilution used |
| CRH | Corticotropin-releasing hormone | Affinity purified antibody forN-terminus of human CRH | Santa Cruz Biotechnology, CA; goat; polyclonal; sc-21675 | 1∶100 |
| Oxytocin | Neurophysin I, a peptidederived from oxytocin precursor | Affinity purified antibody forC-terminus of mouse neurophysin I | Santa Cruz Biotechnology, CA; goat; polyclonal; sc-7810 | 1∶50 |
| Vasopressin | Copeptin, a peptide derivedfrom vasopressin precursor | Affinity purified antibody forC-terminus of mouse copeptin | Santa Cruz Biotechnology, CA; goat; polyclonal; sc-7812 | 1∶50 |
| Orexin | Orexin A | Affinity purified antibody for C-terminus of human orexin A | Santa Cruz Biotechnology, CA; goat; polyclonal; sc-8070 | 1∶100 |
| AgRP | AgRP | Affinity purified antibody for internal region of AgRP | Santa Cruz Biotechnology, CA; goat; polyclonal; sc-18634 | 1∶50 |
| POMC | POMC | Synthetic peptide: NAIIKNAYKKGE, corresponding toC terminal amino acids 256–267 of human POMC, conjugated to KLH | Abcam, Cambridge, MA; goat; polyclonal; ab32893 | 1∶75 |
| Histamine | Histidine decarboxylase | Synthetic peptide corresponding to C-terminus of mouse histidine decarboxylase | American Research Products, MA; guinea pig; polyclonal; 03-16046 | 1∶60 |
| Dopamine and Noradrenaline | Tyrosine hydroxylase | Tyrosine hydroxylase purified from PC12 cells | Millipore, MA; mouse; polyclonal; MAB318 | 1∶200 |
| Serotonin | Serotonin | Serotonin coupled to bovine serum albumin | ImmunoStar, WI; goat; polyclonal; 20079 | 1∶300 |
| HDAC1 | Synthetic peptide: KEEKPEAKGVKEEVKLA, corresponding to amino acids 466–482 of human HDAC1, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab19845 | 1∶100 | |
| HDAC2 | Synthetic peptide: GEKTDTKGTKSEQLSNP, corresponding to amino acids 471–488 of human HDAC2, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab16032 | 1∶250 | |
| HDAC3 | Synthetic peptide: NEFYDGDHDNDKESDVEI, corresponding to amino acids 411–428 of human HDAC3, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab16047 | 1∶100 | |
| HDAC4 | Synthetic peptide: CISSDPRYWYGKTQHS, corresponding to amino acids 194–209 of human HDAC4, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab79521 | 1∶300 | |
| HDAC5 | Synthetic non-phosphopeptide derived from human HDAC5 around serine 259 | Abcam, Cambridge, MA; rabbit; polyclonal; ab55403 | 1∶100 | |
| HDAC6 | Synthetic peptide: KNIAHQNKFGEDMPH, corresponding to amino acids 1199–1213 of human HDAC6, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab12173 | 1∶50 | |
| HDAC7 | Synthetic peptide: KPRLRQIPSAEDLETDG, corresponding to amino acids 436–452 of human HDAC7 isoform a, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab12174 | 1∶50 | |
| HDAC8 | Synthetic non-phosphopeptide derived from human HDAC8, around serine 39 | Abcam, Cambridge, MA; rabbit; polyclonal; ab39664 | 1∶250 | |
| HDAC9 | Synthetic peptide: EVPVGLEPISPLDLRT, corresponding to 55–70 of human HDAC9, conjugated to KLH | Abcam, Cambridge, MA; rabbit; polyclonal; ab18970 | 1∶50 | |
| HDAC10 | Synthetic peptide corresponding to a part of human HDAC10 | Abcam, Cambridge, MA, Japan; rabbit; polyclonal; ab53096 | 1∶50 | |
| HDAC11 | Synthetic peptide, corresponding to amino acids around glycine 269 of human HDAC 11 | Abcam, Cambridge, MA, Japan; rabbit; polyclonal; ab18973 | 1∶100 | |
| MAP2 | Bovine brain microtubule protein | Millipore, MA; mouse; polyclonal; MAB3418 | 1∶400 | |
| PSD-95 | Recombinant rat PSD-95 | Millipore, MA; mouse; polyclonal; MAB1598 | 1∶400 |
Immunofluorescent Staining
For immunofluorescent staining, mice (n = 3) were deeply anesthetized with sodium pentobarbital and perfused transcardially with phosphate-buffered saline (PBS, 0.1 M, pH 7.4) followed by phosphate-buffered 4% paraformaldehyde (PFA). Brains were rapidly removed, post-fixed overnight in phosphate-buffered 4% PFA, and equilibrated in 30% sucrose for 2 days. Brains were sectioned on a cryostat at 30 µm. Sections were stored in a cryoprotective tissue collection solution (25% glycerol, 30% ethylene glycol, 0.05 M phosphate buffer (PB)) at −20°C until use. Immunofluorescence was performed using a free-floating method. The brain sections were washed 2×10 minutes in PBS and blocked for 1 hour in a blocking solution containing 0.1 M PB, 0.25% Triton X-100, and 5% normal donkey serum. The brain sections were then incubated with primary antibodies for CRH, orexin, neurophysin I, copeptin, AgRP, POMC, histamine, tyrosine hydroxylase, serotonin, MAP-2, or PSD-95 and with antibodies for HDAC1-11 with 0.25% Triton X-100 and 3% normal donkey serum overnight at 4°C.
After washing the sections 2×20 minutes in PBS, the sections were incubated with a fluorescence-conjugated secondary antibody, and Hoechst 33342 (2 µg/ml, H21492, Invitrogen) for 2 hours at room temperature. After washing the sections 2×10 minutes in PBS and 3 minutes in PB, the sections were mounted on a glass slide with Gel/Mount (BioMeda, CA, USA). For immunofluorescent staining of CRH and AgRP neurons, colchicine was intracerebroventricularly injected into the mice (n = 3) one day before decapitation. Colchicine inhibits axonal transport by suppressing microtubule polymerization but does not affect nuclear-cytoplasmic shuttling.
Confocal Laser Scanning Microscopy
Samples were observed using confocal laser scanning microscopy as previously described [32]. In brief, immunofluorescent images were captured using a scanning confocal microscope (LSM510 META, Zeiss, Oberkochen, Germany) with objectives of 40×, 63×, or 100×. The pinhole size was adjusted, so the optical thickness of the sections was 0.6–1.0 µm. To obtain immunofluorescent images, each channel was collected separately with single wavelength excitation and then merged to produce a composite image. Experimental controls were prepared in which one or both of the primary antibodies were omitted from the reaction solution. Confocal laser scanning microscopy showed no immunolabeling of omitted antibodies in the control sections. Photoshop CS5 (Adobe Systems, Mountain View, CA) was used to combine drawings and digital images into plates. The contrast and brightness of images were adjusted. HDAC immunoreactivities localized in dendrites were carefully confirmed using z-stack images.
Image Analysis
To assess the double immunofluorescence data, we observed all CRH, oxytocin, and vasopressin neurons of the bilateral PVN of three brain sections (0.7–0.9 mm posterior to bregma; corresponding to Figures 37, 38 in Franklin and Paxinos [33]); all orexin neurons of the bilateral LHA, and all AgRP and POMC neurons of the bilateral ARC of three brain sections (1.5–1.8 mm posterior to bregma; corresponding to Figures 44–46 in Franklin and Paxinos [33]); all histamine neurons of the bilateral TMN of three brain sections (2.5–2.8 mm posterior to bregma; corresponding to Figures 52–54 in Franklin and Paxinos [33]); all dopamine neurons of the bilateral VTA of three brain sections (3.0–3.2 mm posterior to bregma; corresponding to Figures 56, 57 in Franklin and Paxinos [33]), all serotonin neurons of the DR of three brain sections (4.5–4.7 mm posterior to bregma; corresponding to Figures 68–70 in Franklin and Paxinos [33]); and all noradrenaline neurons of the bilateral LC of three brain sections (5.4–5.6 mm posterior to bregma; corresponding to Figures 76–78 in Franklin and Paxinos [33]) per animal. The percentage of HDAC-immunoreactive cells among the monoaminergic and neuropeptidergic neuron groups was determined. To verify the localization of HDACs at the subcellular level, all brain sections were counterstained with the nuclear dye Hoechst 33342. Nuclear or cytoplasmic localization of the HDACs was determined based on the colocalization with Hoechst 33342. To assess the extent of the HDACs immunoreactivities, we used a four-point scale based on the intensity and area of the immunoreactivities in the following manner: +++, strong HDACs immunoreactivity; ++, moderate HDACs immunoreactivity; +, weak HDACs immunoreactivity; and −, no HDACs immunoreactivity above background. The assessment was independently performed by two observers.
Results
HDAC1
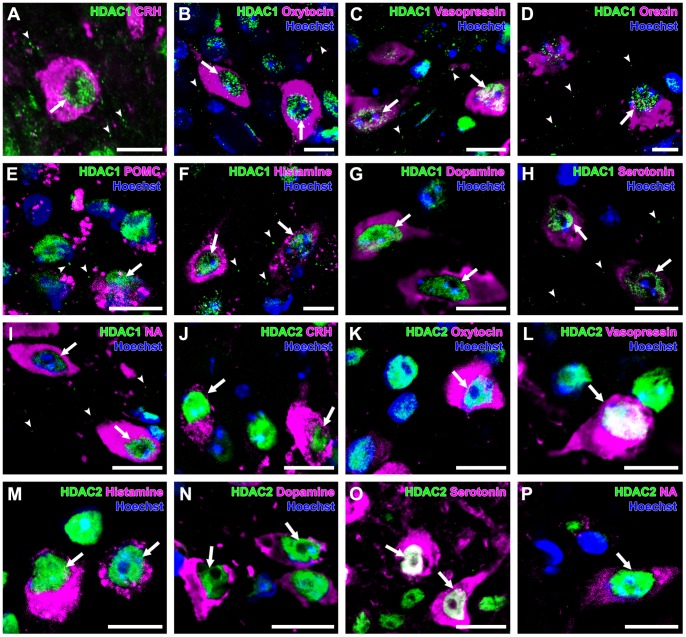
In general, HDAC1 immunoreactivity was recognized mainly in the nuclei of neurons and glial cells (data not shown), with punctate immunoreactivity in neuropils throughout the brain. A few HDAC1-immunoreactive puncta were also observed in the cytoplasm and dendrites. Nuclear HDAC1 immunoreactivity was found in almost all nuclei of the CRH neurons (92±6%), oxytocin neurons (95±1%), and vasopressin neurons (100±0%) of the PVN; the orexin neurons (100±0%) of the LHA; the AgRP neurons (100±0%) and POMC neurons (100±0%) of the ARC; the histamine neurons (91±6%) of the TMN; the dopamine neurons (100±0%) of the VTA; the serotonin neurons (100±0%) of the DR; and the noradrenaline neurons (100±0%) of the LC (Figures 1A–I, Table 2). HDAC1-immunoreactive puncta were uniform in size and widely distributed in the PVN, LHA, ARC, TMN, DR and LC. HDAC1-immunoreactive puncta in the neuropils were often found in dendrites and only a few HDAC1-immunoreactive puncta were colocalized with PSD95-immunoreactive puncta, which correspond to the postsynaptic area.
Figure 1. Expression of HDAC1 and -2 in monoaminergic and neuropeptidergic neurons.
The HDAC immunolocalizations (green) were visualized with a neuronal marker (magenta) and Hoechst stain (blue). A: HDAC1 was detected in the nucleus (arrow) of a CRH neuron, with punctate immunoreactivity (arrowheads) in the PVN. B: HDAC1 was detected in the nuclei (arrows) of oxytocin neurons, with punctate immunoreactivity (arrowheads) in the PVN. C: HDAC1 was detected in the nuclei (arrows) of vasopressin neurons, with punctate immunoreactivity (arrowheads) in the PVN. D: HDAC1 was detected in the nucleus (arrow) of orexin neuron, with punctate immunoreactivity (arrowheads) in the LHA. E: HDAC1 was detected in the nucleus (arrow) of POMC neurons, with punctate immunoreactivity (arrowheads) in the ARC. F: HDAC1 was detected in the nuclei (arrows) of histamine neurons, with punctate immunoreactivity (arrowheads) in the TMN. G: HDAC1 was detected in the nuclei (arrows) of dopamine neurons. H: HDAC1 was detected in the nuclei (arrows) of serotonin neurons, with punctate immunoreactivity (arrowheads) in the DR. I: HDAC1 was detected in the nuclei (arrows) of noradrenaline neurons, with punctate immunoreactivity (arrowheads) in the LC. J: HDAC2 was detected in the nuclei (arrows) of CRH neurons. K: HDAC2 was detected in the nucleus (arrow) of oxytocin neuron. L: HDAC2 was detected in the nucleus (arrow) of vasopressin neuron. M: HDAC2 was detected in the nuclei (arrows) of histamine neurons. N: HDAC2 was detected in the nuclei (arrows) of dopamine neurons. O: HDAC2 was localized in the nuclei (arrows) of serotonin neurons in the DR. P: HDAC2 was detected in the nucleus (arrow) of noradrenaline neuron. Scale bars indicate 10 µm.
Table 2. HDACs Immunoreactivities in Monoaminergic and Neuropeptidergic Neurons.
| CRH (PVN) | Oxytocin (PVN) | ||||||
| Immunoreactive cell (%) | Subcellular immunoreactivity | Immunoreactive cell (%) | Subcellular immunoreactivity | ||||
| HDAC1 | 92±6 | Nucleus | ++ | HDAC1 | 95±1 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC2 | 100±0 | Nucleus | +++ | HDAC2 | 98±1 | Nucleus | +++ |
| Cytoplasm | −∼+/− | Cytoplasm | −∼+/− | ||||
| HDAC3 | 100±0 | Nucleus | ++ | HDAC3 | 95±5 | Nucleus | ++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC4 | 100±0 | Nucleus | +++ | HDAC4 | 98±2 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | −∼+/− | ||||
| HDAC5 | 100±0 | Nucleus | + | HDAC5 | 95±5 | Nucleus | + |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| HDAC6 | 95±2 | Nucleus | +++ | HDAC6 | 70±7 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | + | ||||
| HDAC7 | 97±2 | Nucleus | ++ | HDAC7 | 93±3 | Nucleus | + |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC8 | 0±0 | Nucleus | – | HDAC8 | 4±3 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | +/− | ||||
| HDAC9 | 100±0 | Nucleus | +++ | HDAC9 | 100±0 | Nucleus | +++ |
| Cytoplasm | +/−∼+ | Cytoplasm | + | ||||
| HDAC10 | 0±0 | Nucleus | – | HDAC10 | 0±0 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC11 | 45±10 | Nucleus | ++ | HDAC11 | 89±8 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| Vasopressin (PVN) | Orexin (LHA) | ||||||
| Immunoreactive cell (%) | Subcellular immunoreactivity | Immunoreactive cell (%) | Subcellular immunoreactivity | ||||
| HDAC1 | 100±0 | Nucleus | ++ | HDAC1 | 100±0 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC2 | 100±0 | Nucleus | +++ | HDAC2 | 100±0 | Nucleus | +++ |
| Cytoplasm | −∼+/− | Cytoplasm | −∼+/− | ||||
| HDAC3 | 100±0 | Nucleus | ++ | HDAC3 | 100±0 | Nucleus | ++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC4 | 100±0 | Nucleus | +++ | HDAC4 | 100±0 | Nucleus | +++ |
| Cytoplasm | −∼+/− | Cytoplasm | + | ||||
| HDAC5 | 95±3 | Nucleus | + | HDAC5 | 100±0 | Nucleus | + |
| Cytoplasm | ++ | Cytoplasm | +++ | ||||
| HDAC6 | 80±3 | Nucleus | +++ | HDAC6 | 100±0 | Nucleus | +++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| HDAC7 | 87±7 | Nucleus | +++ | HDAC7 | 100±0 | Nucleus | +++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC8 | 0±0 | Nucleus | – | HDAC8 | 0±0 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC9 | 100±0 | Nucleus | +++ | HDAC9 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC10 | 0±0 | Nucleus | – | HDAC10 | 0±0 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC11 | 100±0 | Nucleus | ++ | HDAC11 | 100±0 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| AgRP (ARC) | POMC (ARC) | ||||||
| Immunoreactive cell (%) | Subcellular immunoreactivity | Immunoreactive cell (%) | Subcellular immunoreactivity | ||||
| HDAC1 | 100±0 | Nucleus | ++ | HDAC1 | 100±0 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC2 | 100±0 | Nucleus | +++ | HDAC2 | 95±1 | Nucleus | ++ |
| Cytoplasm | −∼+/− | Cytoplasm | −∼+/− | ||||
| HDAC3 | 100±0 | Nucleus | ++ | HDAC3 | 79±11 | Nucleus | ++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC4 | 100±0 | Nucleus | +++ | HDAC4 | 92±4 | Nucleus | +++ |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC5 | 100±0 | Nucleus | + | HDAC5 | 85±1 | Nucleus | + |
| Cytoplasm | +++ | Cytoplasm | ++ | ||||
| HDAC6 | 100±0 | Nucleus | +++ | HDAC6 | 88±1 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | ++ | ||||
| HDAC7 | 100±0 | Nucleus | ++ | HDAC7 | 99±1 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC8 | 0±0 | Nucleus | – | HDAC8 | 0±0 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC9 | 100±0 | Nucleus | +++ | HDAC9 | 96±4 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC10 | 98±2 | Nucleus | ++ | HDAC10 | 55±4 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC11 | 100±0 | Nucleus | ++ | HDAC11 | 85±5 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| Histamine (TMN) | Dopamine (VTA) | ||||||
| Immunoreactive cell (%) | Subcellular immunoreactivity | Immunoreactive cell (%) | Subcellular immunoreactivity | ||||
| HDAC1 | 91±6 | Nucleus | ++ | HDAC1 | 100±0 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC2 | 100±0 | Nucleus | +++ | HDAC2 | 100±0 | Nucleus | +++ |
| Cytoplasm | −∼+/− | Cytoplasm | −∼+/− | ||||
| HDAC3 | 92±5 | Nucleus | ++ | HDAC3 | 100±0 | Nucleus | ++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC4 | 85±7 | Nucleus | +++ | HDAC4 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | – | ||||
| HDAC5 | 100±0 | Nucleus | + | HDAC5 | 100±0 | Nucleus | + |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| HDAC6 | 100±0 | Nucleus | ++ | HDAC6 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | ++ | ||||
| HDAC7 | 100±0 | Nucleus | ++ | HDAC7 | 100±0 | Nucleus | +++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC8 | 100±0 | Nucleus | – | HDAC8 | 0±0 | Nucleus | – |
| Cytoplasm | ++ | Cytoplasm | – | ||||
| HDAC9 | 96±2 | Nucleus | +++ | HDAC9 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | ++ | ||||
| HDAC10 | 0±0 | Nucleus | – | HDAC10 | 95±1 | Nucleus | ++ |
| Cytoplasm | – | Cytoplasm | + | ||||
| HDAC11 | 100±0 | Nucleus | ++ | HDAC11 | 100±0 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| Serotonin (DR) | Noradrenaline (LC) | ||||||
| Immunoreactive cell (%) | Subcellular immunoreactivity | Immunoreactive cell (%) | Subcellular immunoreactivity | ||||
| HDAC1 | 100±0 | Nucleus | ++ | HDAC1 | 100±0 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC2 | 100±0 | Nucleus | +++ | HDAC2 | 100±0 | Nucleus | +++ |
| Cytoplasm | −∼+/− | Cytoplasm | −∼+/− | ||||
| HDAC3 | 100±0 | Nucleus | ++ | HDAC3 | 100±0 | Nucleus | ++ |
| Cytoplasm | + | Cytoplasm | +/−∼+ | ||||
| HDAC4 | 93±2 | Nucleus | +++ | HDAC4 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC5 | 100±0 | Nucleus | + | HDAC5 | 100±0 | Nucleus | + |
| Cytoplasm | ++ | Cytoplasm | +++ | ||||
| HDAC6 | 100±0 | Nucleus | +++ | HDAC6 | 100±0 | Nucleus | +++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
| HDAC7 | 100±0 | Nucleus | +++ | HDAC7 | 100±0 | Nucleus | ++ |
| Cytoplasm | +/− | Cytoplasm | +/− | ||||
| HDAC8 | 0±0 | Nucleus | – | HDAC8 | 0±0 | Nucleus | – |
| Cytoplasm | – | Cytoplasm | – | ||||
| HDAC9 | 100±0 | Nucleus | +++ | HDAC9 | 100±0 | Nucleus | +++ |
| Cytoplasm | + | Cytoplasm | + | ||||
| HDAC10 | 0±0 | Nucleus | – | HDAC10 | 100±0 | Nucleus | +++ |
| Cytoplasm | – | Cytoplasm | ++ | ||||
| HDAC11 | 100±0 | Nucleus | ++ | HDAC11 | 100±0 | Nucleus | ++ |
| Cytoplasm | ++ | Cytoplasm | ++ | ||||
The percentage of immunoreactive cells was assessed at the confocal laser scanning microscopy. Values are means ± S.E.M. Nuclear or cytoplasmic localization of HDACs was determined based on the colocalization with Hoechst 33342. The extent of HDACs immunoreactivities was determined on the intensity and area of the immunoreactivities: “+++”, strong immunoreactivity; “++”, moderate immunoreactivity; “+”, weak immunoreactivity; “−“, no immunoreactivity above background.
HDAC2
Similar to HDAC1, strong HDAC2 immunoreactivity was mainly found in the nuclei of neurons, glial cells (data not shown). Only a few HDAC2-immunoreactive puncta were observed in the hypothalamus, VTA, DR, and LC, and there were fewer HDAC2-immunoreactive puncta than for HDAC1. HDAC2 expressions was recognized in a broad range of neuron groups, including the CRH neurons (100±0%), oxytocin neurons (98±1%), and vasopressin neurons (100±0%) of the PVN; the orexin neurons (100±0%) of the LHA; the AgRP neurons (100±0%) and POMC neurons (95±1%) of the ARC; the histamine neurons of the TMN (100±0%); the dopamine neurons (100±0%) of the VTA; the serotonin neurons (100±0%) of the DR; and the noradrenaline neurons (100±0%) of the LC (Figures 1J–P, Table 2). A small number of HDAC2- immunoreactive puncta were colocalized with PSD95 immunoreactive puncta of dendrites.
HDAC3
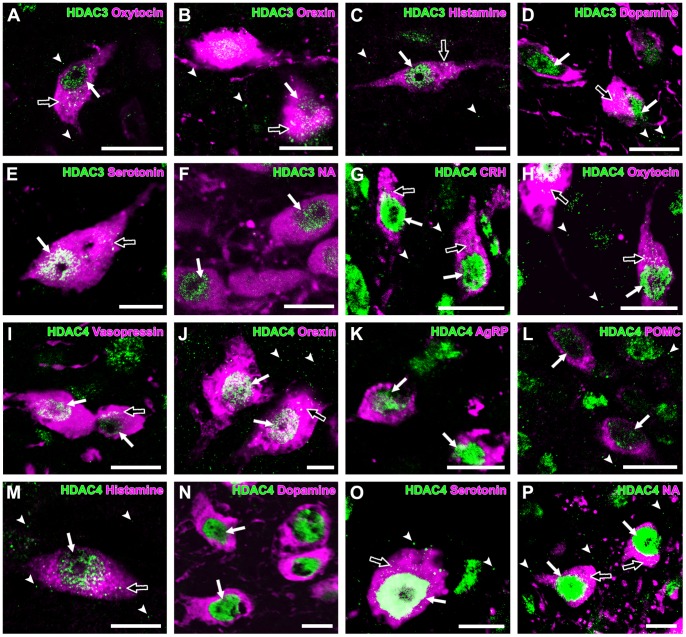
In general, HDAC3 immunoreactivity was observed in the nuclei of neurons throughout the brain. HDAC3-immunoreactive puncta were observed in the cytoplasm and neuropils. HDAC3 immunoreactivity was found in both the nuclei and cytoplasm of neuron groups, including the CRH neurons (100±0%), the oxytocin neurons (95±5%), the vasopressin neurons (100±0%), the orexin neurons (100±0%), the AgRP neurons (100±0%), the histamine neurons (92±5%), the dopamine neurons (100±0%), the serotonin neurons (100±0%), and the noradrenaline neurons (100±0%). However, a small population of POMC neurons (21±11%) did not show any immunoreactivity for HDAC3 (Figures 2A–F, Table 2). HDAC3-immunoreactive puncta in the neuropils were uniform in size and widely distributed in the PVN, LHA, ARC, TMN, and VTA. Similar to HDAC1, a minor population of HDAC3-immunoreactive puncta in the neuropils was found in dendrites. Only a few HDAC3-immunoreactive puncta in the neuropils were colocalized with PSD95-immunoreactive puncta.
Figure 2. Expression of HDAC3 and -4 in monoaminergic and neuropeptidergic neurons.
The HDAC immunolocalizations (green) were visualized with a neuronal marker (magenta). A: HDAC3 was detected in the nucleus (arrow) and cytoplasm (open arrow) of oxytocin neuron, with punctate immunoreactivity (arrowheads) in the PVN. B: HDAC3 was detected in the nucleus (arrow) and cytoplasm (open arrow) of orexin neurons, with punctate immunoreactivity (arrowheads) in the LHA. C: HDAC3 was detected in the nucleus (arrow) and cytoplasm (open arrow) of histamine neuron, with punctate immunoreactivity (arrowheads) in the TMN. D: HDAC3 was detected in the nuclei (arrows) and cytoplasm (open arrow) of dopamine neurons, with punctate immunoreactivity (arrowheads) in the VTA. E: HDAC3 was detected in the nucleus (arrow) and cytoplasm (open arrow) of serotonin neuron. F: HDAC3 was detected in the nuclei (arrows) of noradrenaline neurons. G: HDAC4 was localized in the nuclei (arrows) and cytoplasm (open arrows) of CRH neurons, with punctate immunoreactivity (arrowheads) in the PVN. H: HDAC4 was detected in the nucleus (arrow) and cytoplasm (open arrows) of oxytocin neurons, with punctate immunoreactivity (arrowheads) in the PVN. I: HDAC4 was detected in the nuclei (arrows) and cytoplasm (open arrow) of vasopressin neurons. J: HDAC4 was detected in the nuclei (arrows) and cytoplasm (open arrow) of orexin neurons, with punctate immunoreactivity (arrowheads) in the LHA. K: HDAC4 was detected in the nuclei (arrows) of AgRP neurons. L: HDAC4 was detected in the nuclei (arrows) of POMC neurons, with punctate immunoreactivity (arrowheads) in the ARC. M: HDAC4 was detected in the nucleus (arrow) and cytoplasm (open arrow) of histamine neuron, with punctate immunoreactivity (arrowheads) in the TMN. N: HDAC4 was detected in the nuclei (arrows) of dopamine neurons. O: HDAC4 was detected in the nucleus (arrow) and cytoplasm (open arrow) of serotonin neuron, with punctate immunoreactivity (arrowheads) in the DR. P: HDAC4 was detected in the nuclei (arrows) and cytoplasm (open arrows) of noradrenaline neurons, with punctate immunoreactivity (arrowheads) in the LC. Scale bars indicate 10 µm.
HDAC4
In the hypothalamus, HDAC4 immunoreactivity was found mainly in the nuclei of neurons [29]. HDAC4 immunoreactivity was recognized in the CRH neurons (100±0%), the oxytocin neurons (98±2%), the vasopressin neurons (100±0%), orexin neurons (100±0%), the AgRP neurons (100±0%), the POMC neurons (92±4%), the histamine neurons (85±7%), the dopamine neurons (100±0%), the serotonin neurons (93±2%), and the noradrenaline neurons (100±0%) (Figure 2G–P, Table 2). Confocal laser scanning microscopy showed HDAC4-immunoreactive puncta in the cytoplasm of neurons containing CRH, oxytocin, vasopressin, orexin, histamine, serotonin, and noradrenaline. AgRP and POMC neurons did not show cytoplasmic immunoreactivities for HDAC4 as previously reported [29]. The strong nuclear immunoreactivity for dopamine neurons (100±0%) in the VTA and substantia nigra was consistent with the previous report [26]. A small population of histamine neurons (15±7%) did not show any HDAC4 immunoreactivity. As previously reported [26], HDAC4-immunoreactive puncta were uniform in size and were widely distributed in the neuropil of brain areas, including the PVN, LHA, ARC, TMN, DR, and LC, and a number of HDAC4-immunoreactive puncta were colocalized with PSD95-immunoreactive puncta.
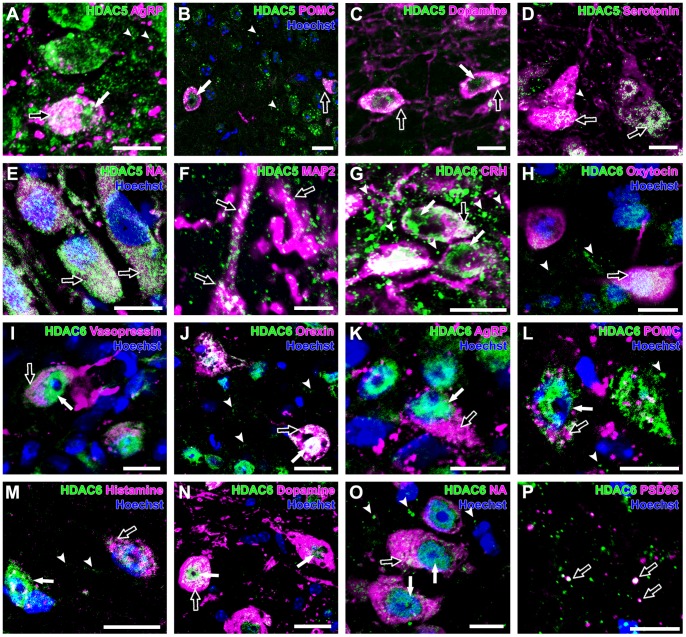
HDAC5
HDAC5 immunoreactivity was generally observed in both the nucleus and cytoplasm. HDAC5 expression was observed in the CRH neurons (100±0%), the oxytocin neurons (95±5%), the vasopressin neurons (95±3%), the orexin neurons (100±0%), the AgRP neurons (100±0%), the POMC neurons (85±1%), the histamine neurons (100±0%), the dopamine neurons (100±0%), and the serotonin neurons (100±0%)(Figures 3A–D). The noradrenaline neurons (100±0%) showed very intense immunoreactivity for HDAC5 in the cytoplasm (Figure 3E). HDAC5 was detected in the cytoplasm and dendrites of orexin neurons. Only a subset of oxytocin neurons (5±3%), vasopressin neurons (5±3%) and POMC neurons (15±1%) did not show any immunoreactivity for HDAC5 (Table 2). HDAC5-immunoreactive puncta were variable in size and distributed in the PVN, LHA, ARC, TMN, and DR (Figures 3A–E). HDAC5-immunoreactive puncta were observed in the MAP2-positive dendrites (Figure 3F). A very small population of HDAC5-immunoreactive puncta in the neuropil was overlapped with PSD95-immunoreactive puncta.
Figure 3. Expression of HDAC5 and -6 in monoaminergic and neuropeptidergic neurons.
The HDAC immunolocalizations (green) were visualized with a neuronal marker (magenta) and Hoechst stain (blue). A: HDAC5 was detected in the nucleus (arrow) and cytoplasm (open arrow) of AgRP neurons, with punctate immunoreactivity (arrowheads) in the ARC. B: HDAC5 was detected in the nucleus (arrow) and cytoplasm (open arrow) of POMC neurons, with punctate immunoreactivity (arrowheads) in the ARC. C: HDAC5 was detected in the nucleus (arrow) and cytoplasm (open arrows) of dopamine neurons. D: HDAC5 was detected in the cytoplasm (open arrows) of serotonin neurons, with punctate immunoreactivity (arrowhead) in the DR. E: Strong HDAC5 immunoreactivity was observed in the cytoplasm (open arrows) of noradrenaline neurons. F: Granular HDAC5 immunoreactivities were observed in the dendrites (open arrows) in the LHA. G: HDAC6 was detected in the nuclei (arrows) and cytoplasm (open arrows) of CRH neurons, with abundant HDAC6-immunoreactive puncta (arrowheads) in the PVN. H: HDAC6 was detected in the cytoplasm (open arrow) of oxytocin neurons, with punctate immunoreactivity (arrowheads) in the PVN. I: HDAC6 was detected in the nucleus (arrow) and cytoplasm (open arrow) of vasopressin neuron. J: HDAC6 was detected in the nucleus (arrow) and cytoplasm (open arrow) of orexin neurons, with punctate immunoreactivity (arrowheads) in the LHA. K: HDAC6 was detected in the nucleus (arrow) and cytoplasm (open arrow) of AgRP neurons. L: HDAC6 was detected in the nucleus (arrow) and cytoplasm (open arrow) of POMC neuron, with punctate immunoreactivity (arrowheads) in the ARC. M: HDAC6 was detected in the nucleus (arrow) and cytoplasm (open arrow) of histamine neurons, with punctate immunoreactivity (arrowheads) in the TMN. N: HDAC6 was detected in the nuclei (arrows) and cytoplasm (open arrow) of dopamine neurons. O: HDAC6 was localized in the nuclei (arrows) and cytoplasm (open arrow) of noradrenaline neurons, with punctate immunoreactivity (arrowheads) in the LC. P: Some of HDAC6-immunoreactive puncta were colocalized with PSD95-immunoreactive puncta (open arrows) in the LHA. Scale bars indicate 10 µm (A–E, G–O) and 5 µm (F, P).
HDAC6
HDAC6 immunoreactivity was observed mainly in the nucleus and weakly in the cytoplasm. HDAC6 immunoreactivity was found in all neurons that expressed orexin, AgRP, histamine, dopamine, serotonin, or noradrenaline. CRH neurons (95±2%), oxytocin neurons (70±7%), vasopressin neurons (80±3%), and POMC neurons (88±1%) showed variable HDAC6 immunoreactivity (Figure 3G–O, Table 2). The HDAC6-immunoreactive puncta in the cytoplasm varied in size, while the puncta in the neuropils were similarly sized and widely distributed in the PVN, LHA, ARC, TMN, VTA, DR, and LC. A small number of HDAC6-immunoreactive puncta were colocalized with PSD95-immunoreactive puncta (Figure 3P).
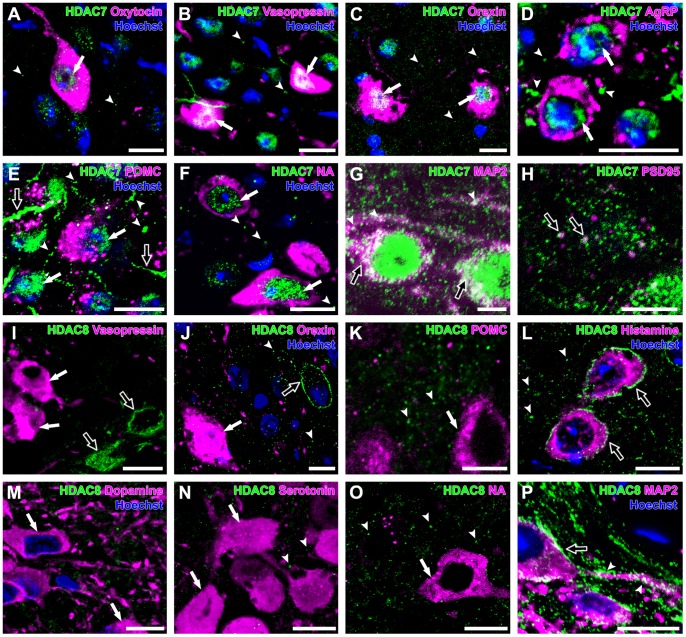
HDAC7
HDAC7 immunoreactivity was found mainly in the nuclei of neurons. HDAC7 was recognized in the CRH neurons (97±2%), the oxytocin neurons (93±3%), the vasopressin neurons (87±7%), the orexin neurons (100±0%), the AgRP neurons (100±0%), the POMC neurons (99±1%), the histamine neurons (100±0%), the dopamine neurons (100±0%), the serotonin neurons (100±0%), and the noradrenaline neurons (100±0%) (Figures 4A–F). A small population of CRH neurons (3±2%), oxytocin neurons (7±3%), and vasopressin neurons (13±7%) did not show HDAC7 immunoreactivity (Table 2). HDAC7-immunoreactive puncta in the neuropil were observed in the PVN, LHA, ARC, TMN, VTA, DR, and LC (Figures 4A–F). Double immunofluorescent observation for MAP2 and HDAC7 showed that HDAC7-immunoreactivity was rich in cytoplasm and dendrites of neurons of the PVN (Figures 4G). HDAC7-immunoreactive puncta were frequently colocalized with PSD95-immunoreactive puncta (Figure 4H).
Figure 4. Expression of HDAC7 and -8 in monoaminergic and neuropeptidergic neurons.
The HDAC immunolocalizations (green) were visualized with a neuronal marker (magenta) and Hoechst stain (blue). A: HDAC7 was detected in the nucleus (arrow) of oxytocin neuron, with punctate immunoreactivity (arrowheads) in the PVN. B: HDAC7 was detected in the nuclei (arrows) of vasopressin neurons, with punctate immunoreactivity (arrowheads) in the PVN. C: HDAC7 was detected in the nuclei (arrows) of orexin neurons, with punctate immunoreactivity (arrowheads) in the LHA. D: HDAC7 was detected in the nuclei (arrows) of AgRP neurons, with punctate immunoreactivity (arrowheads) in the ARC. E: HDAC7 immunoreactivity was detected in the nuclei (arrows) of POMC neuron with dendritic structures (open arrows) and puncta in neuropil (arrowheads). F: HDAC7 was detected in the nuclei (arrows) of noradrenaline neurons, with punctate immunoreactivity (arrowheads) in the LC. G: HDAC7 was detected in the cytoplasm (open arrow) and the dendrites (arrowheads) of neurons of the PVN. H: Some HDAC7-immunoreactive puncta were overlapped with PSD95-immunoreactive puncta (open arrows) of the LHA. I: HDAC8 was detected in cells (open arrows) in the PVN, but not in vasopressin neurons (arrows). J: HDAC8 was detected in a cell in the LHA in a pericellular pattern (open arrow), with punctate immunoreactivity (arrowheads), but not in orexin neuron (arrow). K: HDAC8 was not observed in POMC neuron (arrows). HDAC8-immunoreactive puncta (arrowheads) were widely distributed in the ARC. L: HDAC8 was detected in histamine neurons in a pericellular pattern (open arrows), with punctate immunoreactivity (arrowheads) in the TMN. M: HDAC8 was not observed in dopamine neurons (arrows). N: HDAC8 was not observed in serotonin neurons (arrows). HDAC8-positive punctate immunoreactivities (arrowheads) were widely distributed in the DR. O: HDAC8 was not observed in noradrenaline neuron (arrow). HDAC8-positive punctate immunoreactivities (arrowheads) were widely distributed in the LC. P: HDAC8 was detected in the periphery of the cytoplasm (open arrow) and dendrites (arrowheads) of the TMN neuron. Scale bars indicate 10 µm (A–F, I–P) and 5 µm (G,H).
HDAC8
HDAC8 immunoreactivity showed a clear difference among neuronal groups (Figures 4I–O). Surprisingly, all histamine neurons (100±0%) of the TMN showed HDAC8 immunoreactivity around and adjacent to histamine-immunoreactive cytoplasm, suggesting that HDAC8 was localized adjacent to the plasma membrane (Figure 4L). In addition, a small population of oxytocin neurons (4±3%) showed HDAC8 immunoreactivity in a pericellular pattern, similar to the pattern observed in the histamine neurons. In contrast, HDAC8 immunoreactivity was not recognized in any of the CRH neurons, vasopressin neurons, orexin neurons, AgRP neurons, POMC neurons, dopamine neurons, serotonin neurons, or noradrenaline neurons (Figures 4I–K, M–O, Table 2). Importantly, the majority of HDAC8-positive neurons of the brain, including the PVN, ventromedial hypothalamus, and LHA, showed HDAC8 immunoreactivity throughout the cytoplasm without a pericellular staining pattern, as previously reported (Figure 4I) [29]. HDAC8-immunoreactive puncta were uniform in size and widely distributed in the PVN, LHA, ARC, TMN, VTA, DR, and LC. The pericellular immunoreactivity was also observed along dendrites (Figure 4P). A very small population of HDAC8-immunoreactive puncta in the neuropil was overlapped with PSD95-immunoreactive puncta.
HDAC9
HDAC9 immunoreactivity was mainly observed in the nucleus and weakly in the cytoplasm. HDAC9 immunoreactivity was found in almost all neurons that expressed CRH (100±0%), oxytocin (100±0%), vasopressin (100±0%), orexin (100±0%), AgRP (100±0%), POMC (96±4%), histamine (96±2%), dopamine (100±0%), serotonin (100±0%), or noradrenaline (100±0%), except for a very small subset of POMC neurons (4±4%), histamine neurons (4±2%), and dopamine neurons (5±1%)(Figures 5A–I, Table 2). HDAC9-immunoreactive puncta in the cytoplasm and the neuropils were not uniform in size and were observed in the PVN, LHA, ARC, TMN, DR, VTA, and LC. A small population of HDAC9-immunoreactive puncta in the neuropil was overlapped with PSD95-immunoreactive puncta.
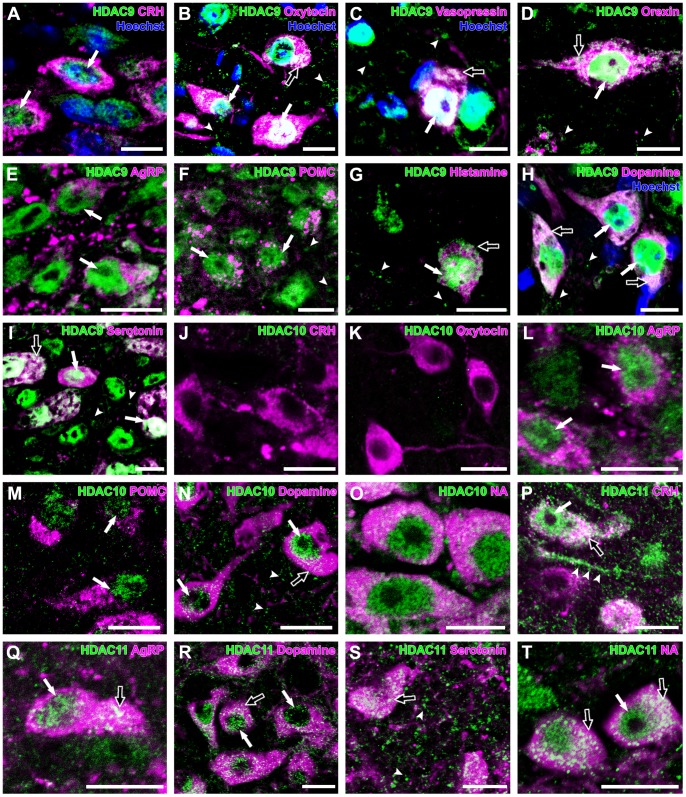
Figure 5. Expression of HDAC9,-10 and -11 in monoaminergic and neuropeptidergic neurons.
The HDAC immunolocalizations (green) were visualized with a neuronal marker (magenta) and Hoechst stain (blue). A: HDAC9 was detected in the nuclei (arrows) of CRH neurons. B: HDAC9 was detected in the nuclei (arrows) and cytoplasm (open arrow) of oxytocin neurons, with punctate immunoreactivity (arrowheads) in the PVN. C: HDAC9 was detected in the nucleus (arrow) and cytoplasm (open arrow) of vasopressin neuron, with punctate immunoreactivity (arrowheads) in the PVN. D: HDAC9 was detected in the nucleus (arrow) and cytoplasm (open arrow) of orexin neuron, with punctate immunoreactivity (arrowheads) in the LHA. E: HDAC9 was detected in the nuclei (arrows) of AgRP neurons. F: HDAC9 was detected in the nuclei (arrows) of POMC neurons, with punctate immunoreactivity (arrowheads) in the ARC. G: HDAC9 was detected in the nucleus (arrow) and cytoplasm (open arrow) of histamine neuron, with punctate immunoreactivity (arrowheads) in the TMN. H: HDAC9 was detected in the nuclei (arrows) and cytoplasm (open arrows) of dopamine neurons, with punctate immunoreactivity (arrowheads) in the VTA. I: HDAC9 was detected in the nuclei (arrows) and cytoplasm (open arrow) of serotonin neurons, with punctate immunoreactivity (arrowheads) in the DR. J: HDAC10 was not detected in the CRH neurons. K: Oxytocin neurons did not show any immunoreactivity for HDAC10. L: HDAC10 was detected in the nuclei (arrows) of AgRP neurons. M: HDAC10 was detected in the nuclei (arrows) of POMC neurons. N: HDAC10 was detected in the nuclei (arrows) and cytoplasm (open arrow) of dopamine neurons, with punctate immunoreactivity (arrowheads) in the VTA. O: HDAC10 was detected in the nuclei and cytoplasm of noradrenaline neurons. P: HDAC11 was detected in the nucleus (arrow) and cytoplasm (open arrow) of CRH neuron and also detected in the neuropils and dendritic structures (arrowheads) in the PVN. Q: HDAC11 was detected in the nucleus (arrow) and cytoplasm (open arrow) of AgRP neuron. R: HDAC11 was detected in the nuclei (arrows) and cytoplasm (open arrow) of dopamine neurons. S: HDAC11 was detected in the cytoplasm (open arrow) of serotonin neurons, with punctate immunoreactivity (arrowheads) in the DR. T: HDAC11 was detected in the nucleus (arrow) and cytoplasm (open arrows) of noradrenaline neurons. Scale bars indicate 10 µm.
HDAC10
HDAC10 showed differential expression patterns among the monoaminergic and neuropeptidergic neuron groups. HDAC10 immunoreactivity was observed in almost all neurons that expressed AgRP (98±2%), dopamine (95±1%), or noradrenaline (100±0%), whereas no HDAC10 immunoreactivity were found in CRH neurons, oxytocin neurons, vasopressin neurons, orexin neurons, histamine neurons, and serotonin neurons (Figures 5J–O). Half of POMC neuron population (55±4%) was immunoreactive for HDAC10. Whereas HDAC10 immunoreactivity within the AgRP neurons and POMC neurons was confined to the nuclei, the HDAC10 immunoreactivity of dopamine neurons and noradrenaline neurons was observed in both the nucleus and cytoplasm (Figures 5N, O, Table 2). HDAC10-immunoreactive puncta in the neuropil were observed in the PVN, LHA, ARC, TMN, DR, and LC. A small population of HDAC10-immunoreactive puncta in the neuropils was overlapped with PSD95-immunoreactive puncta.
HDAC11
HDAC11 immunoreactivity was observed in both the nucleus and cytoplasm of almost all neurons that expressed oxytocin (89±8%), vasopressin (100±0%), orexin (100±0%), AgRP (100±0%), POMC (85±5%), histamine (100±0%), dopamine (100±0%), serotonin (100±0%), or noradrenaline (100±0%)(Figures 5P–T, Table 2). Half of CRH neurons (45±10%) were immunoreactive for HDAC11. HDAC11 immunoreactivity was also found in dendritic structures in the hypothalamus (Figure 5P). HDAC11-immunoreactive puncta were uniform in size and widely distributed in the PVN, LHA, ARC, TMN, VTA, DR, and LC, and were frequently colocalized with PSD95-immunoreactive puncta.
Discussion
In the present study, we examined the expression profile of the HDAC protein family in monoaminergic and neuropeptidergic neurons. The expression patterns of HDAC1,-2,-3,-5,-6,-7,-9, and -11 were very similar among all monoaminergic and neuropeptidergic neurons, while the HDAC4, -8, and -10 immunoreactivity patterns were clearly different among the neuronal groups.
Differential Expression of HDAC10 among Neuron Groups
HDAC10 expression was observed in AgRP neurons, POMC neurons, dopamine neurons and noradrenaline neurons but not in neurons containing CRH, oxytocin, vasopressin, orexin, histamine, or serotonin. Nuclear HDAC10 immunoreactivity was consistent among HDAC10-positive neurons, while cytoplasmic HDAC10 immunoreactivity was clearly observed in the dopamine neurons and noradrenaline neurons.
HDAC10, a member of the class IIb HDAC family, has a catalytic domain in the amino terminal half and is leucine-rich in the carboxyl terminal half. Although the function of HDAC10 remains largely unknown, HDAC10 is associated with hsc70, Pax3, and KAP1 and interacts with histones to enhance the deacetylated status of target molecules [34]. Another member of the class IIb HDAC family, HDAC6, has a highly similar catalytic domain as HDAC10 and deacetylates histones and cytoplasmic proteins such as alpha-tubulin, actin-binding protein, contactin, and heat shock chaperone protein HSP90 [20], [35]. Thus, HDAC10 could alter the acetylation status of a variety of nuclear and cytoplasmic molecules of neurons, resulting in a change in gene transcription and cellular function. The clear difference in HDAC10 expression among neuronal groups suggests that HDAC10 regulates gene expression levels, which are pivotal for the functional and biological specificity of AgRP, POMC, dopamine, and noradrenaline neurons.
HDAC8 Expression in Histamine Neurons
Although HDAC8 was not expressed in the neuropeptidergic and monoaminergic neurons we examined in the current study, the histamine neuron is a unique exception. HDAC8 immunoreactivity was found in the cytoplasm of all histamine neurons with a pericellular pattern. Surprisingly, the HDAC8 immunoreactivity within the histamine neurons was confined to the cytoplasmic periphery, sparing a cytoplasmic region positive for histamine. We observed that the subcellular immunoreactive pattern for HDAC8 in neurons of the mouse brain displays nucleocytoplasmic, cytoplasmic, and pericellular distribution patterns. In the amygdala, cerebral cortex, hippocampus, and hypothalamus, a small population of neurons showed moderate to strong HDAC8 immunoreactivity in the cytoplasm and dendrites [29]. Confocal observation of HDAC8 immunoreactivity also identified cytoplasmic expression of HDAC8 in the amygdala, hippocampus, and cerebral cortex (data not shown).
HDAC8 belongs to the class I HDAC family; can deacetylase all core histones; and is associated with EST1B, Hsp70, Hsp90 and STIP [20], [36]. In smooth muscle cells, HDAC8 is co-localized with alpha-smooth muscle actin filament [37] and is interacts directly with it [38]. Although the function of HDAC8 in neurons remains unknown, the abundant HDAC8 localization in the peripheral region of the cytoplasm and dendrites of a specific subset of neurons, including histamine neurons, suggests that an undiscovered role of HDACs is in intracellular signaling rather than gene transcription. Although we previously found that a subset of neurons in the anterior parvicellular and periventricular subdivisions of the PVN changed HDAC8 immunoreactivity in response to fasting and high-fat diet feeding [29], none of the PVN neurons that expressed CRH, oxytocin, or vasopressin were positive for HDAC8. This result is consistent with the localization of only a few neurons containing CRH, oxytocin, or vasopressin in the anterior parvicellular and periventricular subdivisions of the PVN [39].
Differential Expression Profiles among Neuron Groups
All groups of monoaminergic and neuropeptidergic neurons showed immunoreactivity for HDAC4, but the subcellular distribution and intensity varied. Cytoplasmic immunoreactivity for HDAC4 was not observed in AgRP neurons, POMC neurons, or dopamine neurons; however, cytoplasmic HDAC4 was detected in other neuronal groups. Interestingly, almost all neurons showing cytoplasmic HDAC4 immunoreactivity did not have immunoreactivity for HDAC10 (Table 2). The only exception was in the noradrenaline neurons, which showed both HDAC10 immunoreactivity and cytoplasmic HDAC4 immunoreactivity.
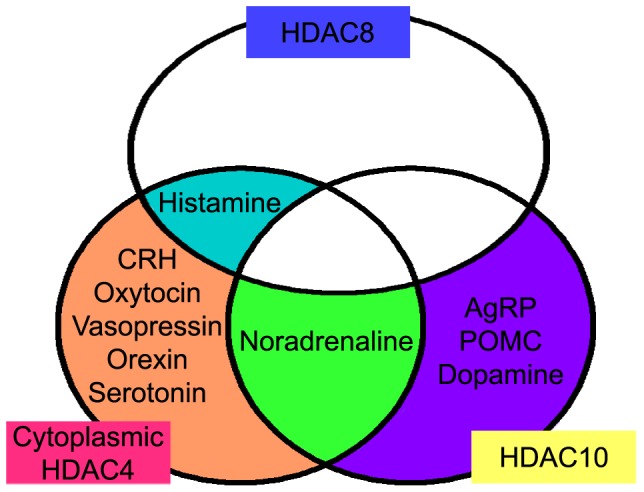
Thus, based on the HDAC4, -8, and -10 immunoreactivities, the monoaminergic and neuropeptidergic neurons we examined were classified into four groups: 1) HDAC8-positive: histamine neurons; 2) HDAC10-positive and cytoplasmic HDAC4-negative: AgRP neurons, POMC neurons and dopamine neurons; 3) HDAC10-positive and cytoplasmic HDAC4-positive: noradrenaline neurons; and 4) HDAC10-negative and cytoplasmic HDAC4-positive: CRH neurons, oxytocin neurons, vasopressin neurons, and serotonin neurons (Figure 6). Although this classification is valid for the adult male mouse under basal conditions, it could differ based on gender, age, stress, and nutrition. Importantly, HDAC4 showed activity-dependent translocation from the nucleus to the cytoplasm in vitro [25], suggesting that the subcellular localization of HDAC4 could be dynamically regulated in response to environmental stimuli. Generally, most monoaminergic and neuropeptidergic neurons express HDACs in an all-or-none manner, but POMC neurons showed a variable population of HDAC-positive cells, especially for HDAC3 and HDAC10. This variable expression may be associated with the differential expression of the anorexigenic genes of POMC neurons, by modulating the acetylation status of genes important for feeding and body weight regulation when a mouse is fasted or fed a high-fat diet.
Figure 6. Differential HDAC expression within the monoaminergic and neuropeptidergic neurons of adult male mice.
HDACs Immunoreactivity in the Neuropils
We observed HDAC-immunoreactive puncta in the neuropil. The number of puncta varied among both HDACs and brain regions. Double immunofluorescent observation of HDACs with MAP2 showed that HDACs-immunoreactive puncta were frequently localized in the dendrites. Puncta immunoreactive for HDAC4 and -11 showed frequent colocalization with PSD95-immunoreactive puncta [26], but the majority of PSD95-immunoreactive puncta were negative for HDACs. Although the substrates and functions of HDACs in the dendrites or spines are unknown, punctate distribution suggests that the HDACs exit in a functional compartment which may be involved in molecular traffic between the cell body and spines, or spine activity [40], [41].
Acknowledgments
The authors thank Hiroko Arai and Akane Iijima for excellent technical assistance for this study.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number 22590228 to HF, MEXT KAKENHI Grant Number 23126526 to HF, the Research Grant from TANITA Healthy Weight Community Trust to HF, the Research Promotion Grant from Toho University Graduate School of Medicine (No.12-02) to HF, and the Research Grant from Toho University School of Medicine (No.21-3) to HF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443: 289–295 Available: http://www.ncbi.nlm.nih.gov/pubmed/16988703. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature reviews Neuroscience 8: 171–181. Available: http://www.ncbi.nlm.nih.gov/pubmed/17299454. Accessed 2010 July 16. [DOI] [PubMed]
- 3.Koob GF (2008) A role for brain stress systems in addiction. Neuron 59: 11–34. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2748830&tool=pmcentrez&rendertype=abstract. Accessed 2012 March 21. [DOI] [PMC free article] [PubMed]
- 4. Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, et al. (2009) Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell metabolism 9: 64–76 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2630400&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andretic R, Franken P, Tafti M (2008) Genetics of sleep. Annual review of genetics 42: 361–388 Available: http://www.ncbi.nlm.nih.gov/pubmed/18983259. [DOI] [PubMed] [Google Scholar]
- 6.Young K a, Gobrogge KL, Wang Z (2011) The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neuroscience and biobehavioral reviews 35: 498–515. Available: http://www.ncbi.nlm.nih.gov/pubmed/20600286. Accessed 2013 Feb 2. [DOI] [PMC free article] [PubMed]
- 7. Donaldson ZR, Young LJ (2008) Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, NY) 322: 900–904 Available: http://www.ncbi.nlm.nih.gov/pubmed/18988842. [DOI] [PubMed] [Google Scholar]
- 8. Zylan KD, Brown SD (1996) Effect of stress and food variety on food intake in male and female rats. Physiology & behavior 59: 165–169 Available: http://www.ncbi.nlm.nih.gov/pubmed/8848477. [DOI] [PubMed] [Google Scholar]
- 9. Iwasaki S, Inoue K, Kiriike N, Hikiji K (2000) Effect of maternal separation on feeding behavior of rats in later life. Physiology & behavior 70: 551–556 Available: http://www.ncbi.nlm.nih.gov/pubmed/11111010. [DOI] [PubMed] [Google Scholar]
- 10. Vazquez-Palacios G, Velazquez-Moctezuma J (2000) Effect of electric foot shocks, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiology & behavior 71: 23–28 Available: http://www.ncbi.nlm.nih.gov/pubmed/11134681. [DOI] [PubMed] [Google Scholar]
- 11.Feng P, Vurbic D, Wu Z, Strohl KP (2007) Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain research 1154: 163–172. Available: http://www.ncbi.nlm.nih.gov/pubmed/17466285. Accessed 2012 April 7. [DOI] [PubMed]
- 12.Takase K, Yamamoto Y, Yagami T (2012) Maternal deprivation in the middle of a stress hyporesponsive period decreases hippocampal calcineurin expression and causes abnormal social and cognitive behaviours in adult male Wistar rats: relevance to negative symptoms of schizophrenia. Behavioural brain research 232: 306–315. Available: http://www.ncbi.nlm.nih.gov/pubmed/22543011. Accessed 2013 Feb 2. [DOI] [PubMed]
- 13. Tsankova N, Renthal W, Kumar A, Nestler EJ (2007) Epigenetic regulation in psychiatric disorders. Nature reviews Neuroscience 8: 355–367 Available: http://www.ncbi.nlm.nih.gov/pubmed/17453016. [DOI] [PubMed] [Google Scholar]
- 14. Dulac C (2010) Brain function and chromatin plasticity. Nature 465: 728–735 Available: http://www.nature.com/doifinder/10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182. Available: http://www.ncbi.nlm.nih.gov/pubmed/17468743. Accessed 2012 March 7.
- 16. Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, et al. (2007) Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56: 517–529 Available: http://www.ncbi.nlm.nih.gov/pubmed/17988634. [DOI] [PubMed] [Google Scholar]
- 17.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, et al. (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science (New York, NY) 328: 753–756. Available: http://www.ncbi.nlm.nih.gov/pubmed/20448184. Accessed 2010 July 13. [DOI] [PubMed]
- 18.Bonthuis PJ, Patteson JK, Rissman EF (2011) Acquisition of sexual receptivity: roles of chromatin acetylation, estrogen receptor-alpha, and ovarian hormones. Endocrinology 152: 3172–3181. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3138229&tool=pmcentrez&rendertype=abstract. Accessed 2012 April 7. [DOI] [PMC free article] [PubMed]
- 19.Ray S, Lee C, Hou T, Boldogh I, Brasier AR (2008) Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic acids research 36: 4510–4520. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2490754&tool=pmcentrez&rendertype=abstract. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed]
- 20. Yang XJ, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nature reviews Molecular cell biology 9: 206–218 Available: http://www.ncbi.nlm.nih.gov/pubmed/18292778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein J a, et al. (2010) Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Developmental cell 19: 807–818. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3003338&tool=pmcentrez&rendertype=abstract. Accessed 2012 Nov 23. [DOI] [PMC free article] [PubMed]
- 22.Li G, Jiang H, Chang M, Xie H, Hu L (2011) HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases. Journal of the neurological sciences 304: 1–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/21377170. Accessed 2012 Nov 23. [DOI] [PubMed]
- 23. De Ruijter AJM, Van Gennip AH, Caron HN, Kemp S, Van Kuilenburg ABP (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. The Biochemical journal 370: 737–749 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1223209&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annual review of biochemistry 76: 75–100. Available: http://www.ncbi.nlm.nih.gov/pubmed/17362198. Accessed 2010 July 17. [DOI] [PubMed]
- 25.Chawla S, Vanhoutte P, Arnold FJL, Huang CLH, Bading H (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. Journal of Neurochemistry 85: 151–159. Available: http://blackwell-synergy.com/doi/abs/10.1046/j.1471-4159.2003.01648.x. Accessed 2010 July 17. [DOI] [PubMed]
- 26. Darcy MJ, Calvin K, Cavnar K, Ouimet CC (2010) Regional and subcellular distribution of HDAC4 in mouse brain. The Journal of comparative neurology 518: 722–740 Available: http://www.ncbi.nlm.nih.gov/pubmed/20034059. [DOI] [PubMed] [Google Scholar]
- 27. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, et al. (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60 Available: http://www.ncbi.nlm.nih.gov/pubmed/19424149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, et al. (2008) Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456: 997–1000. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2742159&tool=pmcentrez&rendertype=abstract. Accessed 2010 July 17. [DOI] [PMC free article] [PubMed]
- 29.Funato H, Oda S, Yokofujita J, Igarashi H, Kuroda M (2011) Fasting and High-Fat Diet Alter Histone Deacetylase Expression in the Medial Hypothalamus. PLoS ONE 6: e18950. Available: http://dx.plos.org/10.1371/journal.pone.0018950. Accessed 2011 April 25. [DOI] [PMC free article] [PubMed]
- 30. Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, et al. (2007) Distribution of Histone Deacetylases 1–11 in the Rat Brain. Brain 31: 47–58 doi:10.1385/JMN/31. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Brailoiu GC, Brailoiu E, Dun SL, Yang J, et al. (2008) Copeptin immunoreactivity and calcium mobilisation in hypothalamic neurones of the rat. Journal of neuroendocrinology 20: 1242–1251. Available: http://www.ncbi.nlm.nih.gov/pubmed/18752653. Accessed 2012 Dec 3. [DOI] [PubMed]
- 32.Oda S, Funato H, Adachi-Akahane S, Ito M, Okada A, et al. (2010) Dopamine D5 receptor immunoreactivity is differentially distributed in GABAergic interneurons and pyramidal cells in the rat medial prefrontal cortex. Brain research 1329: 89–102. Available: http://www.ncbi.nlm.nih.gov/pubmed/20226768. Accessed 2010 Nov 15. [DOI] [PubMed]
- 33.Paxinos G, Franklin KBJ (2008) The Mouse Brain in Stereotaxic Coordinates. Third. Academic Press.
- 34.Lai IL, Lin TP, Yao YL, Lin CY, Hsieh MJ, et al. (2010) Histone deacetylase 10 relieves repression on the melanogenic program by maintaining the deacetylation status of repressors. The Journal of biological chemistry 285: 7187–7196. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2844168&tool=pmcentrez&rendertype=abstract. Accessed 2012 Dec 3. [DOI] [PMC free article] [PubMed]
- 35. Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, et al. (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458 Available: http://www.ncbi.nlm.nih.gov/pubmed/12024216. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Sengupta N, Villagra A, Rezai-Zadeh N, Seto E (2006) Histone deacetylase 8 safeguards the human ever-shorter telomeres 1B (hEST1B) protein from ubiquitin-mediated degradation. Molecular and cellular biology 26: 5259–5269. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1592721&tool=pmcentrez&rendertype=abstract. Accessed 2012 Dec 3. [DOI] [PMC free article] [PubMed]
- 37. Waltregny D, De Leval L, Glénisson W, Ly Tran S, North BJ, et al. (2004) Expression of histone deacetylase 8, a class I histone deacetylase, is restricted to cells showing smooth muscle differentiation in normal human tissues. The American journal of pathology 165: 553–564 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1618574&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waltregny D, Glénisson W, Tran SL, North BJ, Verdin E, et al. (2005) Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB journal?: official publication of the Federation of American Societies for Experimental Biology 19: 966–968 Available: http://www.ncbi.nlm.nih.gov/pubmed/15772115. [DOI] [PubMed] [Google Scholar]
- 39.Simmons DM, Swanson LW (2009) Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. The Journal of comparative neurology 516: 423–441. Available: http://www.ncbi.nlm.nih.gov/pubmed/19655400. Accessed 2010 Aug 3. [DOI] [PubMed]
- 40.Hirokawa N, Niwa S, Tanaka Y (2010) Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron 68: 610–638. Available: http://linkinghub.elsevier.com/retrieve/pii/S0896627310007816. Accessed 2010 Nov 18. [DOI] [PubMed]
- 41.Kennedy MJ, Ehlers MD (2006) Organelles and trafficking machinery for postsynaptic plasticity. Annual review of neuroscience 29: 325–362. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1876664&tool=pmcentrez&rendertype=abstract. Accessed 2012 Nov 12. [DOI] [PMC free article] [PubMed]