Abstract
Cell-penetrating peptides (CPPs) are short peptides which can carry various types of molecules into cells; however, although most CPPs rapidly penetrate cells in vitro, their in vivo tissue-targeting specificities are low. Herein, we describe cell-binding, internalization, and targeting characteristics of a newly identified 10-residue CPP, denoted ECP32–41, derived from the core heparin-binding motif of human eosinophil cationic protein (ECP). Besides traditional emphasis on positively charged residues, the presence of cysteine and tryptophan residues was demonstrated to be essential for internalization. ECP32–41 entered Beas-2B and wild-type CHO-K1 cells, but not CHO cells lacking of cell-surface glycosaminoglycans (GAGs), indicating that binding of ECP32–41 to cell-surface GAGs was required for internalization. When cells were cultured with GAGs or pre-treated with GAG-digesting enzymes, significant decreases in ECP32–41 internalization were observed, suggesting that cell-surface GAGs, especially heparan sulfate proteoglycans were necessary for ECP32–41 attachment and penetration. Furthermore, treatment with pharmacological agents identified two forms of energy-dependent endocytosis, lipid-raft endocytosis and macropinocytosis, as the major ECP32–41 internalization routes. ECP32–41 was demonstrated to transport various cargoes including fluorescent chemical, fluorescent protein, and peptidomimetic drug into cultured Beas-2B cells in vitro, and targeted broncho-epithelial and intestinal villi tissues in vivo. Hence this CPP has the potential to serve as a novel vehicle for intracellular delivery of biomolecules or medicines, especially for the treatment of pulmonary or gastrointestinal diseases.
Introduction
Cell-penetrating peptides (CPPs) are peptides derived from proteins that can transport cargo such as nanoparticles, low molecular weight compounds, other peptides, proteins, and nucleic acids into cells [1]. CPPs may potentially be used during clinical procedures such as gene therapy and cancer treatment, and thus substantial efforts have been made to discover CPPs with suitable carrier properties [1], [2].
Most CPPs are rich in positively charged Arg and/or Lys residues, and are internalized after initially interacting with negatively charged cell surface glycosaminoglycans (GAGs), which cluster CPPs on outer membrane surfaces [3], [4]. Cell-surface GAGs are complex polysaccharides that participate in cell growth, differentiation, morphogenesis, migration, and bacterial/viral infections. Major vertebrate GAGs include heparan sulfate (HS), chondroitin sulfate (CS)/dermatan sulfate (DS), and hyaluronic acid (HA) [5], [6]. It has been shown that syndecan-4, a heparan sulfate proteoglycan (HSPG), accelerates the uptake of cationic CPPs penetratin and octa-arginine into K562 cells [7].
CPPs are usually divided into two groups [1], synthetic peptides such as oligoarginines which penetrate 293T cells [8], [9], and peptides derived from natural proteins such as TAT47–57 (GRKKRRQRRRP) from nuclear transcription activator Tat protein (TAT) of human immunodeficiency virus-1, which penetrates various cell types [10]. In the past two decades, 52 CPPs derived from natural proteins that can translocate across cell membranes have been reported [1], [11]. Twenty-eight of these CPPs including 15 viral protein-derived peptides, 7 animal modulator-derived peptides, 3 antimicrobial peptides, and 3 toxin-derived peptides have been demonstrated or predicted to interact with cell-surface HS before penetrating plasma membranes [1], [11], [12]. Most of these heparin-binding CPPs possess consensus heparin-binding motifs XBBXB or XBBBXXBX, where B is a basic amino acid and X is any amino acid. These peptides are further classified as cationic or amphipathic peptides [13]. Heparin-binding CPPs not only requires electrostatic interactions, but also uses aromatic residues for hydrophobic interactions with target cells [14]. However, little is known about how sequential aromatic and cationic residues affect the interactions of CPPs with cell-surface molecules.
Human eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) are secretory ribonucleases (RNases) released by activated eosinophils [15]. Both ECP (RNase 3) and EDN (RNase 2) possess antiviral and antiparasitic activities [15]. Interestingly, the RNase activity of ECP is much lower than that of EDN [16], although ECP has stronger antibacterial [15], [17] and cytotoxic activities [18]. In addition, ECP binds lipopolysaccharides and peptidoglycans tightly [19]. The N-terminal domain of ECP (residues 1–45) retains most of the antimicrobial properties [20]. Boix and colleagues identified residues 1–38 as responsible for the bactericidal activity [21] and found that a cavity created by residues A8–Q14, Y33–R36, Q40–L44, and H128–D130 could bind a HS disaccharide [22]. We have previously reported that cell-surface GAGs, especially HSPGs, act as receptors to promote ECP internalization via the macropinocytic pathway [23], resulting in apoptosis in Beas-2B cells [24]. The cytotoxicity of ECP was significantly reduced in mutant cell lines that lacked cell-surface HS or GAGs [23]. A sequential segment of ECP, 34RWRCK38, was subsequently identified as a core heparin-binding motif [25].
Very few CPPs derived from heparin-binding regions in proteins have been reported. Here two 10-residue peptides, ECP32–41 (RYRWRCKNQN) containing a novel heparin-binding motif of ECP, and EDN32–41 (NYQRRCKNQN) possessing a consensus heparin-binding motif in EDN [25], were synthesized and their cell-binding, GAG-binding, cell-penetrating, and cargo-transport activities were analysed. Interestingly, only ECP32–41 displayed CPP-like properties. The main endocytotic routes for ECP32–41 internalization were found to be temperature-sensitive and energy-dependent. ECP32–41 was able to deliver a small fluorescent molecule, a recombinant protein, and a peptidomimetic drug into cells. Moreover, an ECP32–41-tagged protein was preferentially routed to broncho-epithelial and intestinal villi tissues in rat. Here we demonstrate that ECP32–41 is the first heparin-binding CPP derived from a secretory human RNase, and we propose that it may serve as a new vehicle for intracellular cargo delivery and tissue targeting. It is a promising candidate for further molecular and cellular engineering investigations.
Results
ECP32–41 Internalization
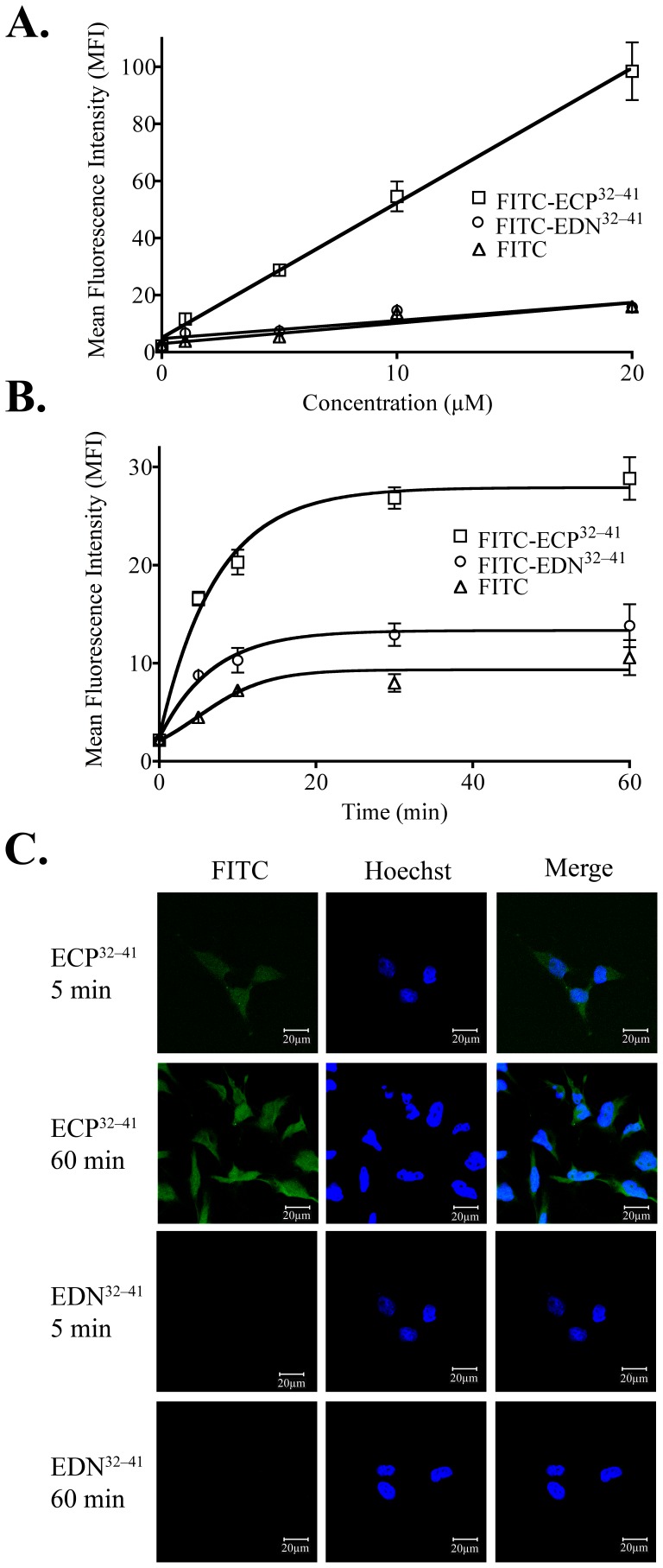
Internalization of FITC-ECP32–41 and FITC-EDN32–41 was measured as the median fluorescence intensity (MFI) of 6.0×105 Beas-2B cells that had been treated with one of the FITC-labelled peptides (1 to 20 µM) at 37°C for 1 h, and then treated with trypsin to remove surface-bound peptides. FITC-ECP32–41 internalization was concentration dependent (Figure 1A), and at each concentration tested, the signal arising from FITC-EDN32–41 fluorescence was similar to that of the corresponding FITC control (Figure 1A). When Beas-2B cells were treated with 5 µM of a FITC-peptide at 37°C, the fluorescent signal for FITC-ECP32–41 increased within 5 min, and reached plateau at 30 min (Figure 1B). FITC-EDN32–41 penetrated the cells to a lesser extent during the 60 min incubation (Figure 1B). After addition of 5 µM ECP32–41, intercellular fluorescence was clearly detected 5 and 60 min later by CLSM, whereas a signal for intracellular EDN32–41 was not detected even after 1 h (Figure 1C). ECP32–41 therefore penetrated Beas-2B cells in a time- and concentration-dependent manner, whereas EDN32–41 did not act as a CPP, even though it contained a conventional heparin-binding motif.
Figure 1. Internalization of ECP32–41 and EDN32–41.
(A) Beas-2B cells were incubated with 1, 5, 10, or 20 µM FITC-ECP32–41, FITC-EDN32–41, or FITC at 37°C for 1 h. The cells were washed twice with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and then subjected to flow cytometry. (B) Beas-2B cells were incubated with 5 µM FITC-ECP32–41, FITC-EDN32–41, or FITC at 37°C for 5, 10, 30, or 60 min. The cells were then treated as described in (A) and subjected to flow cytometry. The results in (A) and (B) are expressed as the mean ± standard deviation (S.D.), n = 3. (C) Beas-2B cells were incubated with 5 µM FITC-ECP32–41, FITC-EDN32–41, or FITC at 37°C for 5 or 60 min before CLSM. Nuclei were stained with Hoechst 34850. Scale bar: 20 µm.
Influence of Sequence and Length on ECP32–41 Internalization
The sequences of ECP32–41 and EDN32–41 differ only at positions three and four, i.e., Arg3 and Trp4 in ECP32–41 and Gln3 and Arg4 in EDN32–41 (Table 1). Because these two peptides internalized to significantly different extents (Figure 1), the importance of these residues was explored. Two ECP32–41 derivatives, ECP32–41R3Q and ECP32–41W4R, were synthesized, each with a single residue mutated to that found at the same position in EDN32–41, and were then tested for cell binding and internalization. Surprisingly, twice as much ECP32–41W4R bound to Beas-2B cells than did ECP32–41 and ECP32–41R3Q after 1 h incubation at 37°C (Table 2), indicating that an increase in the positive charge at position four led to stronger binding. Furthermore, when penetration of each peptide was assessed by FACS, only ECP32–41 internalized (Table 2). Replacement of an ECP32–41 residue with an EDN32–41 residue affected binding and internalization. The functionality of ECP32–41, as a CPP, could therefore be ascribed to both the W3R4 dipeptide sequence, and a nearly complete heparin-binding motif.
Table 1. Sequences and molecular weights of peptides.
| Peptide | Sequence | Molecular weight (Da) | |
| ECP32–41 | NYRWRCKNQN | 1381 | |
| EDN32–41 | NYQRRCKNQN | 1323 | |
| ECP32–41R3Q | NYQWRCKNQN | 1353 | |
| ECP32–41W4R | NYRRRCKNQN | 1351 | |
| ECP33–41 | YRWRCKNQN | 1268 | |
| ECP32–40 | NYRWRCKNQ | 1267 | |
| ECP33–40 | YRWRCKNQ | 1153 | |
| ECP32–39 | NYRWRCKN | 1139 | |
| ECP34–41 | RWRCKNQN | 1104 | |
| ECP32–38 | NYRWRCK | 1025 | |
| TAT47–57 | GRKKRRQRRRP | 1493 | |
| KLA | KLAKLAKKLAKLAK | 1524 | |
| KLA-TAT47–57 | KLAKLAKKLAKLAKGRKKRRQRRRP | 2999 | |
| KLA-ECP32–41 | KLAKLAKKLAKLAKNYRWRCKNQN | 2887 | |
Table 2. Binding and penetrating activities of synthetic peptides in Beas-2B cells.
| Peptide | Binding (%)a | Penetrating (%)b |
| ECP32–41 | 100 | 100 |
| EDN32–41 | 19.4±0.05*** | 17.0±4.24*** |
| ECP32–41R3Q | 87.2±0.40 | 70.6±3.30* |
| ECP32–41W4R | 549.9±1.00*** | 32.3±6.55** |
| ECP33–41 | 54.7±3.37* | 48.2±2.48* |
| ECP33–40 | 72.4±4.68* | 70.8±4.97* |
| ECP34–41 | 39.3±2.76** | 34.3±2.41** |
| ECP32–40 | 90.7±7.79 | 82.0±9.42 |
| ECP32–39 | 82.6±3.20 | 35.0±6.98** |
| ECP32–38 | 86.6±3.75 | 28.7±6.24** |
X: amino-n-butyric acid. ND: not determined. The result is expressed as the mean ± S.D., n = 4.
P<0.001;
P<0.01;
P<0.05.
Beas-2B cells were incubated with 5 µM FITC-peptides at 4°C for 1 h, washed twice with PBS, and subjected to ELISA. The amount of FITC-ECP32–41 bound to Beas-2B cells was normalized to 100%.
Beas-2B cells were incubated with 5 µM FITC-peptides at 37°C for 1 h. The cells were washed twice with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and then subjected to flow cytometry. The fluorescence of cells treated with ECP32–41 was set as 100%.
To determine the optimal length (core composition) of cell penetrating properties, deletion of residues of ECP32–41 was individually carried out done from N-terminus and C-terminus. As expected, differences in the internalization were observed with the removal of amino acids from the N-terminus or C-terminus in the sequence (Table 2). N-terminally curtailed peptides, ECP33–41, ECP34–41 and ECP34–40 showed significantly 44%, 28% and 61% lower cell binding and thus decreasing internalization by 52%, 30% and 66% (Table 2). However, C-terminally curtailed mutants, ECP32–39 and ECP32–38 showed similar cellular binding as ECP32–41 but lower internalization than wild type ECP32–41 (Table 2). Most importantly, only ECP32–41 showed the highest cellular uptake, strongly suggesting that the length of our ECP-derived CPP was critical for internalization and residues from 32 to 41 were required.
Effects of GAG on ECP32–41 Binding
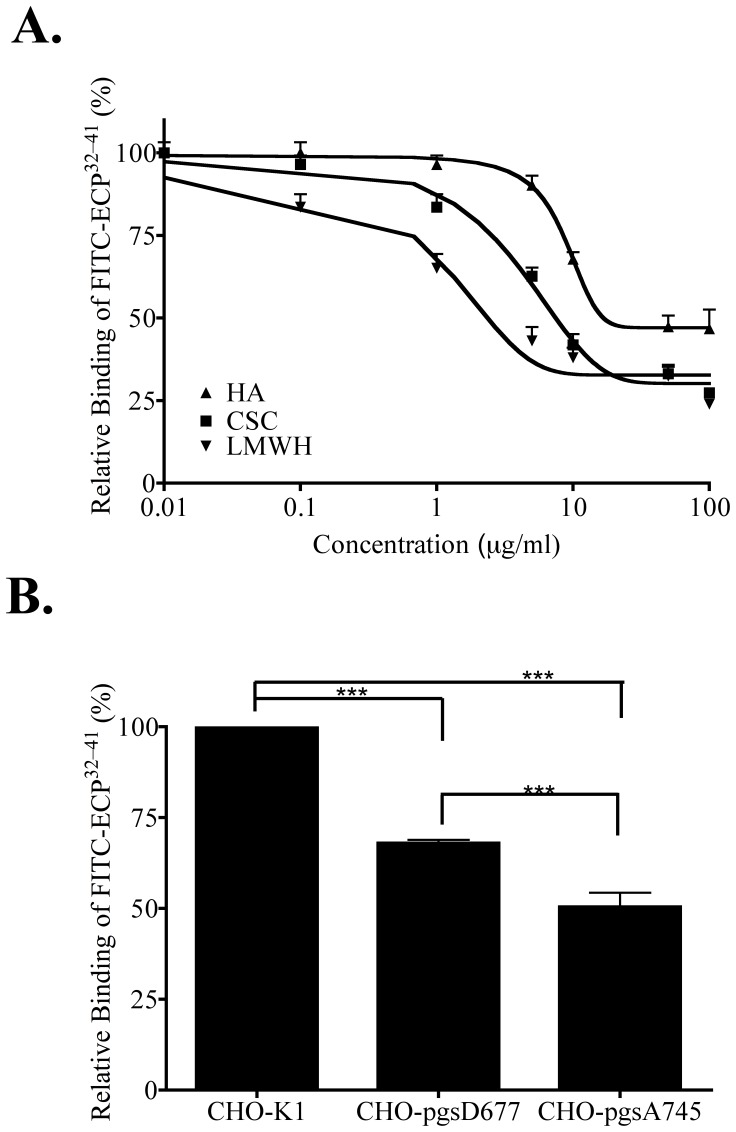
Cell-membrane GAGs including HS, CS/DS, and HA are necessary for CPP internalization [5], [6]. To assess the effect of GAGs on ECP32–41 cellular binding ability, soluble GAGs including LMWH, CSC, and HA were used as competitors to inhibit the attachment of ECP32–41 to Beas-2B cells. At concentrations between 0.01 and 1 µg/ml, LMWH, CSC, and HA inhibited ECP32–41 binding, with LMWH being the most effective and HA being the least. At concentrations exceeding 50 µg/ml, LMWH and CSC prevented approximately 70% of the normal ECP32–41 binding, whereas 53% inhibition was observed for 100 µg/ml HA (Figure 2A). Hence HS and CS might be involved in ECP32–41 binding to Beas-2B cells. To clarify the roles of cell-surface HS and CS, the binding of ECP32–41 to wild-type and two mutant strains of Chinese hamster ovary (CHO) cells was assessed by ELISA. CHO-pgsD677 cells do not express N-acetylglucosaminyltransferase and glucuronyltransferase, and therefore lack HS, but produce three times more CS than wild-type CHO-K1 cells [26]. CHO-pgsA745 cells are deficient in xylosyltransferase so that no GAG was present on the surface [27]. The amount of ECP32–41 bound to CHO-pgsA745 cells was found to be 52% less than that bound to CHO-K1 cells, suggesting that GAG was required for binding (Figure 2B). Additionally, a 31% reduction in ECP32–41 binding was observed for CHO-pgsD677 cells, even though the cells expressed much more CS than CHO-K1 cells (Figure 2B). GAGs, and especially HSPGs, are therefore crucial for the initial interaction of ECP32–41 with cell surfaces.
Figure 2. Cell-surface GAG-dependent binding of ECP32–41.
(A) Beas-2B cells were treated with the indicated concentrations of LMWH, HA, or CS for 30 min prior to incubation with 5 µM FITC-ECP32–41 at 37°C for 1 h. After washed twice with PBS, cells were subjected to ELISA. The result is expressed as the mean ± S.D., n = 3. (B) CHO cells were incubated with 5 µM FITC-ECP32–41 at 4°C for 1 h, washed twice with PBS, and subjected to ELISA. The amount of FITC-ECP32–41 bound to CHO-K1 cells was normalized to 100%. The result is expressed as the mean ± S.D., n = 3. ***, P<0.001.
Effect of HS on ECP32–41 Internalization
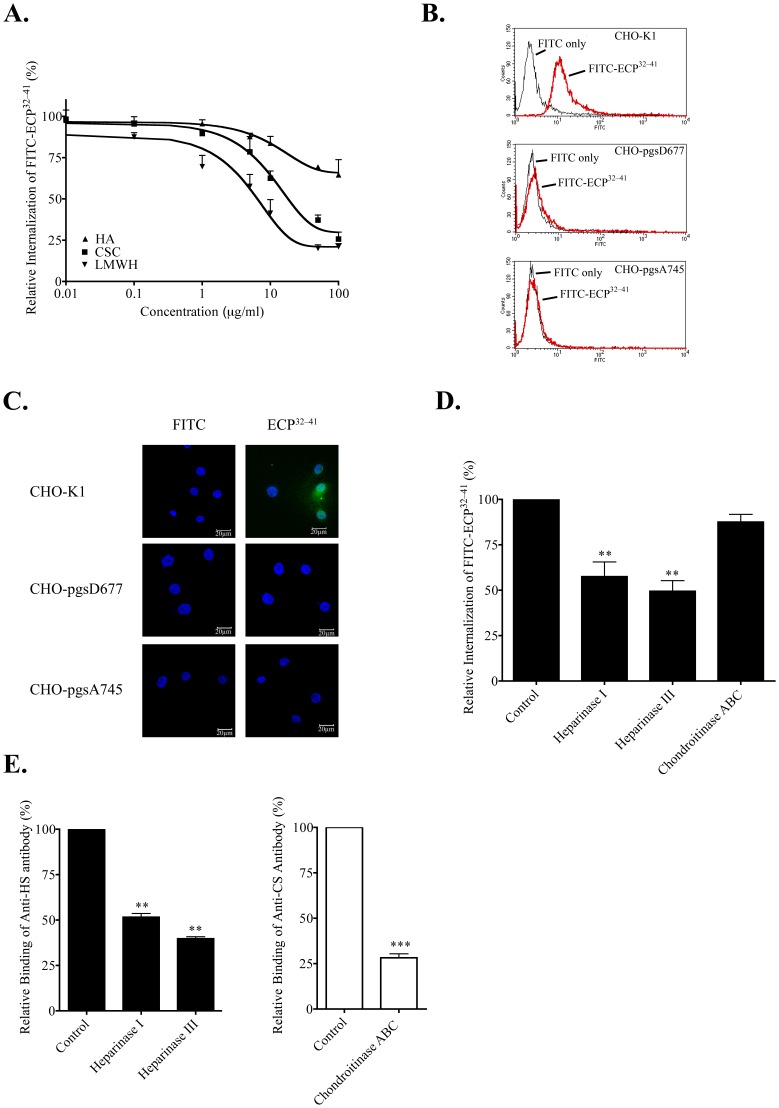
To further investigate the involvement of GAG in ECP32–41 internalization, Beas-2B cells were incubated with LMWH, CS, and HA prior to treatment with ECP32–41. The resulting inhibition profiles (Figure 3A) were similar to those for binding Beas-2B cells (Figure 2A), and the effectiveness of LMWH, CS, and HA as inhibitors decreased in the same order. LMWH and CS (each at 10 µg/ml) decreased ECP32–41 internalization by 58% and 38%, respectively. HA treatment was less effective however, and only a 35% decrease was observed at high concentration of 100 µg/ml. Both HS and CS appear to facilitate ECP32–41 binding and internalization.
Figure 3. HS-dependent ECP32–-41 internalization.
(A) Beas-2B cells were treated with the indicated concentrations of LMWH, CS, or HA for 30 min prior to incubation with 5 µM FITC-ECP32–41 at 37°C for 1 h. The cells were washed twice with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and subjected to flow cytometry. The result is expressed as the mean ± S.D., n = 3. (B) Samples of wild-type and mutant CHO cells were each incubated with 5 µM FITC-ECP32–41 at 37°C for 1 h and then subjected to flow cytometry. (C) Samples of wild-type and mutant CHO cells were incubated with 5 µM FITC-ECP32–41 at 37°C for 1 h., then washed twice with 1 ml PBS, and fixed for CLSM. Nuclei were stained with Hoechst 34850. Scale bar: 20 µm. (D) Beas-2B cells were treated with heparinase I, heparinase III, or chondroitinase ABC for 2 h prior to incubation with 5 µM FITC-ECP32–41 at 37°C for 1 h. The cells were washed twice with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and subjected to flow cytometry. Untreated cells served as the controls. The fluorescence of cells treated with FITC-ECP32–41 was set to 100%. The result is expressed as the mean ± S.D., n = 3. **, P<0.01. (E) Beas-2B cells were treated with heparinase I, heparinase III, or chondroitinase ABC for 2 h. After stained with anti-HS or anti-CS monoclonal antibodies, washed twice with 500 µl PBS, and hybridized with FITC-conjugated anti-mouse secondary antibody, cells were suspended in 500 µl PBS and subjected to flow cytometry. Untreated cells served as the control. The fluorescence of the untreated cells was set to 100%. The result is expressed as the mean ± S.D., n = 3. **, P<0.01 and ***, P<0.001.
A significant fluorescence shift reflecting FITC-ECP32–41 internalization was observed for CHO-K1 cells but not for CHO pgsD-677 or CHO pgsA-745 cells (Figure 3B). ECP32–41 internalization was also clearly observed for CHO-K1 cells but not for CHO pgsD677 or pgsA745 cells, when monitored by CLSM (Figure 3C). HS, instead of CS, is therefore the major ECP32–41 receptor.
To further confirm that cell-surface HS, rather than CS, was involved in ECP32–41 internalization, Beas-2B cells were treated with heparinase I, heparinase III or chondroitinase ABC for 2 h, and then with FITC-ECP32–41 for 1 h prior to measuring the cell fluorescence signal (Figure 3D). Pre-treatment of Beas-2B cells with heparinase I or heparinase III decreased ECP32–41 internalization by 43% and 51%, respectively. CS depletion had little effect on ECP32–41 internalization, thus heparinase, but not chondroitinase ABC, inhibits ECP32–41 internalization.
To verify that selective cell-surface polysaccharides had been enzymatically removed, the treated cells were subsequently incubated with anti-HS or anti-CS monoclonal antibodies. As expected, heparinase I and heparinase III had decreased the amount of HS on Beas-2B cell surfaces by 48% and 61%, respectively (Figure 3E, left panel), and chondroitinase ABC removed 72% of the cell-surface CS (Figure 3E, right panel). The HS that remained on Beas-2B cell surface accounted for the observed ECP32–41 internalization into heparinase-treated cells. Conversely, even though 70% of the initial cell-surface CS had been removed, ECP32–41 internalization was hardly affected–thus as concluded above, HS, rather than CS, is responsible for ECP32–41 internalization.
Temperature and Energy Dependences of ECP32–41 Internalization
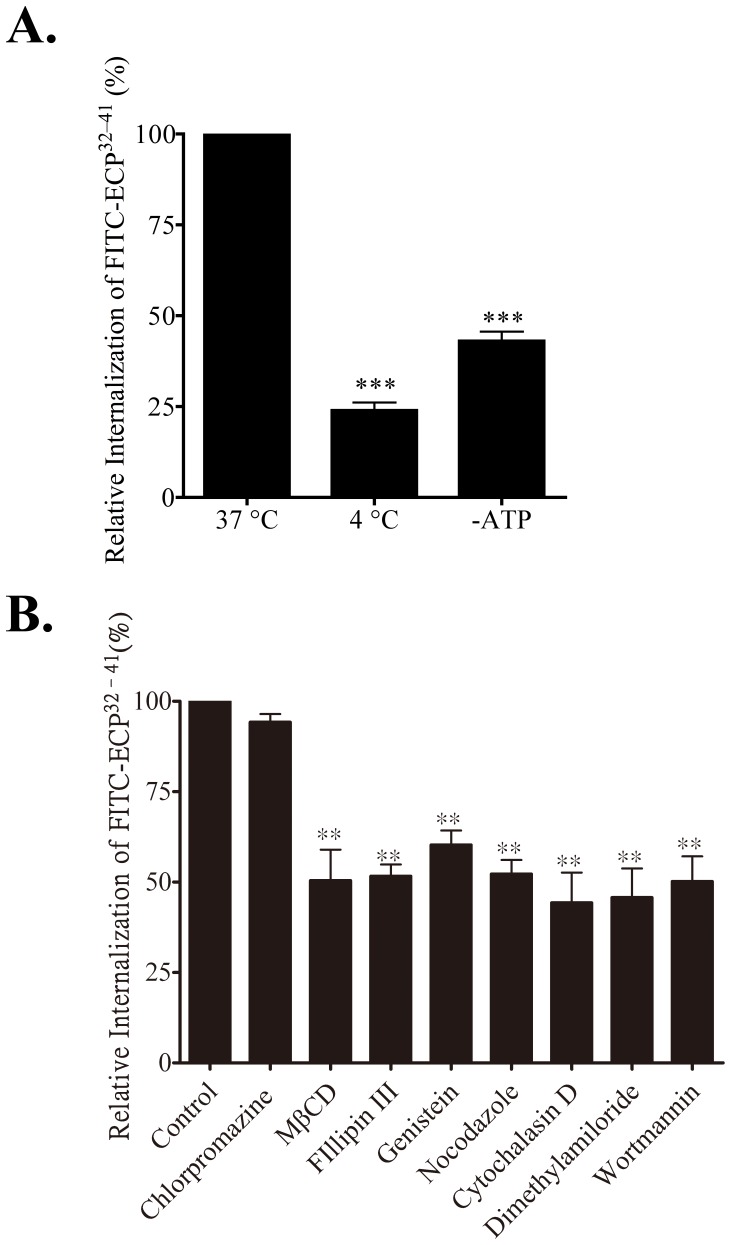
CPPs enter cells by two routes: direct translocation through lipid bilayers or energy-dependent vesicular mechanisms referred to as endocytosis [28]. Direct CPP translocation is usually observed when the CPP concentration is above 10 µM [28]. To characterize the mechanism(s) of ECP32–41 internalization at low concentrations (≤5 µM), we investigated the effect of cellular ATP depletion and low incubation temperature–both of which were expected to inhibit endocytosis. FITC-ECP32–41 internalization was inhibited by 76% at 4°C, compared to 37°C (Figure 4A), when cell samples were first incubated at these temperatures for 30 min prior to addition of 5 µM FITC-ECP32–41. Pre-incubation with sodium azide and deoxyglucose, which depleted the cellular ATP pool, inhibited FITC-ECP32–41 internalization by 57%. ECP32–41 internalization is therefore, temperature- and energy-dependent, indicating that, at low concentrations of ECP32–41, the main internalization route is endocytic in nature.
Figure 4. Internalization pathway of ECP32–41.
(A) Beas-2B cells were incubated at 37°C, 4°C, or with ATP depletion at 37°C for 30 min and then incubated with of 5 µM FITC-ECP32–41 for 1 h. The cells were washed twice with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and subjected to flow cytometry. The fluorescence of cells treated with ECP32–41 was set to 100%. The result is expressed as the mean ± S.D., n = 3. ***, P<0.001; **, P<0.01. (B) Beas-2B cells were incubated with the indicated endocytic inhibitors at 37°C for 30 min, followed by addition of 5 µM FITC-ECP32–41 at 37°C for 1 h. Cells were then treated as described in (A). The result is expressed as means ± S.D., n = 3. **, P<0.01.
ECP32–41 Internalization via Lipid-raft Dependent Macropinocytosis
Endocytic pathways are generally grouped into four categories: clathrin- and caveolin-mediated pathways, macropinocytosis, and other less-well characterized clathrin- and caveolin-independent mechanisms [29]. Some of these pathways are also lipid-raft dependent [29]. We pretreated Beas-2B cells with endocytic inhibitors to identify the pathways involved in ECP32–41 internalization. Chlorpromazine, an inhibitor of clathrin-mediated endocytosis, did not affect FITC-ECP32–41 internalization (Figure 4B), suggesting that clathrin-mediated endocytosis was not involved. The lipid-raft pathway inhibitors methyl-β-cyclodextrin and genistein inhibited FITC-ECP32–41 internalization by 48% and 40%, respectively. Cellular uptake of ECP32–41 reduced 50% in the presence of filipin III which depleted lipid raft on cell membrane, also suggesting that lipid raft-dependent endocytosis was involved in ECP32–41 internalization. Nocodazole and cytochalasin D, which blocked cytoskeleton polymerization and consequently phagosome and macropinosome formation, respectively, reduced FITC-ECP32–41 internalization by 48% and 56%, respectively. Dimethyl amilorides, an inhibitor of the Na+/H+ ion exchange pump resulting in the cessation of macropinocytosis, and wortmannin, an inhibitor of both macropinocytosis and clathrin-mediated endocytosis, inhibited internalization by 50% and 53%, which indicated that macropinocytosis was involved. Lipid rafts are therefore involved in ECP32–41 internalization, and two pathways appear to govern ECP32–41 internalization: actin-dependent endocytosis and lipid-raft macropinocytosis.
Cytotoxic Effects of ECP32–41
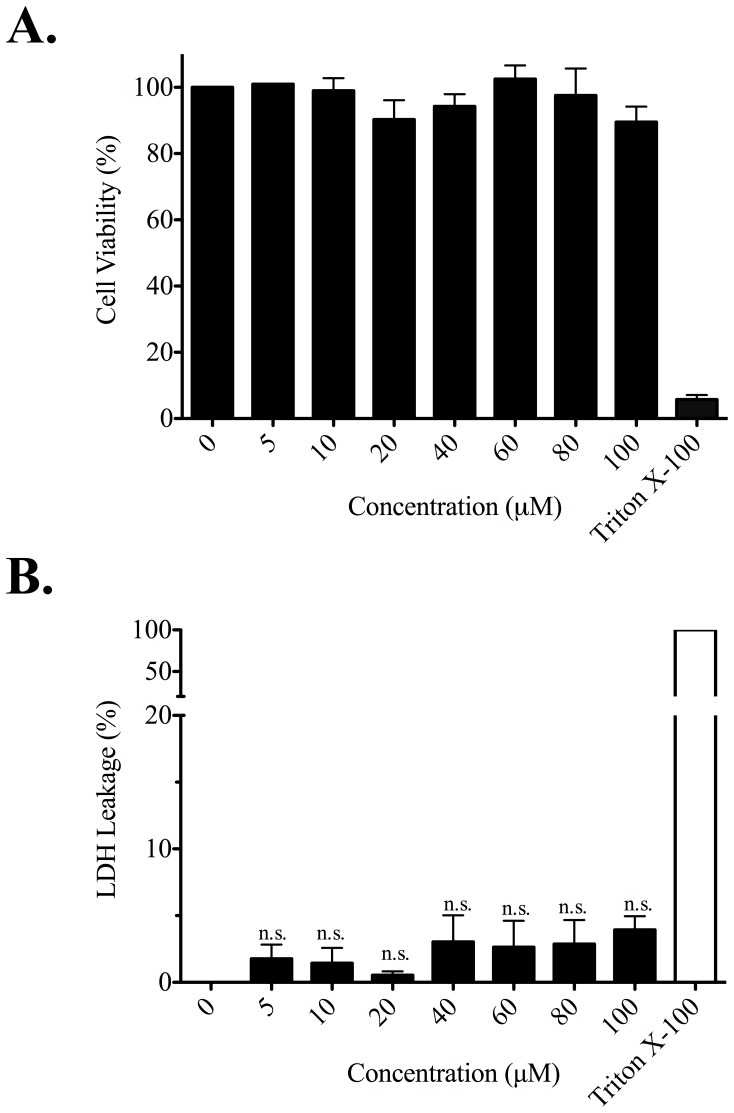
To get a comprehensive analysis of toxic profiles induced by ECP32–41, cytotoxic and membrane disruptive properties of ECP32–41 were analysed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) and lactate dehydrogenase (LDH) leakage assay, respectively. Beas-2B was treated with ECP32–41 up to 100 µM at 37°C for 24 h. No sign of any negative effects in cell viability were observed after treatment with ECP32–41 (Figure 5A) and no significant changes (P>0.05) in LDH levels were found between ECP32–41 treated and untreated cells (Figure 5B). These results demonstrated that treatment of cells with ECP32–41 had no effects on cytotoxicity and membrane disruption.
Figure 5. Cytotoxicity and membrane disruption by ECP32–41.
Beas-2B cells were grown in serum-free medium in the presence of ECP32–41 at indicated concentrations for 24 h. (A) The cytotoxic effect of ECP32–41 was measured by MTT assay. The cell viability of untreated cells was set to 100%. Cells treated with 0.1% Triton X-100 was used as a positive control. (B) The membrane disruption by ECP32–41 was measured by LDH assay. LDH released from cells lysed with 0.1% Triton X-100 in medium was defined as 100% leakage and LDH released from untreated cells was set as 0% leakage. The result is expressed as means ± S.D., n = 3. no significance (n.s.).
In vitro Delivery of Proteins and Peptides by ECP32–41 into Cells
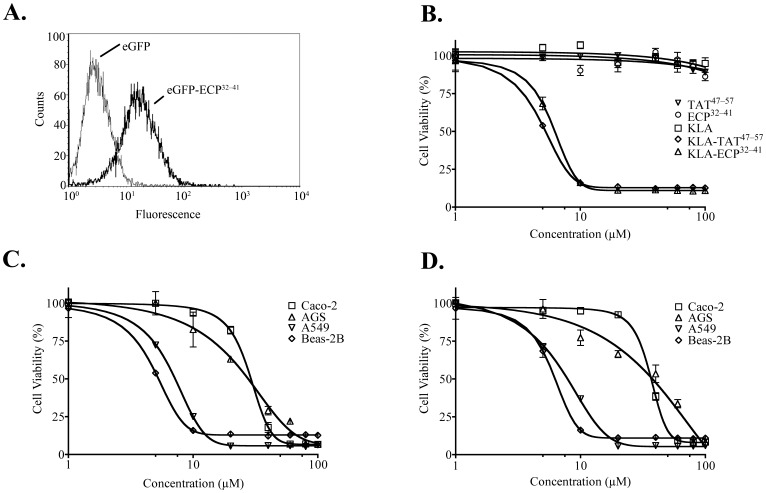
The ability to mediate cellular uptake of normally impermeable small molecules, proteins, and peptides is an important functional characteristic of CPPs [28]. To determine what type(s) of cargo ECP32–41 could deliver, first, eGFP (28 kDa) was fused to ECP32–41 so that internalization of ECP32–41 could be monitored by flow cytometry. A fluorescent signal shift was clearly observed after incubating Beas-2B cells with eGFP-ECP32–41, indicating that ECP32–41 successfully delivered eGFP into the cells (Figure 6A).
Figure 6. In vitro delivery of a protein and a peptide by ECP32–41.
Beas-2B cells were incubated with 10 µM eGFP or eGFP-ECP32–41 at 37°C for 1 h. The cells were then washed with 500 µl PBS, trypsinized at 37°C for 15 min, suspended in 500 µl PBS, and subjected to flow cytometry. (B) Beas-2B cells were treated with the indicated concentrations of TAT47–57, ECP32–41, KLA, KLA-TAT47–57 or KLA-ECP32–41 at 37°C for 24 h, and their cytotoxic effects were determined by the MTT assay. Cytotoxic effects of (C) KLA-TAT47–57 and (D) KLA-ECP32–41, at the indicated concentrations, on Beas-2B, A549, Caco-2, and AGS cells were assessed by the MTT assay after incubation at 37°C for 24 h. The cell viability untreated cells was set to 100%. The result is expressed as the mean ± S.D., n = 3.
To determine ECP32–41 penetration into the cell in terms of time and intracellular localization, the cytosolic and endosomal fractions of Beas-2B cells were isolated by subcellular fractionation after treatment with eGFP-ECP32–41. Beas-2B cells were incubated with eGFP or eGFP-ECP32–41 (20 µM) at 4°C for 1 h and then shifted to 37°C for further incubation for 1 h, 2 h, 3 h and 4 h separately. Cells were homogenized and fractionated by floatation in Percoll gradients separating cytoplasmic and endosomal fractions. Neither eGFP-ECP32–41 nor eGFP signal was detected along with Actin in cytoplasm even after 4 h incubation (Figure S1A). In terms of endosomal fraction, eGFP-ECP32–41 signal was detected along with LAMP-1 in endosomal fraction after 1 h incubation, the accumulated amount reached maximum at 2 h and then gradually decreased (Figure S1B). In contrast, eGFP signal was not detected even after 4 h treatment. These results suggested that larger cargo (eGFP-ECP32–41) remained in endocytic vesicles for at least 4 h.
In general, CPPs should not be cytotoxic when serving as viable delivery vehicles, the effect of ECP32–41 on cell viability was assayed using MTT assay with a well characterized CPP, TAT47–57, as a control [10]. At 100 µM, neither peptide affected cell viability (Figure 5A, Figure 6B); thus ECP32–41, unlike full-length ECP [23], [24], could potentially serve as a delivery vehicle. To assess the ability of ECP32–41 to deliver a small cargo, a peptidomimetic drug, KLA, which contained a proapoptotic domain that induces mitochondrial swelling but did not affect the plasma membrane, was chosen as the cargo [30], [31]. The in vitro internalization of KLA conjugated to TAT47–57 and ECP32–41 was evaluated by monitoring cell viability. As expected, KLA alone was not cytotoxic, whereas KLA-TAT47–57 and KLA-ECP32–41 had similar cytotoxic effects on Beas-2B cells when incubated at 37°C for 24 h (Figure 6B). KLA-TAT47–57 and KLA-ECP32–41 induced cell death in a concentration-dependent manner with half maximal effective concentrations (EC50) of 5.64 µM and 6.08 µM, respectively. ECP32–41 may therefore be useful as a delivery vehicle for functional peptides.
To investigate whether ECP32–41 maintains its penetration property after coupling with cargos, KLA-ECP32–41 was mixed with indicated concentrations of LMWH, CSC or HA prior to incubation with Beas-2B cells, followed by MTT assay. In the presence of 25 µg/ml of LMWH or CSC, the cytotoxicity of KLA-ECP32–41 was significantly reduced (Figure S2), inferring that KLA-ECP32–41 penetration decreased due to competition with LMWH or CSC. However, HA had no inhibitory effect on cytotoxicity of KLA-ECP32–41, similar to GAG influence on FITC-ECP32–41 internalization (Figure 3A). These results suggested that intracellular delivery of cargo-ECP32–41 relied on cell surface HS, resembling the case of ECP32–41 peptide alone.
To further explore which cell types ECP32–41 could enter, the cytotoxic effects of KLA-TAT47–57 (Figure 6C) and KLA-ECP32–41 (Figure 6D) on the human cell lines, lung A549, and digestive-tract Caco-2 and AGS cell lines were examined. The EC50 values for KLA-TAT47–57 and KLA-ECP32–41 in Beas-2B, A549, Caco-2 and AGS cells were summarized in Table 3. For the lung cell lines, the EC50 values of KLA-ECP32–41 and KLA-TAT47–57 on Beas-2B and A549 cells were quite similar (5 to 7 µM. However, for the digestive-track cell lines, EC50 values of KLA-ECP32–41 were respectively 1.6- and 2.42- fold higher than those of KLA-TAT47–57 in Caco-2 and AGS cells, presumably due to higher expression of HSPGs on lung cells or higher KLA resistance of gastrointestinal cell lines.
Table 3. Half maximal effective concentration of KLA-TAT49–57 and KLA-ECP32–41.
| Cell line | EC50 (µM) | |
| KLA-TAT47–57 | KLA-ECP32–41 | |
| Beas-2B | 5.64±0.37 | 6.08±0.26 |
| A549 | 6.84±0.13 | 7.17±0.39 |
| Caco-2 | 21.79±0.63 | 35.07±0.77 |
| AGS | 24.67±5.54 | 59.75±6.82 |
Tissue Targeting of ECP32–41 in an Animal Model
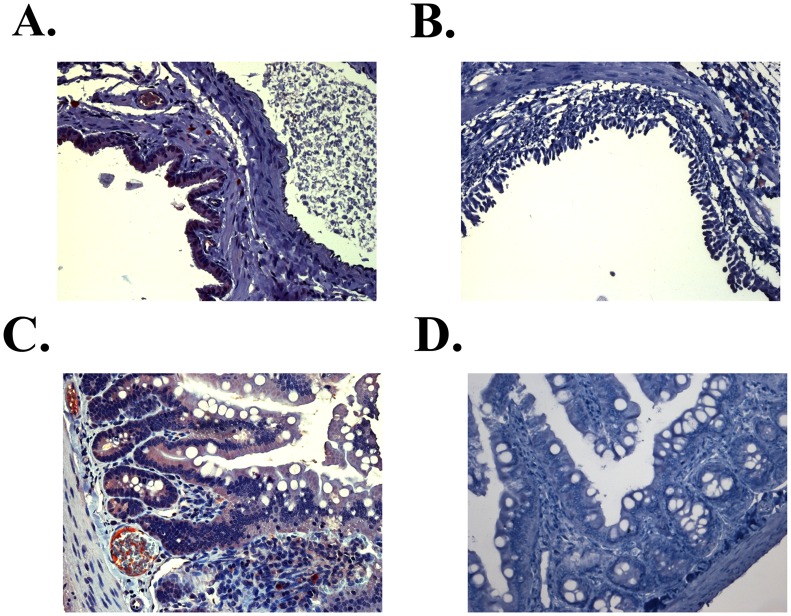
GAG expression is related to cell differentiation and growth [32], and specific HPSGs are differentially expressed in different cell types [33]. To delineate tissue targeting by ECP32–41 in vivo and to develop potential applications, eGFP-ECP32–41 and eGFP were separately injected into the circulatory system of specific-pathogen-free rats through tail veins. The tissues were immunohistochemically stained with anti-eGFP antibody. Interestingly, 1 h after injection, significant eGFP-ECP32–41 signals were detected in broncho-epithelial and intestinal villi tissues (Figure 7A, 7C), which was quite similar to tissue distribution as ECP [23]. eGFP alone was not detected in these tissues (Figure 7B, 7D). As is known that mammalian mucosal cells are rich in HSPGs, ECP32–41 may potentially be used for in vivo targeting of broncho-epithelial and intestinal villi tissues.
Figure 7. Tissue-specific localization of ECP32–41.
Immunohistochemical staining of ECP32–41 was detected by Supersensitive Non-Biotin HRP Detection System. Representative images of eGFP-ECP32–41 (red) in (A) broncho-epithelial and (C) and the epithelium of intestinal villi 1 h after intravenous injection of eGFP-ECP32–41 were shown. Signals of eGFP were not detected in (B) broncho-epithelial and (D) intestinal villi tissue sections 1 h after intravenous injection of eGFP. (Magnification in all panels, 200×).
Discussion
CPPs are a class of peptides differing in sequence, size, and charge that can translocate across plasma membranes. In this study, a newly identified CPP corresponding to residues 32–41 of human ECP (ECP32–41) was characterized. ECP32–41 delivered a small, fluorescent compound (Figure 1), a recombinant protein (Figure 6A), and a peptidomimetic drug (Figure 6D) into Beas-2B cells, and targeted specific rat tissues in vivo (Figure 7), showing that it can act as a delivery vehicle in both types of environments.
ECP is a multifunctional protein with ribonucleolytic, cytotoxic, membrane-disrupting, antibacterial, antiparasitic, antiviral, heparin-binding, and cell-penetrating activities. Boix and colleagues identified the heparin-binding residues in ECP as A8–Q14, Y33–R36, Q40–L44, and H128–D130 [22], and we have previously shown that residues 34–38 comprise a critical heparin-binding sequence, and substitution of residues in the ECP sequence 34RWRCK38 with alanine resulted in decreased cell-penetrating activity [25]. Here ECP32–41 is identified as the first CPP derived from a human RNase sequence.
Previous reports have suggested that the guanidinium group of arginine rather than lysine or histidine side-chains is necessary for CPP activity [34], [35]. Addition of tryptophan to heptaarginine peptide increases its uptake efficiency [36]. And further, cellular internalization of tryptophan distributed along (RWRRWRRWRRWR) shows higher efficiency than heptaarginine with tryptophan at the N-terminus [37]. Interestingly, although both ECP32–41 and EDN32–41 possess heparin-binding sequences differing only at two positions, they have very dissimilar cell-binding and internalization activities. ECP32–41R3Q and ECP32–41 bound Beas-2B cells similarly (Table 2), but ECP32–41R3Q did not penetrate cells as ECP32–41 did (Table 2). Additionally, although ECP32–41W4R had the strongest affinity for Beas-2B cell among those peptides tested (Table 2), it did not penetrate the cells (Table 2), possibly due to its tight binding to cell-surface GAGs [38]. Residues R3 and W4 in ECP32–41 thus appeared to be crucial for internalization. The two arginines adjacent to W4 in ECP32–41 possibly interacted with negatively charged cell-surface HSPGs, thereby promoting binding. Taken together, the positively charged arginines and the aromatic tryptophan are necessary for ECP32–41 internalization.
Most viral-derived CPPs are rich in basic amino acids [1]. For example, flock house virus coat peptide (residues 35–49, RRRRNRTRRNRRRVR) is extensively used as a CPP, it can interact with sulfated proteoglycans and negatively charged cell-membrane phospholipids [39]. Interestingly, internalization of the amphipathic peptide penetratin (RQIKIWFQNRRMKWKK) requires electrostatic interactions between basic residues and HS and the presence of aromatic residues, especially tryptophan, for insertion into a lipid bilayer [40]. Although the length of ECP32–41 is comparable to that of TAT47–57 and the antimicrobial CPP SynB3 (RRLSYSRRRF), physical characteristics of these three CPPs differ. TAT47–57 and SynB3 both have pI values of 12, owing to the presence of many cationic residues, whereas ECP32–41 has only two arginines and one lysine with a pI value of 10.05. Interestingly, the percentage of basic residues (50%) in CyLoP-1 (CRWRWKCCKK) from crotamine [41], one of the main toxins in rattlesnake venom, is similar to that of ECP32–41 (40%). A consensus motif RWRXK (where X is any amino acid) is present in both CyLoP-1 [41] and ECP32–41. Likewise, internalization of both CyLoP-1 and ECP32–41 requires positively charged residues and non-polar residues, especially tryptophan [41]. Moreover, tryptophan residues are preferentially oriented parallel to the membrane and required for membrane penetration of CPP [42]. ECP32–41, located in a flexible loop structure in intact ECP [22], showed random coil property as determined by circular dichroism spectroscopy (data not shown). Flexibility of structure has been suggested as a favorable property for direct membrane penetration for CPPs, as it might allow efficient cell entry [34], [40]. In summary, the presence of aromatic W4, the binding by cationic R3 and R5 to cell surface GAGs, and flexible backbone structure probably all contribute to ECP32–41 internalization.
Three lines of direct evidence indicated that cell-surface HS was involved in ECP32–41 endocytosis. First, soluble HS significantly decreased FITC-ECP32–41 internalization into Beas-2B cells (Figures 2A, 3A). Second, cell-surface HSPGs facilitated FITC-ECP32–41 binding because wild-type CHO cells uptook more FITC-ECP32–41 than did HS- and GAG-deficient CHO cells (Figures 2B, 3B). Third, removal of cell surface HS reduced FITC-ECP32–41 internalization into Beas-2B cells (Figures 3C, 3D). Therefore, cell surface HSPGs mediate ECP32–41 internalization.
In general, three steps are involved in CPP internalization: CPPs first bind to cell surface GAGs, then they move through the cell membrane, and finally they are released into the cytoplasm [43]. Initially, CPPs were thought to bind and directly cross plasma membranes via a receptor- and energy-independent path [44], [45]. However, internalization mechanisms, in addition to direct translocation, e.g., clathrin-mediated, caveolin-mediated, macropinocytotic, and clathrin- and caveolin-independent endocytosis, have been reported [29]. Moreover, most CPPs employ two or more internalization pathways [44]. We have previously demonstrated that ECP internalization into Beas-2B cells occurs via HS-facilitated and lipid raft-dependent macropinocytic routes [23]. We have now found that HSPGs act as receptors or attachment factors for ECP32–41 internalization (Figure 3). Additionally, ECP32–41 internalized at 4°C, albeit with a lower efficiency than at 37°C, and endocytotic inhibitor screening suggested that lipid raft-dependent macropinocytic routes were also involved (Figure 4B). The internalization routes of ECP and ECP32–41 therefore, appear to be similar [23]. However, nocodazole blocked internalization of only ECP32–41 (Figure 4B) but not that of ECP [23], thus multi-endocytic routes should be involved in ECP32–41 internalization.
Previous studies have emphasized that the use of CPPs should improve drug delivery to cells, although CPPs usually target cells promiscuously [46]. Most CPPs have high internalization rates in vitro but low target specificity in vivo [47]. Certain peptides, denoted cell-targeting peptides, specifically target a certain type(s) of cell(s) and bind to their target(s) strongly [48]. CPP fusion with cell-targeting peptide might therefore, prove useful as drug delivery systems, although however, TAT linked to antibody did not retain the cell-targeting ability of the antibody [46]. Nevertheless, Kuniyasu and colleagues, using phage display technology, isolated the peptide, CAYHRLRRC that contained a lymph node-homing sequence (CAY) and a cell-penetrating motif (RLRR) [49], which selectively penetrated leukaemia and lymphoma cells in vitro. Notably, we found that ECP32–41 could penetrate cells in vitro and selectively penetrate broncho-epithelial and intestinal villi tissues in vivo (Figure 7). ECP32–41 targets specific cells and tissues effectively and thus may be used in the development of innovative biomaterials for molecular detection and diagnosis purposes.
Most known CPPs are non-human in origin, which means that the adaptive immune response to these molecules will be of particular concern during the development of biomedical applications, especially if the CPP is conjugated to a protein or nanoparticle. The overall sequence identity among 13 primate eosinophilic RNases, each containing approximately 130 amino acids, is 67%. The sequence identities for ECP32–41 and the correspondent regions of human RNase 2 and RNase 8 are 80% and 50%, respectively, and those for other human RNases are smaller (Table S1). Interestingly, the corresponding 10-amino acid sequences of eosinophil RNases from higher primates, Pan troglodytes and Gorilla gorilla, the closest living relatives to humans [50], are identical to that of ECP32–41. In addition, the corresponding sequences in Macaca fascicularis and Macaca nemestrina RNases are 80% identical to ECP32–41 but are completely identical to that of EDN32–41 (Table S2). Therefore, residues 32–41 in ECP may have evolved from those in EDN. Apparently, residues 32–41 are not conserved in the members of the human RNase A superfamily, but represent a specific motif present in higher primates.
In summary, ECP32–41 is not cytotoxic and can be covalently coupled to many different molecules, it has a substantial cargo delivery potential as an attractive candidate for intracellular delivery of therapeutic molecules. GAG-mediated internalization may be the major pathway for ECP32–41 internalization. Finally, ECP32–41 is a human-derived peptide and specifically targets certain tissues, we expect that, with or without modification, it can be useful as a drug delivery system.
Methods
Peptide Design and Synthesis
Peptides with or without an N-terminally conjugated fluorescein isothiocyanate (FITC) group (Table 1) were synthesised by Genemed Synthesis Inc. and their purities (>90%) were assessed by analytical high-performance liquid chromatography. FITC was conjugated to N-terminus of ECP32–41 through a 5-carbon linker, which gave a spacer of approximately 10 angstroms in length. Peptide sequences were confirmed by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry in Genemed Synthesis Inc.
Cell Cultures
Beas-2B cells were cultured in RPMI 1640 medium, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 1% (v/v) Glutamine-Penicillin-Streptomycin (Biosera). Chinese hamster ovary (CHO) and AGS cells were maintained in Ham's F-12, 10% heat-inactivated FBS, 1% (v/v) Glutamine-Penicillin-Streptomycin. A549 cells were cultured in Dulbecco’s Modified Eagle Medium, 10% heat-inactivated FBS, 1% (v/v) Glutamine-Penicillin-Streptomycin. Caco-2 cells were grown in minimum essential medium, 10% FBS, 1% non-essential amino acids, 1% l-glutamine (Biowhittaker), 1% (v/v) Glutamine-Penicillin-Streptomycin. All cells were grown in a 5% CO2, humidified atmosphere at 37°C. Cell culture media, non-essential amino acids, and FBS were purchased from Invitrogen. Beas-2B, CHO, AGS, A549 and Caco-2 cells were purchased from ATCC.
Cell-based ELISA
Cells (2×104/well) were seeded into 96-well black plate and incubated under a 5% CO2 atmosphere at 37°C for 24 h. Each well was then washed with 150 µl ice-cold PBS. To prevent non-specific antibody binding, BSA was used as a blocking agent, and PBS containing 2% (w/v) BSA was added to each well at 4°C and incubated for 1 h. The wells were then washed with 100 µl ice-cold PBS. FITC-conjugated peptides were diluted to 10, 20, or 100 µM in PBS and then, medium (95 µl) and a peptide/PBS solution (5 µl) were gently mixed, added into a well, and the plate was placed on ice for 1 h. Each well was then washed with 100 µl PBS and the FITC fluorescent intensity for each sample was measured using a fluorescence spectrophotometer (Wallac Victor II, Perkin Elmer, USA) and excitation and emission wavelengths of 485 nm and 521 nm respectively.
Flow Cytometry
Cells (3.0×105/well) were added into six-well plates and cultured in the indicated medium. After 24 h, each FITC-peptide, dissolved in medium, was added into a well and the samples incubated for 1 h. Cells were then harvested, washed, and suspended in PBS. The fluorescent intensities of the cell samples were measured using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ) and excitation and emission wavelengths of 488 nm and 515–545 nm respectively. The relative internalization of each peptide is reported as the mean fluorescent signal for 10,000 cells.
Confocal Laser-scanning Microscopy (CLSM)
Cells were cultured on coverslips (1.0×104/coverslip) in indicated medium. After 24 h, cell samples were each incubated at 37°C for 1 h with an FITC-peptide. The cells were then washed twice with PBS, fixed with 2% (w/v) paraformaldehyde and incubated, first in PBS for 15 min, then with 50 mM NH4Cl in PBS for 10 min, and finally permeabilised with 0.5% (v/v) Triton-X-100 at 25°C for 5 min. Nuclei were stained with Hoechst 33342 Fluorescent Stain, (Sigma-Aldrich) during the final 5 min of incubation. Cells were then washed twice with 0.05% Triton-X-100, once with PBS, and the coverslips were mounted in a Vectashield anti-fade mounting medium (Vector Labs). CLSM was performed using LSM510 META (Carl Zeiss, Göttingen, Germany) to assess the distribution of the FITC-peptides in the cells.
GAG Competition Assay
Beas-2B cells (2×104/well) in RPMI 1640 medium were seeded into 96-well black plate for cell binding test, while cells (3.0×105/well) incubated in the wells of six-well plates were used for internalization test. After 24 h, cells were treated for 30 min with 0.01, 0.1, 1, 5, 10, 50, or 100 µg/ml of low molecular weight heparin (LMWH; average Mr ∼3,000), chondroitin sulfate C (CSC; average Mr ∼50,000–58,000), or HA (Mr ∼3,000,000–5,800,000), all obtained from Sigma-Aldrich (St. Louis, MO, USA). The cells were then incubated with FITC-ECP32–41 and assayed for cell binding or internalization of ECP32–41using cell-based ELISA or flow cytometry, respectively, as described above.
Heparinase and Chondroitinase ABC Depletion of GAGs
Beas-2B cells (3.0×105/well) were incubated with RPMI 1640 medium overnight in six-well plates and then treated with 5 U/ml of heparinase I, 2.4 U/ml of heparinase III, or 10 U/ml of chondroitinase ABC (Sigma-Aldrich) at 37°C for 2 h. After a PBS wash, the cells were incubated with FITC-ECP32–41 and assayed for internalization of the peptide by flow cytometry as described above.
Cell Internalization Pathway
The influence of energy on ECP32–41 internalization was measured at 4°C, 37°C, and while depleting ATP with 10 mM sodium azide and 6 mM 2-deoxy-d-glucose (Sigma-Aldrich) at 37°C for 30 min. Beas-2B cells were treated with 10 µM chlorpromazine, 5 mM methyl-β-cyclodextrin, 25 µM genistein, 2 µM filipin III, 4 µM cytochalasin D, 20 µM nocodazole, 50 nM wortmannin or 50 µM dimethyl amiloride at 37°C for 30 min (Sigma-Aldrich). After treatment, the cells were incubated with FITC-ECP32–41 and assayed by flow cytometry as described above.
Cell Viability Assay
The effects of the peptides on cell viability were determined colourimetrically using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (US Biological). Cells (1.0×104/well) were seeded into the wells of 96-well plates and incubated overnight. Cell samples were then exposed to different concentrations of TAT47–57, ECP32–41, KLA, KLA-TAT47–57 or KLA-ECP32–41. After 24 h, 100 µl of 0.5 mg/ml MTT in medium was added into each well, and the cells were incubated at 37°C for 3 h. The incubation medium was removed, and the remaining purple crystal formazan was dissolved in dimethylsulphoxide. Cells treated with 0.1% Triton X-100 was used as a positive control for cell viability. A540 values were measured using a multiwall plate reader (Molecular Devices).
Membrane Disruption Assay
Lactate dehydrogenase (LDH) was used to quantify membrane disruption. The release of LDH from cells was measured by Promega CytoTox-ONE assay (Promega, USA). Cells (1.0×104/well) were seeded into the wells of 96-well plates and incubated overnight. Cell samples were then exposed to different concentrations of ECP32–41. After 24 h, 100 µl of extracellular medium was transferred to a black 96-well plate containing 100 µl of CytoTox-ONE reagent, incubated at RT for 10 min. Fluorescent intensity for each sample was measured using a fluorescence spectrophotometer (Wallac Victor II, Perkin Elmer, USA) and excitation and emission wavelengths of 540 nm and 590 nm respectively. LDH released from cells lysed with 0.1% Triton X-100 in medium was defined as 100% leakage and LDH released from untreated cells was set as 0% leakage.
Subcellular Fractionation
Beas-2B cells (8×105/dish) were cultured in 10 cm dish for 24 h, followed by incubation with 20 µM eGFP or eGFP-ECP32–41 at 4°C for 1 h. The cells were washed twice with PBS and then incubated at 37°C for 1 h, 2 h, 3 h and 4 h, separately. Cells were then homogenized and fractionated by floatation in Percoll gradients (GE Healthcare, USA) separating cytoplasm and endosomes [51]. In brief, cells were scraped off in 1 ml PBS with a rubber policeman and pelleted at 300×g for 5 min. The pellet was resuspended in 1 ml homogenization buffer (0.25 M sucrose, 3 mM Imidazole and 0.5 mM EDTA, pH 7.3) and pelleted again at 800×g for 7 min. The pellet was resuspended in 100 µl homogenization buffer with a syringe until the cells were broken but the nuclei were still intact as observed by light microscopy. The homogenate was diluted to a total volume of 1 ml with homogenization buffer. After homogenization, the gold-filled fraction was pelleted together with the nuclei at 800×g for 7 min. The pellet was resuspended in 650 µl 17% Percoll and loaded onto a 500 µl 64% sucrose cushion in a 2 ml Beckman ultracentrifuge tube. The samples were centrifuged for 90 min at 27,000×g in a Beckman SW55Ti rotor with fast acceleration to distribute the nuclear fraction at the top and the endosome-filled organelles at the bottom of the sucrose cushion. The pellet was resuspended in 100 µl homogenization buffer and referred to endosomal fraction in the results.
Western Blotting
Protein concentration from each fraction was estimated by BCA protein assay kit (Thermo). Proteins were resolved as reported in 12% SDS-PAGE and blotted to BioTrace™ polyvinylidene fluoride Membrane (Pall Life Sciences, USA). The membrane was incubated in blocking solution (5% nonfat dry milk in PBS) for 1 h. Blots were incubated with antibodies for anti-actin (Novus Biologicals, CO), anti-lysosomal-associated membrane protein 1 (LAMP-1) (Santa Cruz Biotechnology, CA) and anti-His (Clontech Laboratories, CA) in PBS with 0.1% Tween 20 (TPBS) for 1 h. After wash with TPBS for 10 min three times, the membrane was incubated with horseradish peroxidase-conjugated anti-mouse IgG in TPBS at 25°C for 1 h. After wash with TPBS for 10 min three times, the protein on membrane was detected using chemiluminescent detection kit (ECL, Amersham Life Science) and chemiluminescence was measured by Kodak X-Omat film. The blotted signal was quantitated using NIH ImageJ software.
Immunohistochemical Staining
Adult female specific-pathogen-free Sprague-Dawley rats (Narl:SD) with body weights between 200 and 300g were purchased from, and maintained at, the National Laboratory Animal Center, Taiwan. The rats were separated into two groups and injected with 5 nmol of enhanced green fluorescence protein (eGFP) or eGFP-ECP32–41 through their tail veins. All animals were asphyxiated with CO2, 1 h after injection. All major organs including brain, heart, lung, trachea, kidney, liver, spleen and intestine were removed and immediately fixed in 10% neutral-buffered formaldehyde. The tissue samples were processed by standard methods to prepare paraffin wax-embedded block samples [25]. The blocks were sectioned into 6 µm slices and were examined using a Super Sensitive Non-Biotin HRP Detection System (BioGenex Laboratories, San Ramon, CA) as previously described [25]. All these slices were then observed by using light microscope (Zeiss-Axioplan, Germany).
Statistical Analysis
Each result is reported as the mean ± standard deviation (SD), where n is the number of experiments. To compare two means, statistical analysis was performed using the unpaired Student’s t-test in GraphPad Prism v4.02 (GraphPad Software, USA). One-way analysis of variance (ANOVA), followed by Dunnett’s test, was used to test for differences among multiple treatments. A P value <0.05 was considered to be statistically significant.
Supporting Information
eGFP-ECP32–41 in endosomal fraction. (A) Beas-2B cells were incubated with eGFP or eGFP-ECP32–41 at 4°C for 1 h. The cells were washed twice with PBS and then shifted to 37°C for further 1 h, 2 h, 3 h or 4 h. Cells were then homogenized and fractionated by floatation in Percoll gradients separating cytoplasm and endosomes. The locations of eGFP or eGFP-ECP32–41 were analysed by Western blot. (B) The blotted signal was quantitated using NIH ImageJ software and normalized to LAMP-1. The internalization of cells treated with eGFP-ECP32–41 for 2 h was set to 100%. The result is expressed as the mean ± S.D., n = 3. *, P<0.05.
(TIF)
Cell-surface GAG-dependent cytotoxicity of KLA-ECP32–41. GAG-mediated inhibition of KLA-ECP32–41 peptide-induced cytotoxicity in Beas-2B cells. Beas-2B cells were treated with increasing concentrations of LMWH, CSC or HA for 30 min prior to addition of 10 µM KLA-ECP32–41 at 37°C for 24 h. The cytotoxicity of KLA-ECP32–41 was determined by an MTT assay. The cell viability untreated cells was set to 100%. The result is expressed as the mean ± S.D., n = 3.
(TIF)
(DOC)
(DOC)
Acknowledgments
We thank Dr. Yuan-Chuan Lee (Department of Biology, Johns Hopkins University, Baltimore, Maryland, United States of America) for research advice and Dr. Hao-Teng Chang (Graduate Institute of Molecular System Biomedicine, China Medical University, Taichung, Taiwan) for critical comments on the manuscript, Dr. Wen-Guey Wu and Dr. Wen-Ching Wang (Department of Life Science, National Tsing Hua University, Taiwan) and Dr. Cheng-Hsun Chiu (Department of Pediatrics, Chang Gung Memorial Hospital, Taiwan) for providing CHO, AGS, and Caco-2 cells, respectively. Dr. Chuang-Rung Chang (Institute of Biotechnology, National Tsing Hua University, Hsinchu, Taiwan), Dr. Chao-sheng Cheng (Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu, Taiwan), Miss Liang-Chi Mao, Miss Hsiu-Hui Chang and Miss Ee Ling Low (Institute of Molecular and Cellular Biology, National Tsing Hua University, Hsinchu, Taiwan) for proofreading the manuscript.
Funding Statement
Grant support: This work was supported by the National Science Council, Taiwan (grant numbers NSC101-2622-B-007-001-CC1 and NSC101-2325-B-007-002-), Toward World-Class Project of National Tsing Hua University (grant number 101N2051E1), Chang-Gung Memorial Hospital-National Tsing Hua University Joint Research Program (grant numbers 100N2710E1 and 100N2711E1), and Veterans General Hospitals, University System of Taiwan, Joint Research Program (grant numbers VGHUST101-G6-2-1 and VGHUST101-G6-2-2). CJC was awarded a scholarship by Apex Biotechnology Corporation, Taiwan, and TJH was supported by the Graduate Students Study Abroad Program of National Science Council of Taiwan (grant number 100IPFA0400015). The financial funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kersemans V, Kersemans K, Cornelissen B (2008) Cell penetrating peptides for in vivo molecular imaging applications. Curr Pharm Des 14: 2415–2447. [DOI] [PubMed] [Google Scholar]
- 2. Bitler BG, Schroeder JA (2010) Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat Anticancer Drug Discov 5: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K (2003) Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem 278: 35109–35114. [DOI] [PubMed] [Google Scholar]
- 4. Deshayes S, Plenat T, Charnet P, Divita G, Molle G, et al. (2006) Formation of transmembrane ionic channels of primary amphipathic cell-penetrating peptides. Consequences on the mechanism of cell penetration. Biochim Biophys Acta 1758: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi NS, Mancera RL (2008) The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 72: 455–482. [DOI] [PubMed] [Google Scholar]
- 6. Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD, et al. (2007) Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem Soc Trans 35: 775–779. [DOI] [PubMed] [Google Scholar]
- 7. Letoha T, Keller-Pinter A, Kusz E, Kolozsi C, Bozso Z, et al. (2010) Cell-penetrating peptide exploited syndecans. Biochim Biophys Acta 1798: 2258–2265. [DOI] [PubMed] [Google Scholar]
- 8. Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, et al. (2006) Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther 14: 343–350. [DOI] [PubMed] [Google Scholar]
- 9. Wollack JW, Zeliadt NA, Ochocki JD, Mullen DG, Barany G, et al. (2010) Investigation of the sequence and length dependence for cell-penetrating prenylated peptides. Bioorg Med Chem Lett 20: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vives E, Brodin P, Lebleu B (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272: 16010–16017. [DOI] [PubMed] [Google Scholar]
- 11. Gautam A, Singh H, Tyagi A, Chaudhary K, Kumar R, et al. (2012) CPPsite: a curated database of cell penetrating peptides. Database (Oxford) 2012: bas015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie W, Liu J, Qiu M, Yuan J, Xu A (2010) Design, synthesis and biological activity of cell-penetrating peptide-modified octreotide analogs. J Pept Sci 16: 105–109. [DOI] [PubMed] [Google Scholar]
- 13. Cardin AD, Weintraub HJ (1989) Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9: 21–32. [DOI] [PubMed] [Google Scholar]
- 14. Christiaens B, Symoens S, Verheyden S, Engelborghs Y, Joliot A, et al. (2002) Tryptophan fluorescence study of the interaction of penetratin peptides with model membranes. Eur J Biochem 269: 2918–2926. [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg HF (1995) Recombinant human eosinophil cationic protein. Ribonuclease activity is not essential for cytotoxicity. J Biol Chem 270: 7876–7881. [DOI] [PubMed] [Google Scholar]
- 16. Egesten A, Alumets J, von Mecklenburg C, Palmegren M, Olsson I (1986) Localization of eosinophil cationic protein, major basic protein, and eosinophil peroxidase in human eosinophils by immunoelectron microscopic technique. J Histochem Cytochem 34: 1399–1403. [DOI] [PubMed] [Google Scholar]
- 17. Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, et al. (1989) Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol 142: 4428–4434. [PubMed] [Google Scholar]
- 18. Rosenberg HF (2008) Eosinophil-derived neurotoxin/RNase 2: connecting the past, the present and the future. Curr Pharm Biotechnol 9: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torrent M, Navarro S, Moussaoui M, Nogues MV, Boix E (2008) Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry 47: 3544–3555. [DOI] [PubMed] [Google Scholar]
- 20. Torrent M, de la Torre BG, Nogues VM, Andreu D, Boix E (2009) Bactericidal and membrane disruption activities of the eosinophil cationic protein are largely retained in an N-terminal fragment. Biochem J 421: 425–434. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez D, Moussaoui M, Carreras E, Torrent M, Nogues V, et al. (2011) Mapping the eosinophil cationic protein antimicrobial activity by chemical and enzymatic cleavage. Biochimie 93: 331–338. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Mayoral MF, Moussaoui M, de la Torre BG, Andreu D, Boix E, et al. (2010) NMR structural determinants of eosinophil cationic protein binding to membrane and heparin mimetics. Biophys J 98: 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan TC, Chang HT, Chen IW, Wang HY, Chang MD (2007) A heparan sulfate-facilitated and raft-dependent macropinocytosis of eosinophil cationic protein. Traffic 8: 1778–1795. [DOI] [PubMed] [Google Scholar]
- 24. Chang KC, Lo CW, Fan TC, Chang MD, Shu CW, et al. (2010) TNF-alpha Mediates Eosinophil Cationic Protein-induced Apoptosis in BEAS-2B Cells. BMC Cell Biol 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan TC, Fang SL, Hwang CS, Hsu CY, Lu XA, et al. (2008) Characterization of molecular interactions between eosinophil cationic protein and heparin. J Biol Chem 283: 25468–25474. [DOI] [PubMed] [Google Scholar]
- 26. Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, et al. (1992) A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci U S A 89: 2267–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esko JD, Stewart TE, Taylor WH (1985) Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A 82: 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart KM, Horton KL, Kelley SO (2008) Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem 6: 2242–2255. [DOI] [PubMed] [Google Scholar]
- 29. Fotin-Mleczek M, Fischer R, Brock R (2005) Endocytosis and cationic cell-penetrating peptides–a merger of concepts and methods. Curr Pharm Des 11: 3613–3628. [DOI] [PubMed] [Google Scholar]
- 30. Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, et al. (1996) Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem 271: 28375–28381. [DOI] [PubMed] [Google Scholar]
- 31. Risso A, Braidot E, Sordano MC, Vianello A, Macri F, et al. (2002) BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol 22: 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grassel S, Cohen IR, Murdoch AD, Eichstetter I, Iozzo RV (1995) The proteoglycan perlecan is expressed in the erythroleukemia cell line K562 and is upregulated by sodium butyrate and phorbol ester. Mol Cell Biochem 145: 61–68. [DOI] [PubMed] [Google Scholar]
- 33. David G (1993) Integral membrane heparan sulfate proteoglycans. FASEB J 7: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 34. Caesar CE, Esbjorner EK, Lincoln P, Norden B (2006) Membrane interactions of cell-penetrating peptides probed by tryptophan fluorescence and dichroism techniques: correlations of structure to cellular uptake. Biochemistry 45: 7682–7692. [DOI] [PubMed] [Google Scholar]
- 35. Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, et al. (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A 97: 13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maiolo JR, Ferrer M, Ottinger EA (2005) Effects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptides. Biochim Biophys Acta 1712: 161–172. [DOI] [PubMed] [Google Scholar]
- 37.Rydberg HA, Matson M, Amand HL, Esbjorner EK, Norden B (2012) Effects of Tryptophan Content and Backbone Spacing on the Uptake Efficiency of Cell-Penetrating Peptides. Biochemistry. [DOI] [PubMed] [Google Scholar]
- 38. Kamide K, Nakakubo H, Uno S, Fukamizu A (2010) Isolation of novel cell-penetrating peptides from a random peptide library using in vitro virus and their modifications. Int J Mol Med 25: 41–51. [PubMed] [Google Scholar]
- 39. Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, et al. (2001) Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem 276: 5836–5840. [DOI] [PubMed] [Google Scholar]
- 40. Derossi D, Joliot AH, Chassaing G, Prochiantz A (1994) The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 269: 10444–10450. [PubMed] [Google Scholar]
- 41. Jha D, Mishra R, Gottschalk S, Wiesmuller KH, Ugurbil K, et al. (2011) CyLoP-1: a novel cysteine-rich cell-penetrating peptide for cytosolic delivery of cargoes. Bioconjug Chem 22: 319–328. [DOI] [PubMed] [Google Scholar]
- 42. Zhang W, Smith SO (2005) Mechanism of penetration of Antp(43–58) into membrane bilayers. Biochemistry 44: 10110–10118. [DOI] [PubMed] [Google Scholar]
- 43. Ram N, Aroui S, Jaumain E, Bichraoui H, Mabrouk K, et al. (2008) Direct peptide interaction with surface glycosaminoglycans contributes to the cell penetration of maurocalcine. J Biol Chem 283: 24274–24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel LN, Zaro JL, Shen WC (2007) Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res 24: 1977–1992. [DOI] [PubMed] [Google Scholar]
- 45. Ziegler A (2008) Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv Drug Deliv Rev 60: 580–597. [DOI] [PubMed] [Google Scholar]
- 46. Vives E, Schmidt J, Pelegrin A (2008) Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta 1786: 126–138. [DOI] [PubMed] [Google Scholar]
- 47. Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, et al. (2010) The pharmacokinetics of cell-penetrating peptides. Mol Pharm 7: 2224–2231. [DOI] [PubMed] [Google Scholar]
- 48. Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, et al. (2006) Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res 12: 3942–3949. [DOI] [PubMed] [Google Scholar]
- 49. Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, et al. (2008) Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem 283: 11752–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miyamoto MM, Koop BF, Slightom JL, Goodman M, Tennant MR (1988) Molecular systematics of higher primates: genealogical relations and classification. Proc Natl Acad Sci U S A 85: 7627–7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T (1996) Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci 109 (Pt 12): 2905–2914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eGFP-ECP32–41 in endosomal fraction. (A) Beas-2B cells were incubated with eGFP or eGFP-ECP32–41 at 4°C for 1 h. The cells were washed twice with PBS and then shifted to 37°C for further 1 h, 2 h, 3 h or 4 h. Cells were then homogenized and fractionated by floatation in Percoll gradients separating cytoplasm and endosomes. The locations of eGFP or eGFP-ECP32–41 were analysed by Western blot. (B) The blotted signal was quantitated using NIH ImageJ software and normalized to LAMP-1. The internalization of cells treated with eGFP-ECP32–41 for 2 h was set to 100%. The result is expressed as the mean ± S.D., n = 3. *, P<0.05.
(TIF)
Cell-surface GAG-dependent cytotoxicity of KLA-ECP32–41. GAG-mediated inhibition of KLA-ECP32–41 peptide-induced cytotoxicity in Beas-2B cells. Beas-2B cells were treated with increasing concentrations of LMWH, CSC or HA for 30 min prior to addition of 10 µM KLA-ECP32–41 at 37°C for 24 h. The cytotoxicity of KLA-ECP32–41 was determined by an MTT assay. The cell viability untreated cells was set to 100%. The result is expressed as the mean ± S.D., n = 3.
(TIF)
(DOC)
(DOC)