Abstract
Corneal transplantation is the most common form of grafting performed worldwide. Corneal endothelial cells (EC) form a monolayer in the posterior portion of the cornea and are essential for corneal transparency. EC loss during storage prior to transplantation is a principal reason for rendering donor tissue unsuitable for transplantation, and apoptosis has been shown to be the major contributor to EC loss during storage and after transplantation. Therefore, the potential use of anti-apoptotic gene therapy to promote both graft storage and graft survival is of major interest. The goal of this study was to transduce human donor corneas in vitro to enhance EC survival during storage conditions used in eye banking. We utilized a lentiviral vector to perform gene transfer of baculoviral p35 or mammalian Bcl-xL to corneal endothelium in different storage conditions utilizing a lentiviral vector. Our results show significantly enhanced survival and prolonged retention of physiological EC morphology in cells expressing either p35 or Bcl-xL. The clinical application of this technology could lead to a higher availability of donor tissue for transplantation, extend storage periods, and reduce graft failure after transplantation.
INTRODUCTION
An estimated 29 million people worldwide suffer from blindness due to corneal disease 1, 80% of which could be prevented or treated with access to current therapeutic interventions 2,3. Presently only 100 000corneal transplantions (keratoplasties) are performed annually, with patients being waitlisted around the world due to short supply of donor corneas [4]. In order to obtain this quantity of donor corneas suitable for transplantation, nearly 220 000 donor corneas have to be processed each year by eye banks in the United States and European countries alone 4.
The major cause of donor tissue failure is loss of endothelial cells (EC), a cellular monolayer critical to maintaining corneal transparency and visual acuity. Due to minimal to no cellular proliferation, EC loss is permanent 5.During storage the density of EC is a key criterion for the acceptance of a donor cornea for clinical use as well as for evaluation of graft quality after transplantation. EC loss during storage is the principal reason for up to 30% of all discarded tissue by tissue banks 4,6. After transplantation, there is considerable and progressive EC loss 7,8; indeed, EC failure contributes to at least 25% of all failed grafts 15 years post-transplantation 9.
Previous studies have suggested that apoptosis is a key factor for EC loss during storage and for graft failure after transplantation 10-17. EC apoptosis appears to determine the appropriateness of a cornea for transplantation, suggesting that inhibition of apoptosis may prolong acceptable storage times 10,12. The cornea is a particularly suitable tissue for gene therapy-based approaches for several reasons: Unlike some other solid tissues, it can be preserved for periods of several weeks. This allows time for ex vivo genetic alteration before surgery thereby minimizing systemic exposure to viral vectors. Moreover, the transparency of the cornea allows for direct visualization of the consequences of gene transfer. EC are readily accessible (as they are in direct contact with the storage medium) and hence amenable to gene transfer. [REF Barcia; Gong, Pleyer]
Bcl-xL, a member of the Bcl-2 family of proteins, controls both mitochondria-initiated intrinsic apoptosis, and receptor-initiated extrinsic cell death 13,18. The balance between pro- (e.g. Bax) and anti-apoptotic components (Bcl-2, Bcl-xL) is thought to function as a stabilizer controlling the cell’s disposition to apoptosis 19. p35 is an anti-apoptotic protein expressed by baculovirus and identified during genetic studies of this virus 20,21. It has broad specificity for members of the caspase family and can potently inhibit apoptosis induced by a wide range of stimuli 22.
Given the critical importance of EC viability in corneal transplantation and graft survival, we demonstrate the effects of anti-apoptotic gene therapy during corneal storage as a first step toward utilizing gene-therapy approaches to reduce donor tissue waste and post-transplantation graft failure.
RESULTS
Kinetics of IZsGreenW expression and apoptosis in EC
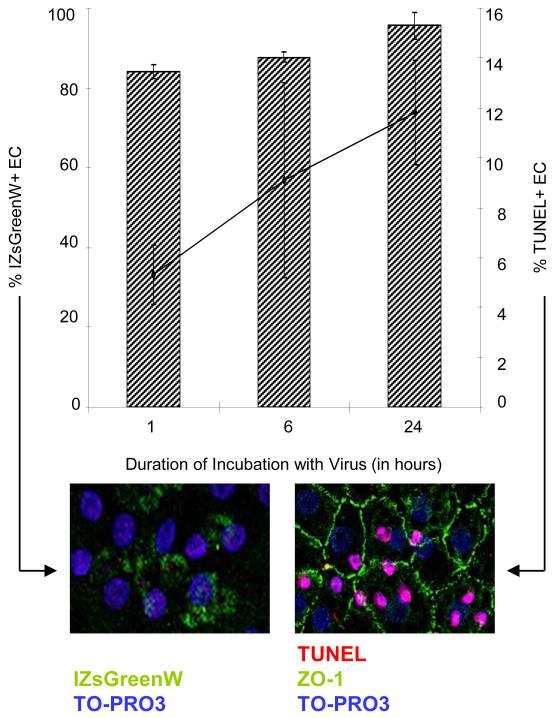
Experimental conditions were established by first assessing the kinetics of expression of the reporter gene IZsGreenW and the impact of transduction on cell viability. Our results indicate that the reporter protein could be detected in the cornea 48 hours after transduction for 30 minutes (data not shown). 48 hours after transduction for 1 hour more than 80% of the cells expressed the reporter gene, and longer exposure to the virus only modestly increased this percentage (Fig. 1). DNA fragmentation as a sign of apoptosis was detected by deoxyuridine-5′-triphosphate-digoxigenin (dUTP) nick-end labeling (TUNEL) and analyzed by confocal laser scanning microscopy. The percentage of TUNEL positive EC steadily increased with duration of transduction (Fig. 1). Consequently, we chose 1 hour of transduction for all subsequent experiments as it was a good balance between transduction efficiency and apoptosis.
Figure 1. Kinetics of IZsGreenW expression and apoptosis in human corneal endothelium.
IZsGreenW expression following corneal transduction with 3 × 10^5 IU/ml of pHAGE-CMV-MCS-IZsGreenW in Biochrome Cornea Medium I in the presence of 8 μg/ml polybrene. IZsGreenW expression was detected by laser scanning microscopy 24 hours after the respective transduction time. DNA fragmentation was subsequently detected with laser scanning microscopy using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Hatched columns display percentage of corneal endothelial cells (mean±SD) expressing IZsGreenW. At each time point, three corneas each cut into seven pieces were examined. Points (connected by black line) represent the percentage of corneal endothelial cells (mean±SD) showing TUNEL-positivity.
Expression of anti-apoptotic proteins results in less EC apoptosis during prolonged storage and in a reduction of Caspase 3
We sought to evaluate the role of apoptosis in storage experiments relevant to eye banking. To assess whether anti-apoptotic gene transfer and subsequent protein expression result in increased viability during corneal preservation, we studied corneas stored under hypothermic conditions (4°C, eye bank practice in North America and Asia) and under organ culture conditions (37°C, eye bank practice in Europe).
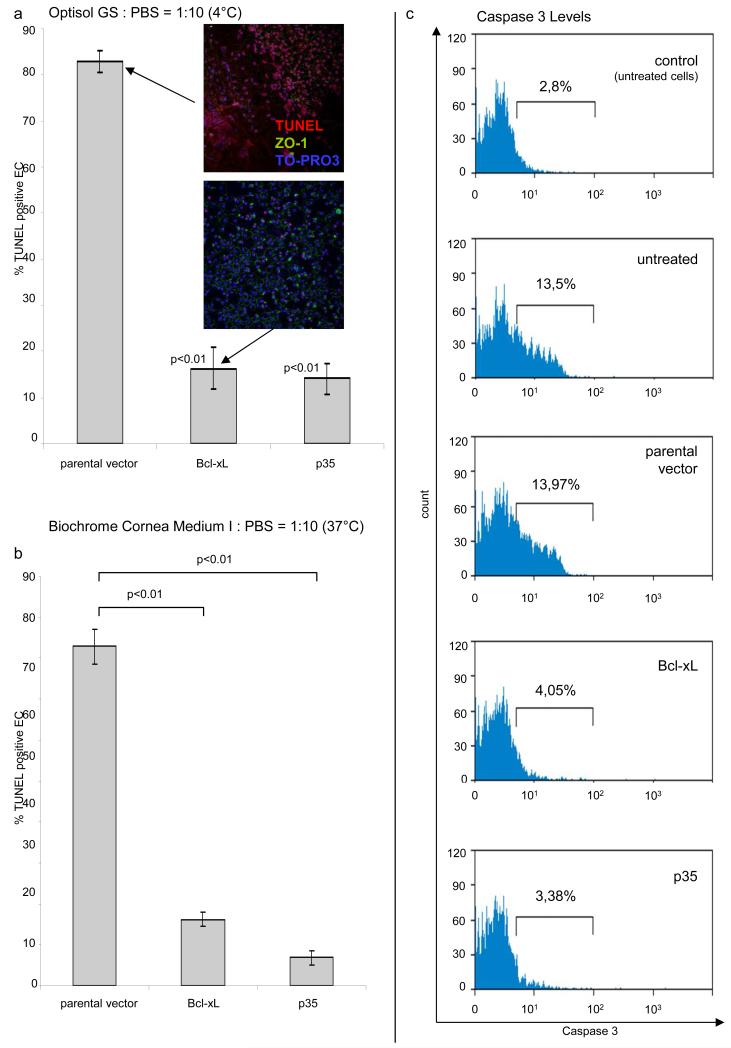
To induce death of corneal endothelial cells, corneas were stored in diluted medium. DNA fragmentation was detected using a TUNEL assay and confocal laser scanning microscopy. Results from these experiments demonstrate that expression of p35 or Bcl-xL leads to a significant decrease in apoptosis relative to parental vector controls (Fig. 2). Transduction of EC with Bcl-xL at a high titer resulted in a significant increase in TUNEL positive EC (data not shown). Findings during storage at 4°C (Fig. 2a) were confirmed in an eye bank setting at 37°C (Fig. 2b). In addition, we could demonstrate that expression of anti-apoptotic proteins leads to a reduction of Caspase 3 in EC (Fig. 2c).
Figure 2. Expression of anti-apoptotic proteins by EC leads to a reduction of apoptosis during long-term storage.
DNA fragmentation, a late sign of apoptosis, was detected by TUNEL (red) and laser scanning microscopy. Corneas were stored at 4°C for 12 days (a; Optisol GS:PBS=1:10) and at 37°C for 9 days (b, Biochrome Culture Medium I:PBS=1:10). Nuclei were stained blue with TO-PRO3, a dicycanine dye which binds to DNA. The cell borders were visualized using an antibody against the tight junction protein 1 (ZO-1, green). Insert images show TUNEL positivity in EC expressing an parental vector or Bcl-xL (six analyzed visual fields at each time point). Expression of anti-apoptotic proteins leads to a decrease in Caspase 3 in EC while apoptosis is induced using Actinomycin D (c).
Assessment of corneal endothelial cell density and morphology during hypothermic storage at 4°C
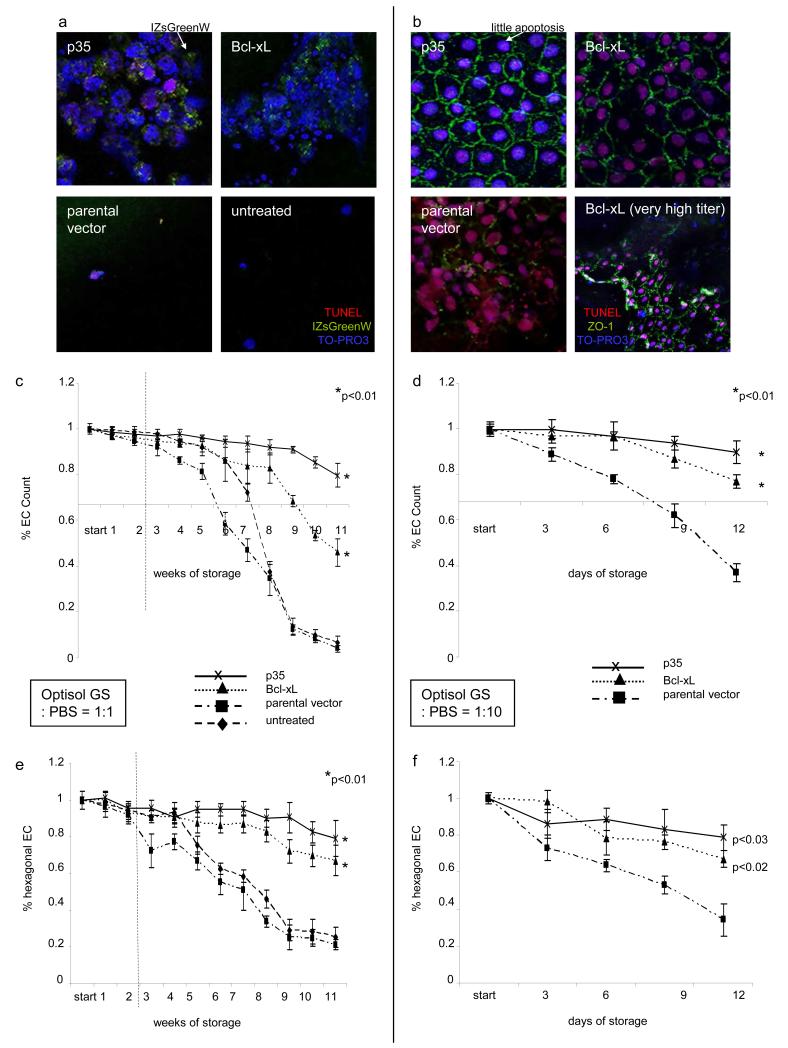
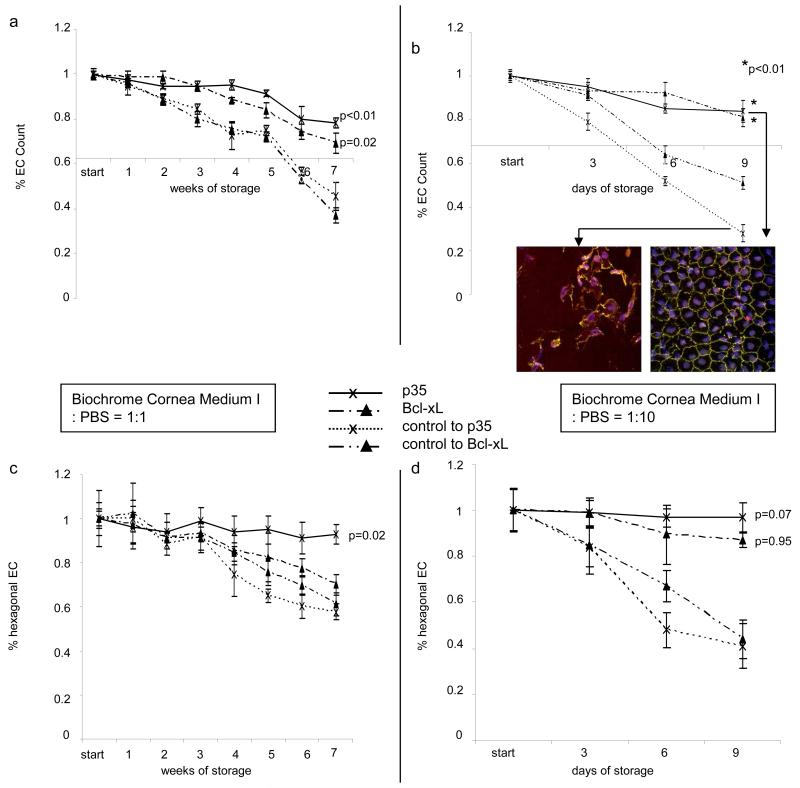
EC expressing anti-apoptotic proteins IZsGreenW-p35 and IZsGreenW-Bcl-xL retained a significantly higher EC count during long-term storage at 4°C compared to untreated EC or EC expressing IZsGreenW-only (parental vector) (Fig. 3). We expected the result given our finding that apoptosis is reduced in EC expressing both anti-apoptotic protein and considering that EC count is an independent measure of cell viability. As demonstrated in Fig. 3a protein expression (IZsGreenW) could still be detected after 11 weeks of storage using laser scanning microscopy. In addition, EC expressing p35 showed less apoptosis and intact cell morphology compared to Bcl-xL (more apoptosis) or the parental vector (cell lysis) (Fig. 3b). Studying cell density, untreated EC dropped below the cell count of 2000 EC/mm2 (critical threshold for eye banks to retain donor tissue) after 7 weeks while EC expressing Bcl-xL achieved this point after 9 weeks, and EC expressing p35 retained counts above this threshold even after 11 weeks (p< 0.01, Fig. 3c). EC enumerations during storage in highly diluted medium confirmed the protective effect of anti-apoptotic protein expression on EC survival (Fig. 3d). Of the two anti-apoptotic proteins tested, expression of the p35 protein was maximally protective against EC loss during storage.
Figure 3. Assessment of corneal endothelial cell density and morphology during hypothermic storage at 4°C.
Untreated EC were compared to those expressing an parental vector (3 × 10^5 IU/ml), Bcl-xL (3 × 10^5 IU/ml) or p35 (3 × 10^5 IU/ml). EC densities and morphology of untreated endothelial cells and those expressing p35, Bcl-xL or an parental vector after storage for 11 weeks (a: Optisol GS:PBS=1:1) or for 12 days (b: Optisol GS:PBS=1:10) are demonstrated. Immunocytochemistry imaging in (a) and (b): TO-PRO3 (blue, nuclei), TUNEL (red, DNA fragmentation), IZsGreenW (a, green, co-expressed with the gene of interest) or ZO-1 (b, green, zonula occludens antibody). Endothelial cell count was enumerated in EC stored in diluted Optisol GS as indicated above. The point of interception of x- and y-axis in (c) and (d) corresponds to 2000 EC/mm2, the minimum EC count used by eye banks to release donor corneas for transplantation. Corneal endothelial cell morphology was evaluated by enumeration of hexagonal EC (e. Optisol GS:PBS=1:1, f. Optisol GS:PBS=1:10; the higher this ratio the faster the decrease of physiological hexagonality and EC numbers; six analyzed visual fields at each time point). The dotted black line in c and e indicates a period of 14 days during which the use of Optisol GS, the most widespread culture medium for hypothermic corneal storage, is currently accepted. P-values are relative to untreated controls only at the final timepoints (* = p<0.01).
Transduction with high titers lead to EC toxicity
We also sought to evaluate whether transduction with lenti-IZsGreenW-Bcl-xL using a significantly higher titer (1.2 × 10^8 IU/ml) resulted in increased EC protection compared to those transduced with lenti-IZsGreenW-Bcl-xL at a titer of 3 × 10^5 IU/ml. Given that transduction with a very high titer of Bcl-xL increases the percentage of EC apoptosis, results from those experiments demonstrated significantly lower EC counts, and lower percentages of EC with physiological morphology likely due to vector toxicity (Fig. 3b, representative image).
EC expressing anti-apoptotic proteins retain physiological cell morphology
Corneal EC morphology is a reliable indicator of cell viability and may be altered under stress conditions and pre-apoptotic states well before cell death and a decrease in EC number. Typically, cells that are compromised lose the characteristic hexagonal cell shape. To assess the effect of these gene therapy approaches on EC morphology, the percentage of EC retaining hexagonality was analyzed (Figs. 3e, f). Expression of p35 or Bcl-xL (3 × 10^5 IU/ml, each) preserved physiological morphology while EC in the other groups developed pathological cell shapes. Interestingly, EC expressing p35 consistently retained higher percentages of hexagonal cell morphology compared to Bcl-xL.
Corneal endothelial cell count and morphology during organ storage at 37°C
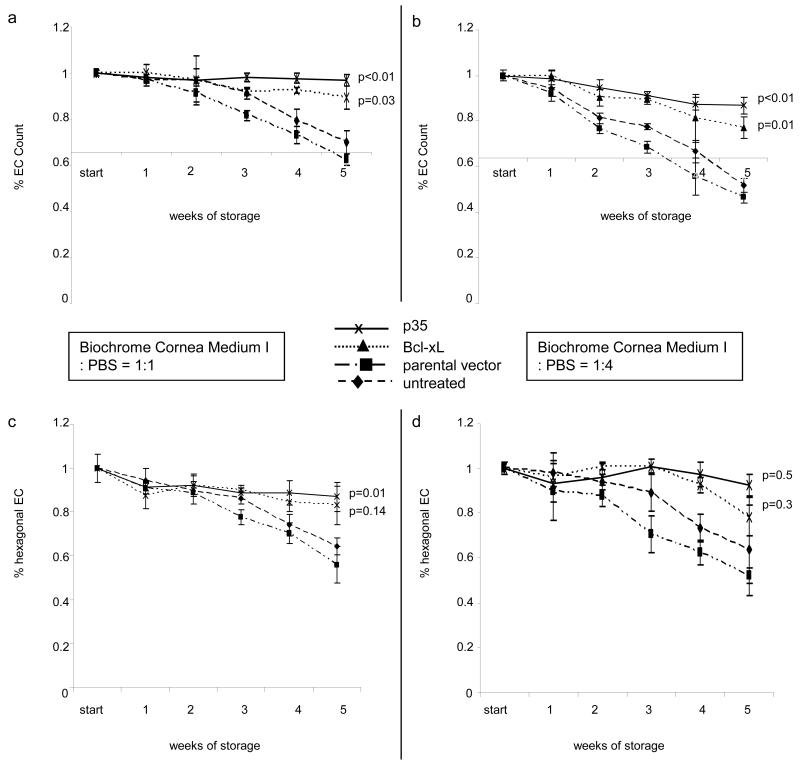
As organ storage at 37°C is widespread outside of the United States, especially in Europe, we assessed the protective effects of p35 and Bcl-xL during corneal donor tissue storage at 37°. Results from these experiments were similar to those observed at 4°C storage, in that we found that EC expressing p35 or Bcl-xL retained significantly higher EC counts [Figs. 4a, b; p35: p< 0.01, Bcl-xL: p< 0.05] and higher percentages of hexagonal cells relative to untreated controls [Figs. 4c, d; p35: p< 0.005, Bcl-xL: p< 0.05]. To exclude donor-related EC variations, these experiments were performed on a single cornea cut into several pieces.
Figure 4. Assessment of corneal endothelial cell density and morphology during organ storage at 37°C.
Untreated EC were compared to those expressing an parental vector, Bcl-xL or p35. Dilutions of the storage medium were used to provoke cell loss. Endothelial cell counts were enumerated in EC stored in diluted organ culture medium (a. Biochrome Culture Medium I:PBS=1:1, b. Biochrome Culture Medium I:PBS=1:4). The point of interception of x- and y-axis in (a) and (b) corresponds to 2000 EC/mm2, the threshold for EC count used by eye banks to release donor tissue for transplantation. Corneal endothelial cell morphology was evaluated by enumeration of hexagonal EC (c. Biochrome Culture Medium I:PBS=1:1, d. Biochrome Culture Medium I:PBS=1:4; six analyzed visual fields at each time point). P-values are relative to untreated controls only at the final timepoints.
Expression of anti-apoptotic proteins extends EC survival in an eye bank setting using donor pairs
To test our hypothesis that EC survival can be promoted by anti-apoptotic gene transfer in an eye bank environment, we compared both corneas (untreated versus treated) from the same donors in the next experiment.
To test our hypothesis, standard operating procedures of ISO 9001/2000 (ISO = International Organization of Standardization) certified organ culture eye banks were adopted 23. Intact corneas were clipped to Boehnke cornea holders and cultured in a standardized volume of medium using respective culture flasks under conditions according to the guidelines of the European Eye Bank Association 24. Transduction of lenti-IZsGreenW was accomplished in one cornea of a donor pair, whereas the other cornea was transduced with the gene of interest. Expression of both anti-apoptotic molecules p35 and Bcl-xL resulted in significantly increased EC counts during long-term storage (p<0.01, Figs. 5a, b). Assessment of cell morphology, however, showed that EC expressing Bcl-xL develop pathological cell morphology almost as rapidly as parental vector controls (Fig. 5c). In contrast, expression of p35 resulted in the maintenance of physiological EC morphology (p<0.01, Fig. 5d).
Figure 5. Expression of anti-apoptotic proteins leads to extended EC survival comparing paired corneas from the same donors cultured under conditions alike in an eye bank.
Organ storage in Biochrome Culture Medium I (1:1 or 1:10 dilution with PBS) was performed in donor pairs. One cornea of a pair was transduced with lenti-IZsGreenW, the other cornea with the lenti-IZsGreenW-Bcl-xL or lenti-IZsGreenW-p35. Enumeration of corneal endothelial cells is demonstrated in (a) and (b). The point of interception of x- and y-axis in (a) and (b) corresponds to 2000 EC/mm2, the minimum EC count used by eye banks to approve donor corneas for transplantation. Representative images of EC layers expressing IZsGreenW or p35 are shown in the respective inserts (TUNEL, TO-PRO3, ZO-1 (green) staining). Corneal endothelial cell morphology was evaluated by enumeration of hexagonal EC (c. Biochrome Culture Medium I:PBS=1:1, d. Biochrome Culture Medium I:PBS=1:10; eight analyzed visual fields at each time point). P-values are relative to untreated controls only at the final timepoints (* = p<0.01).
Evaluation of EC viability during long-term storage
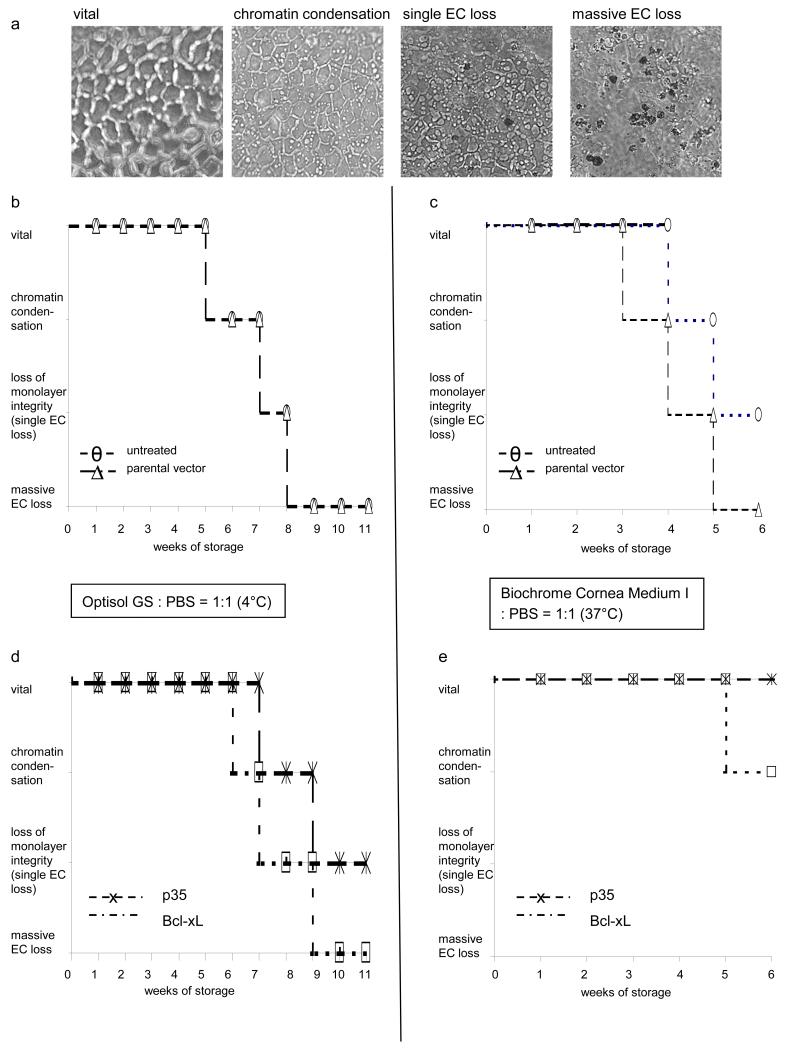
We also determined whether storage at 4°C or at 37°C is more favorable in regard of survival of EC within corneas subjected to gene therapy. To this end, we established how long it took for ECs to die (vital EC, chromatin condensation, single cell loss, massive cell loss) during long-term hypothermic and organ storage (Fig. 6a). Untreated EC and those expressing a parental vector (Figs. 6b (4°C), c (37°C)) were compared to EC expressing p35 or Bcl-xL (Figs. 6d (4°C), e (37°C)). Untreated EC or those expressing the parental vector showed massive EC loss after 8 weeks of storage at 4°C (Fig. 6b). However, EC expressing p35 showed chromatin condensation after 8 weeks and single cell loss after 11 weeks (Fig. 6d). Similarly, during storage at 37°C, untreated cells showed massive cell loss after 5 weeks (Fig. 6c). EC expressing p35, however, maintained vital appearance with no signs of chromatin condensation (Fig. 6e).
Figure 6. Evaluation of cell viability during long-term storage.
Defined phases of cell death (vital, chromatin condensation, single cell loss, massive cell loss) were evaluated by brightfield microscopy according to reference images (a) in corneas stored at 4°C (b, d) and 37°C (c, e). Controls (untreated cells and EC expressing IZsGreenW, b, c) were compared to EC expressing Bcl-xL or p35 (d, e). EC expressing p35 or Bcl-xL show phases of cell death at later points in time compared to untreated EC or those expressing IZsGreenW only.
DISCUSSION
The results hereby presented demonstrate clear advantages to EC expressing anti-apoptotic proteins p35 or Bcl-xL during storage, both in terms of retention of cell viability and physiologic cell morphology. Prolongation of corneal endothelial cell viability during storage by means of anti-apoptotic gene transfer is a novel finding that confirms our hypothesis that EC apoptosis is a pivotal process to loss of donor tissue quality. Furthermore, we demonstrate that gene transfer of the pan-caspase inhibitor p35 prevents apoptosis in EC more effectively than Bcl-xL during both organ (37°) and hypothermic (4°C) storage conditions. Application of this technology in eye banking could lead to an increase of donor corneas available for transplantation, and may lead to a decrease in graft failure since EC deterioration is a common denominator for all forms of corneal graft failure in humans.
Previous studies have suggested protective effects of anti-apoptotic proteins in the rat 25, in the mouse 13, and in murine or human corneal EC lines 13,16,26. Interestingly, such studies have not been performed in human corneas under adapted eye bank conditions which would be crucial for application in grafted donor corneas. The findings of the present study delineate the efficacy of EC protection by anti-apoptotic gene transfer under clinical conditions both regarding the optimal type of gene and elucidating the potential impact of viral dose.
Gene transfer using a lentiviral vector is well known to lead to high gene expression in transduced cells 25,27. Application of lentiviral vectors results in retained cell viability after infection and a rapid onset of gene expression 27,28. Previous studies have shown that this vector can be used to successfully transduce rat, bovine, murine and human corneal endothelial cells 13,25,27. As demonstrated in our experiments, lentiviral-mediated gene expression resulted in high IZsGreenW expression 48 hours post-infection after as few as 30 minutes. We investigated the effects of two virus titers on cell viability 29 by showing that transduction using a very high titer of Bcl-xL does not protect, but derogates, EC vitality due to increased apoptosis.
The functional relevance of EC expression of p35 or Bcl-xL is confirmed by the novel finding of prolonged cell viability and maintenance of physiological morphology under eye bank conditions. Currently, up to 30% of all donor corneas are considered unusable for corneal transplantation due to EC loss during organ preservation 4,6, and up to 16% during hypothermic storage 11. Our data suggest that anti-apoptotic gene transfer could increase the yield of limited donor tissue by decreasing the rate at which eye banks reject donor tissues based on EC quality.
Our findings consistently show higher EC counts and a higher percentage of physiological morphology in EC expressing p35 compared to Bcl-xL. This difference might be explained by the different ways the two proteins interact within the cell-intrinsic suicide signaling cascade. A key characteristic of the Bcl-2 protein family is that its members share sequence homology with four domains (BH1, 2, 3, 4). The BH3 domain is essential for the promotion of the death function of pro-apoptotic molecules 30. Interestingly, the anti-apoptotic proteins Bcl-xL or Bcl-2 merely inhibit pro-apoptotic molecules of the BH3-only subfamily [such as Bad, Bid or Bim] 31. Hence, multidomain pro-apoptotic molecules like Bax, Bak or Bok can still trigger the death program leading to apoptosis, while p35 directly inhibits a broad spectrum of caspases. Cleavage of the reactive site loop of p35 leads to translocation of its N-terminus into the active site of caspases resulting in irreversible caspase inhibition 32,33. Our data suggest that broad suppression of caspases seems to have a higher impact on preventing EC death than inhibition of select pro-apoptotic proteins of the Bcl-2 family.
Previous studies identify apoptosis, an act of cellular “self-destruction”, as the main contributor to cell death during preservation 10-12,34. Depending on the approach of cell death evaluation, different percentages of EC undergoing apoptosis have been reported 10,12,35. As apoptotic cells can be entirely removed by phagocytosis within an estimated time of 30 to 60 minutes 36, detection is only possible within a specific timeframe and results in an underestimation of overall apoptosis. In our current study, we demonstrate a significant decrease in apoptotic EC expressing anti-apoptotic genes p35 or Bcl-xL.
An important concern when applying anti-apoptotic strategies to EC is whether replication can be induced leading to local tumors in the target cell type. Thanks to the very low proliferative capacity of the corneal endothelial monolayer there are no reports of corneal EC tumors described in the literature ever. EC are not even forming a bilayer, not even in in vitro experiments. Therefore, the chance to induce local tumors is minimal to null. Secondly, approaches targeting EC have been of two types: a) To induce proliferation of EC and b) To suppress EC death which is the strategy employed herein. Whereas by promoting proliferation there could be a viable concern of inducing certain pathologies, by suppression of EC death, tumor induction is not possible. This is confirmed by the ‘proof of principle’ experiment in the mouse model transplanting corneas with EC treated with the same viral vector construct used in this study (lenti-IZsGr-Bcl-xL). The data showed that corneal grafts with EC expressing Bcl-xL resulted in a significantly enhanced graft survival compared to controls without signs of pathological proliferation,of EC or other cell types (routine follow-up time in this model is 8 weeks) 13. However, longer follow-up periods in this model might be necessary to further evaluate the safety of this approach.
Another important question is whether or how many viral vector particles remain on the donor tissue after washing the endothelium with PBS at the end of the transduction period. Residual viral vector could lead to transduction of other cell types in the anatomical vicinity of the corneal endothelium raising safety concerns of induction of tumorigenic processes. However, the neighboring cells to the EC in the cornea are either stromal keratocytes (with a low proliferative capacity and with no predilection for tumorogenesis) or epithelial cells (which are entirely replaced by epithelial cells of the host soon after surgery). To study potential hazards, further studies are required. In our study, we were able to detect gene expression without appreciable diminution up to 11 weeks after transduction at readily detectable levels. These data are confirmed by previous work of this group in the mouse transplantation model [13, expression after 8 weeks readily detectable].
Given the biology of the EC as a monolayer, it is conceivable that this approach could be translated to other cell sheet transplantations. Examples could include cultured mesenchymal stem cells to enhance bone formation 37 or transplantation of tissue engineered corneal epithelial sheets 38. Given the risk that uncontrollable cell division of cells expressing anti-apoptotic genes could result in tumor formation, the transfer of this methodology to cell types with a high proliferative capacity should be exercised with caution.
In summary, these findings provide new insights on the roles of both the mammalian anti-apoptotic protein Bcl-xL and the baculoviral protein p35 in prolonging survival of EC during preservation. To our knowledge, we have shown for the first time that cell survival of human EC can be enhanced during preservation by means of gene therapy. Moreover, application of anti-apoptotic gene transfer may be further synergized with the recent advancements in the transplantation of the corneal endothelial monolayer, which is currently being globally implemented in corneal transplantation clinics 39. In addition, application of anti-apoptotic gene therapy in eye banks prior to transplantation could lead to an increase of available donor corneas, which could impact the availability of donor tissue for millions of people suffering from corneal blindness .
METHODS AND MATERIALS
Lentivirus expression system
The cDNA of mammalian anti-apoptotic protein Bcl-xL or the baculoviral anti-apoptotic molecule p35 was subcloned into the lentiviral vector pHAGE-CMV-MCS-IZsGreenW by the Harvard Gene Therapy Initiative. Both genes were cloned into the multiple cloning site that is followed by an internal ribosomal entry site (IRES) and the coding sequence for the green fluorescent protein IZsGreenW, which is derived from a reef coral. The IRES sequence allows for two open reading frames on one mRNA. The vectors lenti-IZsGreenW (parental vector), lenti-IZsGreenW-Bcl-xL and lenti-IZsGreenW-p35 constructs are replication incompetent 40.
Gene transfer in cultured corneas
Gene transfer in human donor corneas was carried out either on intact corneas or on single corneas cut into several pieces: To exclude donor-related variation, experiments were carried our cutting single corneas into five pieces. One piece served as untreated control, one was transfduced with lenti-CMV-MCS-IZsGreenW (parental vector, 3 × 10^5 IU/ml), one with lenti-CMV-IZsGreenW-bclxL (3 × 10^5 IU/ml) and one with lenti-CMV-IZsGreenW-p35 (3 × 10^5 IU/ml). In order to detect the relevance of viral vector titer on EC protection against cell loss and apoptosis, the fifth corneal piece was transduced with lenti-CMV-IZsGreenW-Bcl-xL using a titer of 1.2 × 10^8 IU/ml. In addition, to simulate eye bank conditions, experiments with intact pairs of cornea from the same donor were performed. Cornea was transduced with the parental vector lenti-CMV-MCS-IZsGreenW, the other cornea with the gene of interest (3 × 10^5 IU/ml, each).
Intact corneas or corneal pieces were incubated with the gene of interest / parental vector for one hour at 37°C in Biochrome Cornea Culture Medium I containing 8μg/ml polybrene. To remove excess virus, corneas were washed with sterile PBS prior to storage in the respective culture medium.
Corneal storage and induction of corneal endothelial cell loss
The aspects of corneal preservation were adapted from standard operating procedures and good laboratory practices published by the Eye Bank Association of America (EBAA) and the European Eye Bank Association (EEBA) 24,41,42. Corneas were obtained from eye banks and stored under organ culture (37°C, Biochrome Cornea Medium I, Berlin, Germany) or under hypothermic (4°C, Optisol GS™, Bausch & Lomb, Rochester, NY, USA) conditions. Both storage media are internationally well established in eye banks and represent current standards of practice conditions for corneal transplantation. To promote cell death, the respective storage media were diluted with calcium-free PBS at a ratio of 1:1, 1:4 or 1:10 (organ culture) or 1:1 or 1:10 (hypothermic storage). Depending on the dilution, corneas were stored between 9 days and 11 weeks and were evaluated every 72 hours or every week (Table 1, n=22).
Table 1.
Overview of storage experiments
| Storage Method |
Dilution medium:PBS |
Cornea pairs / cut single corneas |
Duration of storage |
Follow-up interval |
No. of analyzed visual fields |
|---|---|---|---|---|---|
| Organ culture | 1:10 | pairs | 9 days | 72 hours | 8 per cornea |
| (37C) | 1:1 | pairs | 7 weeks | weekly | 8 per cornea |
| 1:4 | single (5 pieces) |
5 weeks | weekly | 3 per corneal piece, 15 per cornea |
|
| 1:1 | single (5 pieces) |
5 weeks | weekly | 3 per corneal piece, 15 per cornea |
|
| Pure PBS | single (5 pieces) |
1 week | daily | 3 per corneal piece, 15 per cornea |
|
| Hypothermic culture |
1:10 | single (5 pieces) |
12 days | 72 hours | 3 per corneal piece, 15 per cornea |
| (4C) | 1:1 | single (5 pieces) |
11 weeks | weekly | 3 per corneal piece, 15 per cornea |
Detection of transgene expression, measurement of apoptosis and caspase 3
Transgene expression in cultured corneas was analyzed 0 (control), 1, 6 and 24h after transduction (n=2, single corneas cut into equal pieces). In addition, apoptosis was detected using a terminal deoxyribonucleotidyl transferase (TdT)-mediated deoxyuridine-5′-triphosphate-digoxigenin (dUTP) nick-end labeling (TUNEL) assay (In Situ Cell Death Detection Kit, Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instruction. Vital nuclei were visualized using To-Pro 3 iodide (Invitrogen, Eugene OR, USA). Cell borders were visualized using rabbit anti-ZO-1 (N term) (Invitrogen, Carlsbad CA, USA). Corneas were washed and mounted with Vectashield’s medium for fluorescence (Vector Laboratories, Burlingame CA, USA). IZsGreenW expression, TUNEL positivity was detected by a Leica TSC-SP2 confocal laser scanning microscope (40X). Caspase 3 in human EC was detected by flow cytometry after induction of apoptosis with 5 ng/ml Actinomycin D (for 24h) using the antibody cleaved Caspase-3 (Asp175) (Cell Signaling, Beverly MA, USA).
Detection of corneal endothelial cell loss and measurement of EC morphology
EC were visualized using the inverted research microscope Nikon Eclipse TE-2000S (10X, bright field mode), a microscope also used in eye banks. Enumeration of EC and analysis of hexagonality was performed using eye bank software allowing automated cell counts and simultaneous measurement of cell shape variation from hexagonal morphology (Endothelium Analysis Tool, Rhine-Tec GmbH, Krefeld, Germany). To obtain representative data, three visual fields were examined and analyzed in each corneal piece during experiments using single corneas cut into equal pieces. In intact corneas a total of eight visual fields were examined and analyzed (two in the center, three in the mid-periphery, three in the periphery of the cornea). Evaluation of stored corneas was performed every three days (1:10 dilutions) or weekly (1:4, 1:1 dilutions). In addition to the parameters above, certain phases of EC loss could be determined by comparison of the microscopy images to reference images.
Computations and statistical analysis
To verify the significance the Student’s t-test and the Mann-Whitney test was applied (p-values <0.05 were considered significant, p-values <0.01 highly significant). Regarding statistical analyses, error bars displayed in the figures were calculated from ± Standard Deviation (SD).
ACKNOWLEDGMENTS
This work was supported by NIH R01EY012963 (R.D.), K24EY019098 (R.D.), Research to Prevent Blindness Lew R. Wasserman Merit Award (R.D.), the German Research Foundation (DFG/FU 726/1-1, T.F.) and the Eye Bank Association of America (T.F.). We thank Tissue Banks International and the Lions Eye Institute for Transplant and Research (Tampa, Florida) for providing corneas for research purposes.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Garg P, Krishna PV, Stratis AK, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye (London, England) 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Jones GL, Ponzin D, Pels E, Maas H, Tullo AB, Claerhout I. European eye bank association. Developments in ophthalmology. 2009;43:15–21. doi: 10.1159/000223835. [DOI] [PubMed] [Google Scholar]
- 5.Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Investigative ophthalmology & visual science. 2002;43:2152–2159. [PubMed] [Google Scholar]
- 6.Armitage WJ, Easty DL. Factors influencing the suitability of organ-cultured corneas for transplantation. Investigative ophthalmology & visual science. 1997;38:16–24. [PubMed] [Google Scholar]
- 7.Vasara K, Setala K, Ruusuvaara P. Follow-up study of human corneal endothelial cells, photographed in vivo before enucleation and 20 years later in grafts. Acta ophthalmologica Scandinavica. 1999;77:273–276. doi: 10.1034/j.1600-0420.1999.770305.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruusuvaara P, Setala K. Long-term follow-up of cryopreserved corneal endothelium. A specular microscopic study. Acta Ophthalmol (Copenh) 1988;66:687–691. doi: 10.1111/j.1755-3768.1988.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 9.Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008;86:1720–1724. doi: 10.1097/TP.0b013e3181903b0a. [DOI] [PubMed] [Google Scholar]
- 10.Komuro A, Hodge DO, Gores GJ, Bourne WM. Cell death during corneal storage at 4 degrees C. Investigative ophthalmology & visual science. 1999;40:2827–2832. [PubMed] [Google Scholar]
- 11.Means TL, Geroski DH, Hadley A, Lynn MJ, Edelhauser HF. Viability of human corneal endothelium following Optisol-GS storage. Archives of ophthalmology. 1995;113:805–809. doi: 10.1001/archopht.1995.01100060131047. [DOI] [PubMed] [Google Scholar]
- 12.Albon J, Tullo AB, Aktar S, Boulton ME. Apoptosis in the endothelium of human corneas for transplantation. Investigative ophthalmology & visual science. 2000;41:2887–2893. [PubMed] [Google Scholar]
- 13.Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant. 2007;7:2082–2089. doi: 10.1111/j.1600-6143.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 14.Okumura N, Ueno M, Koizumi N, Sakamoto Y, Hirata K, Hamuro J, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investigative ophthalmology & visual science. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 15.Bourges JL, Torriglia A, Valamanesh F, Benezra D, Renard G, Behar-Cohen FF. Nitrosative stress and corneal transplant endothelial cell death during acute graft rejection. Transplantation. 2007;84:415–423. doi: 10.1097/01.tp.0000275378.45133.82. [DOI] [PubMed] [Google Scholar]
- 16.Gong N, Ecke I, Mergler S, Yang J, Metzner S, Schu S, et al. Gene transfer of cyto-protective molecules in corneal endothelial cells and cultured corneas: analysis of protective effects in vitro and in vivo. Biochemical and biophysical research communications. 2007;357:302–307. doi: 10.1016/j.bbrc.2007.03.146. [DOI] [PubMed] [Google Scholar]
- 17.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 18.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 19.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science (New York, NY. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 20.Xue D, Horvitz HR. Caenorhabditis elegans CED-9 protein is a bifunctional cell-death inhibitor. Nature. 1997;390:305–308. doi: 10.1038/36889. [DOI] [PubMed] [Google Scholar]
- 21.Clem RJ. Baculoviruses and apoptosis: the good, the bad, and the ugly. Cell death and differentiation. 2001;8:137–143. doi: 10.1038/sj.cdd.4400821. [DOI] [PubMed] [Google Scholar]
- 22.Clem RJ. Baculoviruses and apoptosis: a diversity of genes and responses. Current drug targets. 2007;8:1069–1074. doi: 10.2174/138945007782151405. [DOI] [PubMed] [Google Scholar]
- 23.Toniolo M, Camposampiero D, Griffoni C, Jones GL. Quality management in European eye banks. Developments in ophthalmology. 2009;43:70–86. doi: 10.1159/000223840. [DOI] [PubMed] [Google Scholar]
- 24.Pels E, Rijneveld WJ. Organ culture preservation for corneal tissue. Technical and quality aspects. Developments in ophthalmology. 2009;43:31–46. doi: 10.1159/000223837. [DOI] [PubMed] [Google Scholar]
- 25.Parker DG, Kaufmann C, Brereton HM, Anson DS, Francis-Staite L, Jessup CF, et al. Lentivirus-mediated gene transfer to the rat, ovine and human cornea. Gene therapy. 2007;14:760–767. doi: 10.1038/sj.gt.3302921. [DOI] [PubMed] [Google Scholar]
- 26.Beutelspacher SC, Ardjomand N, Tan PH, Patton GS, Larkin DF, George AJ, et al. Comparison of HIV-1 and EIAV-based lentiviral vectors in corneal transduction. Experimental eye research. 2005;80:787–794. doi: 10.1016/j.exer.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Appukuttan B, Ott S, Patel R, Irvine J, Song J, et al. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vector. Gene therapy. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- 28.McMahon JM, Conroy S, Lyons M, Greiser U, O’Shea C, Strappe P, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem cells and development. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XY, La Russa VF, Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. Journal of virology. 2004;78:1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell death and differentiation. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 31.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science (New York, NY. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q, Krebs JF, Snipas SJ, Price A, Alnemri ES, Tomaselli KJ, et al. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase/p35 complex. Biochemistry. 1998;37:10757–10765. doi: 10.1021/bi980893w. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H. Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature. 2001;410:494–497. doi: 10.1038/35068604. [DOI] [PubMed] [Google Scholar]
- 34.Gain P, Thuret G, Chiquet C, Dumollard JM, Mosnier JF, Burillon C, et al. Value of two mortality assessment techniques for organ cultured corneal endothelium: trypan blue versus TUNEL technique. The British journal of ophthalmology. 2002;86:306–310. doi: 10.1136/bjo.86.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crewe JM, Armitage WJ. Integrity of epithelium and endothelium in organ-cultured human corneas. Investigative ophthalmology & visual science. 2001;42:1757–1761. [PubMed] [Google Scholar]
- 36.Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science (New York, NY. 1998;281:1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Ohgushi H, Dohi Y, et al. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2009 doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi R, Yamato M, Takayanagi H, Oie Y, Kubota A, Hori Y, et al. Validation System of Tissue Engineered Epithelial Cell Sheets for Corneal Regenerative Medicine. Tissue engineering. 2009 doi: 10.1089/ten.TEC.2009.0277. [DOI] [PubMed] [Google Scholar]
- 39.Lombardo M, Lombardo G, Friend DJ, Serrao S, Terry MA. Long-term anterior and posterior topographic analysis of the cornea after deep lamellar endothelial keratoplasty. Cornea. 2009;28:408–415. doi: 10.1097/ICO.0b013e31818d33c7. [DOI] [PubMed] [Google Scholar]
- 40.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11:RA110–121. [PubMed] [Google Scholar]
- 41.Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. International ophthalmology. 2008;28:155–163. doi: 10.1007/s10792-007-9086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goren MB. The Eye Bank Association of America. Comprehensive ophthalmology update. 2006;7:261–262. [PubMed] [Google Scholar]