Abstract
Prepulse inhibition (PPI) of the acoustic startle reflex refers to the reduction of the startle response to an intense acoustic pulse stimulus when it is shortly preceded by a weak non-startling prepulse stimulus and provides a cross-species measure of sensory-motor gating. PPI is typically impaired in schizophrenia patients, and a similar impairment can be induced in rats by systemic scopolamine, a muscarinic cholinergic receptor antagonist that can evoke a range of cognitive and psychotic symptoms in healthy humans that are commonly referred to as the “anti-muscarinic syndrome” resembling some clinical features of schizophrenia. Scopolamine-induced PPI disruption has therefore been proposed as an anti-muscarinic animal model of schizophrenia, but parallel investigations in the mouse remain scant and the outcomes are mixed and often confounded by an elevation of startle reactivity. Here, we distinguished the PPI-disruptive and the confounding startle-enhancing effects of scopolamine (1 and 10 mg/kg, i.p.) in C57BL/6 wild-type mice by showing that the latter partly stemmed from a shift in spontaneous baseline reactivity. With appropriate correction for between-group differences in startle reactivity, we went on to confirm that the PPI-disruptive effect of scopolamine could be nullified by clozapine pre-treatment (1.5 mg/kg, i.p.) in a dose-dependent manner. This is the first demonstration that scopolamine-induced PPI disruption is sensitive to atypical antipsychotic drugs. In concert with previous data showing its sensitivity to haloperidol the present finding supports the predictive validity of the anti-muscarinic PPI disruption model for both typical and atypical antipsychotic drug action.
Keywords: muscarinic receptor, scopolamine, clozapine, prepulse inhibition, schizophrenia, mice
1. INTRODUCTION
Muscarinic cholinergic dysfunction has been implicated in the pathophysiology of schizophrenia (for reviews, see Bymaster et al. 2002; Dean et al. 2003; Raedler et al. 2007; Scarr and Dean, 2008). The muscarinic receptor antagonist, scopolamine, can evoke multiple cognitive and psychotic disturbances in healthy individuals similar to the symptoms of schizophrenia – collectively known as the “anti-muscarinic syndrome” (Marchlewski, 1994; Perry et al. 1978; Perry and Perry, 1995; Yeomans, 1995), and consistently disrupts prepulse inhibition (PPI) in rats (see Table 1) – a form of sensorimotor gating that is commonly reported to be deficient in schizophrenia patients (Braff and Geyer, 1990; Braff et al., 1992, 2001). PPI is a cross-species phenomenon referring to the reduction of the startle response to an intense acoustic pulse stimulus when it is shortly preceded by a weak non-startling prepulse stimulus (Graham, 1975; Hoffman and Searle, 1965).
Table 1.
Reported effects of scopolamine on PPI and startle reaction in mice and rats.
| Strain | Scopolamine Dose |
Control Treatment |
PPI | Startle | References | |
|---|---|---|---|---|---|---|
| Rats | Wistar | 0.33 mg/kg | Saline | ↓ | ≈ | Hohnadel et al. 2007 |
| 0.14 and 1 mg/kg | Saline | ↓ | ? | Tadros et al. 2009 | ||
| 1 mg/kg | Saline | ↓ | ↓ | Yeomans et al. 2010 | ||
| Sprague Dawley | 1 mg/kg | Saline | ↓ | ≈ | Wu et al. 1993 | |
| 0–40 nmol/0.5µl* | Saline | ↓ | ≈ | Fendt and Koch, 1999 | ||
| 0.3–1.0 mg/kg | Saline | ↓ | ≈ | Jones and Shannon, 2000a | ||
| 0.3–1.0 mg/kg | Saline mSCP | ↓ | ≈ | Jones and Shannon, 2000b | ||
| 0.1 mg/kg | Saline mSCP | ↓ | ≈ | Stanhope et al. 2001 | ||
| 0.3 and 0.56 mg/kg | Saline | ↓ | ↑ | Sipos et al. 2001 | ||
| 0.5 mg/kg | Saline | ↓ | ≈ | Jones et al. 2005 | ||
| 0.3 mg/kg | Saline | ↓ | ≈ | Andrus et al. 2007 | ||
| 0.4 mg/kg | Saline | ↓ | ≈ | Mitchell and Neumaier, 2008 | ||
| Mice | C57BL/6 | 3, 10 and 30 mg/kg | Saline | ≈ | ≈ | Ouagazzal et al. 2001 |
| 1 mg/kg | Saline | ↓ | ? | Yeomans et al. 2010 | ||
| C57BL/6NTac | 1, 1.8 mg/kg | Saline | ↓ | ↑ | Thomsen et al., 2010 | |
| ddY | 0.3 mg/kg | Saline | ≈ | ↑ | Ukai et al. 2004 | |
| Swiss | 0.3 mg/kg | Saline | ≈ | ↑ | Eleore et al. 2007 |
↑: PPI/startle enhanced, ↓: PPI/startle reduced, “≈” PPI/startle unaltered, “n/a” data not available.
bilateral microinfusion in caudal pontine reticular nucleus. “mSCP” refers to methyl-scopolamine.
It has been shown that the PPI-disruptive effect of scopolamine is reversible by the typical antipsychotic haloperidol, a dopamine D2 receptor (D2R) agonist, while the PPI disruption induced by apomorphine, a non-specific dopamine receptor agonist, can be antagonized by muscarinic agonists (Jones et al. 2005). This pattern of results highlights the relevance of muscarinic-dopaminergic interaction in the regulation of PPI, and the contribution of its imbalance to PPI deficiency in schizophrenia (Davis et al. 1975). Scopolamine-induced PPI disruption has therefore been proposed as an antimuscarinic model of schizophrenia (Barak 2009). However, its sensitivity to atypical antipsychotic drugs, such as clozapine, has never been examined which is of importance with respect to the identification of novel drugs targeting the negative and cognitive symptoms of schizophrenia which are largely resistant to typical antipsychotics (e.g., Geyer and Ellenbroek, 2003).
Examination of existing mouse studies however raises some concern over the cross-species consistency of the anti-muscarinic PPI model of schizophrenia. Table 1 shows that only two out of the five existing mouse studies reported a disruption of PPI by scopolamine, compared to twelve out of twelve rat studies reporting a disruption of PPI. Given the known differences between mice and rats in the modulation of PPI by different subtypes of dopamine receptors (e.g., Doherty et al. 2008; Ralph-Williams et al. 2003), it is of fundamental importance to ascertain whether the robust PPI-disruptive effect of scopolamine seen in rats is translatable to mice. Moreover, three of the five mice studies quoted in Table 1 reported a confounding increase in startle reactivity that could complicate interpretation of PPI data because there is ample evidence for a dependency of PPI on the baseline startle reactivity in mice as well as humans (Csomor et al. 2006, 2008; Yee et al. 2005).
The present study validated the PPI-disruptive effect of scopolamine in C57BL/6 mice using a PPI protocol with multiple pulse stimuli in order to control for a potential confounding effect on startle reaction (see Pietropaolo et al. 2008; Singer et al. 2009; Yee et al. 2005). To dissociate central from peripheral effects of scopolamine we included its methyl analogue methylscopolamine, which does not cross the blood-brain barrier, as a peripheral control in addition to the standard saline control. Finally, we evaluated for the first time whether scopolamine-induced PPI disruption was reversible by clozapine, the prototypical antipsychotic drug.
2. MATERIALS AND METHODS
2.1. Animals
Experimentally naïve, 12 weeks old, male C57BL/6NCrl mice were generated at our in-house specific pathogen free facility (ETH Zurich, Schwerzenbach, Switzerland) from breeding pairs obtained from Charles River (France). They were group-housed, 4–5 to a cage, in Type III Macrolon cages (Techniplast, Milan, Italy), kept in a temperature- and humidity-controlled vivarium (21°C, 55% relative humidity) with a reversed 12:12h light-dark cycle (lights off: 07:00–19:00) and ad libitum access to food and water. All experiments were conducted in the dark phase of the cycle. The manipulations and procedures described in the present study had been previously approved by the Cantonal Veterinary Office in Zurich, and conformed to Directive 86/609/EEC on animal experimentation and the Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985).
2.2. Drugs
All solutions for injection were freshly prepared on the day of testing, and administered via the intraperitoneal route at a volume of 5 ml/kg. Scopolamine (SCP) and methylscopolamine (mSCP) were obtained from Sigma-Aldrich (Germany) and dissolved in sterile saline to achieve the desired doses of 1 and 10 mg/kg. SCP and mSCP were administered 30 min before testing. Clozapine (CLZ) was obtained from Sigma-Aldrich (Germany) and dissolved in 0.1 N HCl in physiological saline to prepare the appropriate dose of either 0.5 or 1.5 mg/kg. The acidity was adjusted to pH 5.5 by adding solid Na2CO3. An equivalent concentration of saline/HCl mixture (pH 5.5) served as vehicle control. CLZ was administered 45 min before testing.
2.3. Assessment of PPI
Apparatus and procedure have been fully described by Yee et al. (2005). In brief, four acoustic startle chambers for mice (SR-LAB, San Diego Instruments, San Diego, CA, USA) were used to assess PPI. A testing session began after a 2-min acclimatization period. The first six trials consisted of pulse-alone trials in order habituate and stabilize the animals’ startle response, which were not analysed. The animals were subsequently tested across 10 blocks of test trials to assess PPI, with each block consisting of three pulse-alone trials, three prepulse-alone trials, the nine possible combinations of prepulse-plus-pulse trials (3 levels of pulse × 3 levels of prepulse), and one no-stimulus trial (i.e., background alone). The 16 discrete trials within each block were presented in a pseudorandom order, with a variable inter-trial interval averaging 15 s (ranging from 10 to 20 s). The sound intensity of the pulse stimulus was 100, 110 or 120 dBA and that of the prepulse 71, 77 or 83 dBA. The duration of the pulse and prepulse was 40 and 20 ms, respectively. All trials were presented against a constant background noise of 65 dBA. In prepulse-plus-pulse trials, the stimulus onset asynchrony between the two stimuli was 100 ms.
Whole body acceleration was measured by a stabilimeter on each trial within a 65-ms response window (from the onset of the pulse in pulse-alone and prepulse-plus-pulse trials, or the onset of the prepulse on prepulse-alone trials). This output (in arbitrary units) was referred to as reactivity score. Individual average reactivity scores per trial type formed a highly skewed data distribution that deviated significantly from a normal distribution [Shapiro-Wilk Test: all p<0.05] as expected, and therefore the data set was subjected to a logarithmic transformation [transformed score = ln(reactivity score + e)] as recommended by Csomor et al. (2008) in order to improve data distribution especially in terms of skewness and kurtosis as well as and variance homogeneity (i.e., homoscedasticity). The improvement contributed to both increasing statistical power and preventing systematic misinterpretation as explained in full by Csomor et al. (2008), even though the distribution of the transformed data still significantly deviated from a normal distribution according to the Shapiro-Wilk test of normality [all p’s < 0.05].
Since scopolamine treatment led to a confounding elevation of baseline spontaneous activity on no-stimulus trials (see Results below), the critical analyses of PPI were performed on normalized reactivity data, i.e. corrected by subtraction of baseline activity recorded on no-stimulus trials. Due to the confounding effect on startle reactivity by scopolamine, PPI was not indexed by percent inhibition, but by means of direct comparison between pulse-alone trials and prepulse-plus-pulse trials (see Swerdlow et al. 2000). In this approach, PPI is indexed by the rate of reduction in startle reaction as a function of increasing prepulse intensity and the PPI magnitude is reflected by the steepness (slope) of the startle reactivity curve.
To further minimize the confounding effect of unequal startle reactivity in the assessment of PPI we matched the startle reactivity of the different drug conditions across the three pulse intensities, as previously demonstrated (see Pietropaolo et al. 2008; Singer et al. 2009). This was conducted in all experiments to enhance consistency and avoid any arbitrariness in application. This is in line with our PPI protocol incorporating multiple pulse intensities (100, 110 and 120-dB pulse stimuli), which was designed specifically to overcome such interpretative concerns related to divergent baseline startle reactivity. To this end, we followed a general selection procedure as follows. In a comparison with k treatments, the objective is to select, out of the three possible pulse intensities, one for each treatment so that group differences in “mean pulse-alone reactivity” are minimized. To this end, all possible combinations [= k3] were examined by calculating all possible [= k(k+1)/2] pairwise (group) differences in “mean pulse-alone reactivity”, and then the associated sum of squared differences. The combination that yielded the minimal “sum of squared differences” was then selected as the “matched combination” regardless of whether it involved any cross-pulse comparison.
2.4. Statistical analysis
The data were analysed by parametric analysis of variance (ANOVA). To assist the interpretation of the statistical outcomes, significant effects were further investigated by supplementary restricted analyses applied to subsets of the data included in the overall ANOVA, and pair-wise Student-Newman-Keuls or Fisher’s least significant difference (LSD) post hoc comparisons. All statistical analyses were carried out using IBM SPSS Statistics (version 18, SPSS Inc. Chicago IL, USA). A two-tailed criterion of p<0.05 was taken as the cut-off for statistical significance.
3. RESULTS
3.1. Scopolamine dose-dependently impaired PPI by blocking central muscarinic receptors
The effect of SCP (1 and 10 mg/kg) was evaluated against equivalent doses of mSCP in addition to saline control. The two doses were tested in the same animals. The lower dose was assessed first followed by a 2-week washout period before testing the higher dose with prior drug experience fully counterbalanced. The sample size per treatment group was 8. To control for a potential confounding effect of repeated drug treatment we originally included the between-subject factor drug experience in the analysis of the second experiment. Since no statistically significant outcomes were detected involving this factor, it was dropped in the final analyses presented below.
3.1.1. Effects on single-stimulus and no-stimulus trials
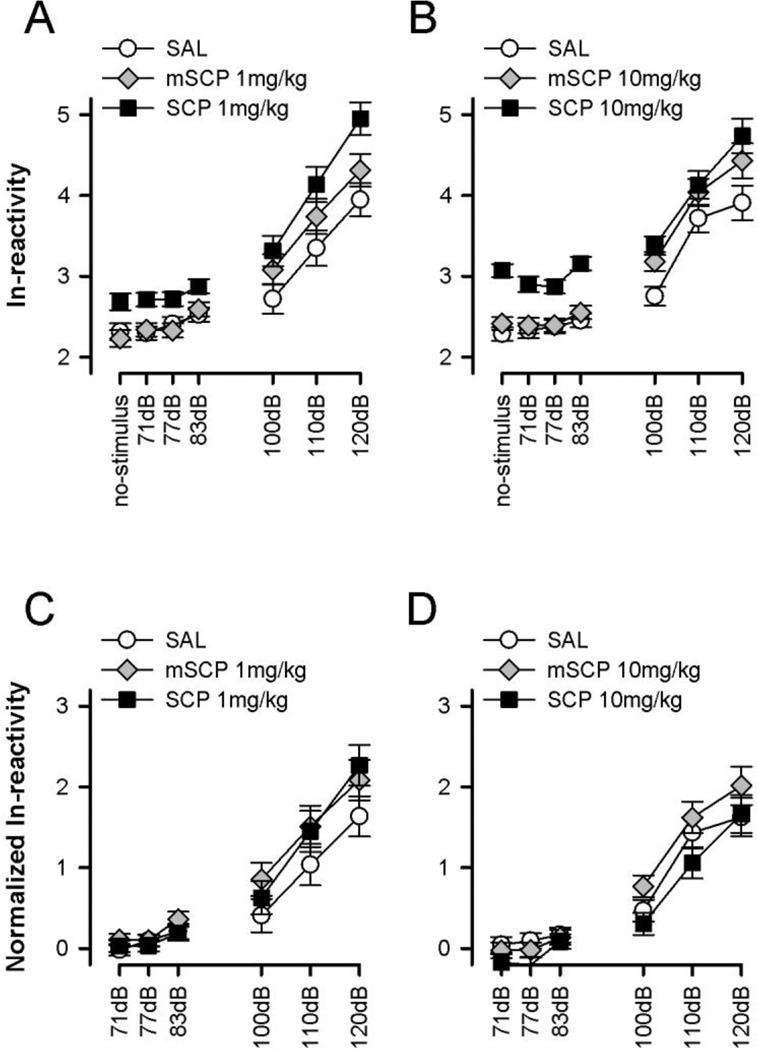
First, the spontaneous activity recorded on no-stimulus trials and the reactivity obtained on prepulse-alone, and pulse-alone trials were examined. As expected, the reactivity levels generally increased with increasing stimulus intensity (Figures 1A and 1B). In both experiments, SCP led to an overall increase in the reactivity scores evident across all seven trial-types, including baseline spontaneous activity on no-stimulus trials. Consistent with this observation, separate 3 × 7 (Treatment × Trial-type) ANOVAs revealed a significant main effect of treatment [Exp. 1: F(2,21)=10.60, p<0.001, Exp. 2: F(2,21)=20.11, p<0.001] and trial-type [Exp. 1: F(6,126)=103.34, p<0.001 Exp. 2: F(6,126)=101.69, p<0.001] in both experiments. Subsequent Student-Newman-Keuls post-hoc comparisons indicated that the 1mg/kg SCP group differed significantly (p<0.05) from the 1mg/kg mSCP and SAL groups in the first experiment, while all three groups significantly differed from each other (p<0.05) in the second experiment.
Figure 1.
Spontaneous activity on no-stimulus and the reaction on single-stimulus trials. Scopolamine (SCP), but not methylscopolamine (mSCP) led to an upward-shift in the reactivity curve, mainly due to an increase in baseline spontaneous activity on no-stimulus trials (A and C). This effect of SCP could be eliminated by normalizing the ln-transformed reactivity against baseline reactivity using a difference score (B and D). All values refer to mean values ± standard error (SE).
It is obvious that the enhancing effect of scopolamine on prepulse-elicited reactivity and startle reaction (on pulse-alone trials) was mainly due to an increase in baseline spontaneous activity on no-stimulus trials causing an upward shift of the reactivity curve. In support of this, one-way ANOVA of baseline reactivity yielded a significant main effect of treatment in both experiments [Exp. 1: F(2,21)=5.54, p<0.05, Exp. 2: F(2,21)=27.38, p<0.001]. In each case, Newman-Keuls post-hoc comparisons verified higher baseline reactivity in SCP-treated mice relative to the mSCP and SAL groups (p’s<0.05) which did not differ significantly from each other (see Figures 1A and 1B). Importantly, the significant treatment effect of scopolamine was eliminated (p>0.15) when the reactivity scores recorded on prepulse-alone and pulse-alone trials were normalized as deviation from baseline reactivity and then subjected to a 3 × 6 (Treatment × Trial-type) ANOVA (see Figures 1C and 1D). Thus, it appears that the nature of the startle-potentiating effect of scopolamine reported in some studies (Table 1) was to a large extent accounted for by an increase in baseline reactivity rather than a genuine potentiation of the startle magnitude. To eliminate this potential confound, all analyses of PPI were performed on normalized reactivity scores.
3.1.2. Effects on PPI
The expression of PPI is reflected by a decrease in the startle reactivity on prepulse-plus-pulse trials relative to pulse-alone trials which was analysed by a 3 × 3 × 4 (Treatment × Pulse intensity × Prepulse intensity) ANOVA that included reactivity data from pulse-alone and prepulse-plus-pulse trials, respectively. Comparison of the two PPI experiments suggests that scopolamine dose-dependently disrupted PPI via its central action.
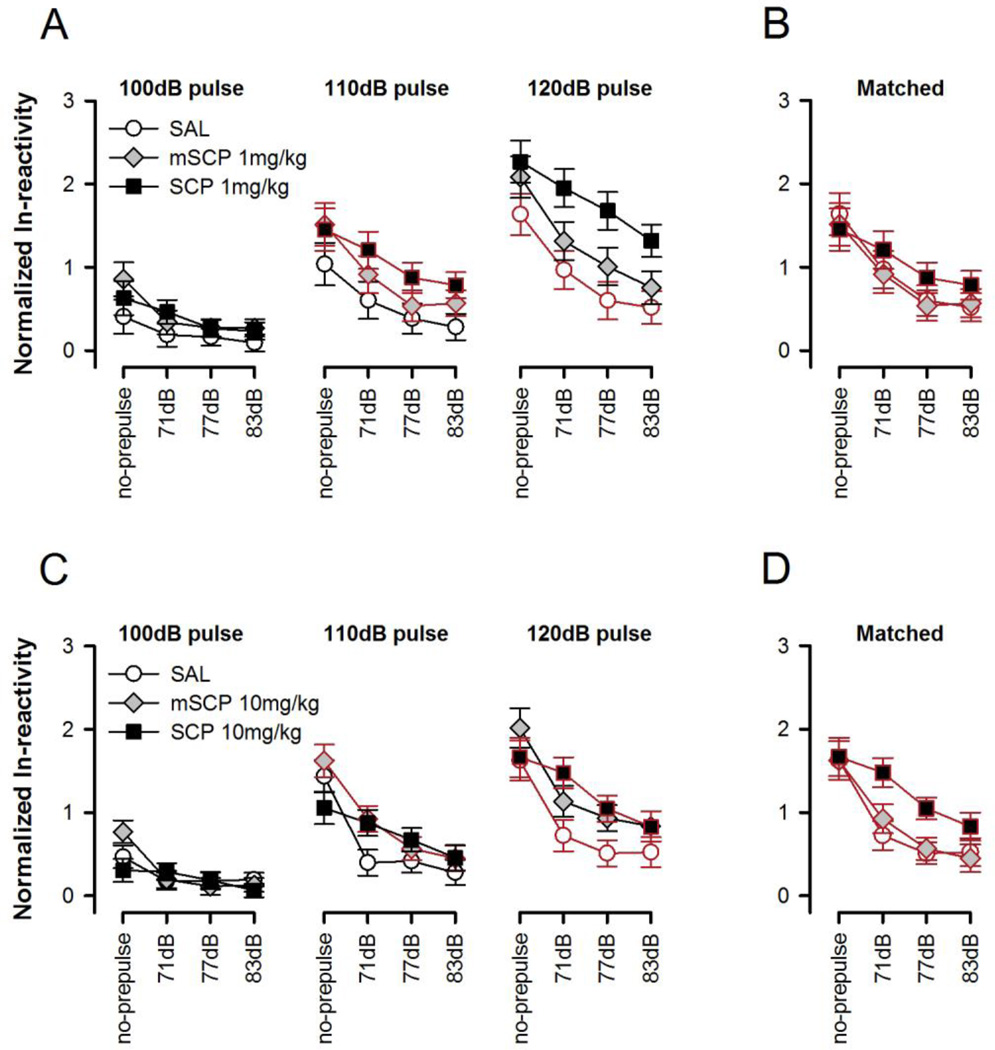
In both experiments, the overall presence of PPI was confirmed by a significant decrease of startle reactivity with increasing prepulse intensity [Exp. 1: F(3,63)=72.85, p<0.001, Exp. 2 F(3,63)=112,46, p<0.001] (see Figure 2). The magnitude of the PPI effect also depended on the prepulse intensity yielding a significant prepulse intensity × pulse intensity interaction [Exp. 1: F(6,126)=13.63, p<0.001, Exp. 2: F(6,126)=12.72, p<0.001]. As expected, more intense pulse stimuli generally yielded a stronger reaction, thus giving rise to a highly significant pulse effect [Exp. 1: F(2,42)=101.11, p<0.001, Exp. 2: F(2,42)=81.02, p<0.001].
Figure 2.
Prepulse inhibition (PPI) is indexed by the reduction in startle reaction as a function of increasing prepulse intensity, and is depicted separately for each of the three pulse intensities (as indicated at the top) in A and C. The different prepulse intensities are denoted along the abscissa in dB units. Pulse-alone trials are denoted as ‘no-prepulse’. The respective pulse conditions that are explicitly compared in the cross-pulse analysis are highlighted in red and are re-plotted in B and C. Scopolamine (SCP) led to a non-significant decrease in PPI at 1 mg/kg (A and B) whilst the drug significantly reduced PPI at 10 mg/kg (C and D) as indicated by a significant drug × prepulse intensity interaction revealed by both the overall (three pulse × three prepulse) and cross-pulse analysis (p’s < 0.05). By contrast, methylscopolamine (mSCP) did not alter PPI at either dose. All values refer to mean values ± SE.
In experiment 1, drug treatment modified the pulse-dependency of the startle reactivity as indicated by a significant treatment × pulse intensity interaction [F(4,42)=4.55, p<0.005] but there was no statistical evidence that it affected PPI given that both the treatment × prepulse intensity and the three-way interaction were far from statistical significance. To improve the power to detect a possible drug effect on PPI we matched the startle reactivity on pulse alone-trials across drug conditions (see Figure 2B). The best matched data (110-dB pulse condition for SCP and mSCP, and 120-dB pulse condition for SAL) were then subjected to 3 × 4 (Treatment × Prepulse intensity) ANOVA which yielded only a significant main effect of prepulse intensity [F(3,63)=68.60, p<0.001]. Thus, the absence of a significant treatment × prepulse intensity interaction (p=0.10) indicated that SCP did not disrupt PPI at a dose of 1 mg/kg.
The initial three-way ANOVA indicated that the reactivity profile as a function of prepulse intensity (Figure 2C) differed significantly between groups [F(6,63)=7.35, p<0.001]. This was pursued further in the cross-pulse analysis next that matched the startle reaction between groups to improve comparability and minimize confound due to startle reactivity difference (see Figure 2D), even though overall reactivity was not significantly affected by drug treatment here. A significant treatment × prepulse intensity interaction [F(3,63)=2.56, p<0.05] emerged again in the cross-pulse analysis that compared the 110-dB pulse condition of mSCP against the 120-dB pulse condition of SCP and SAL groups. This thus confirmed that the three groups differed in terms of the impact of the prepulse to diminish the startle response towards the pulse stimulus (Figure 2D). To further delineate the pattern of between-groups difference, restricted analyses were conducted with one group eliminated at a time. These 'post-hoc ANOVAs' revealed that the critical treatment × prepulse intensity interaction was solely attributable to weaker PPI in the SCP group relative to the two control groups (mSCP and SAL), which did not differ from each other. These conclusions were supported by the fact that the treatment × prepulse intensity interaction remained significant so long as the restricted analysis included the SCP group, but was rendered non-significant only when the SCP group was dropped.
3.2. Clozapine dose-dependently reversed scopolamine-induced PPI disruption
A four-group factorial design was used to assess the efficacy of clozapine pretreatment (CLZ 0.5mg/SCP: n=9 and CLZ 1.5mg/SCP: n=10) to counter the PPI-disruptive effect of 10 mg/kg scopolamine (VEH/SCP, n=10). The VEH/SAL condition (n=8) allowed the demonstration of scopolamine-induced PPI disruption and the PPI-corrective effect of clozapine.
3.2.1. Effects on single stimulus and no-stimulus trials
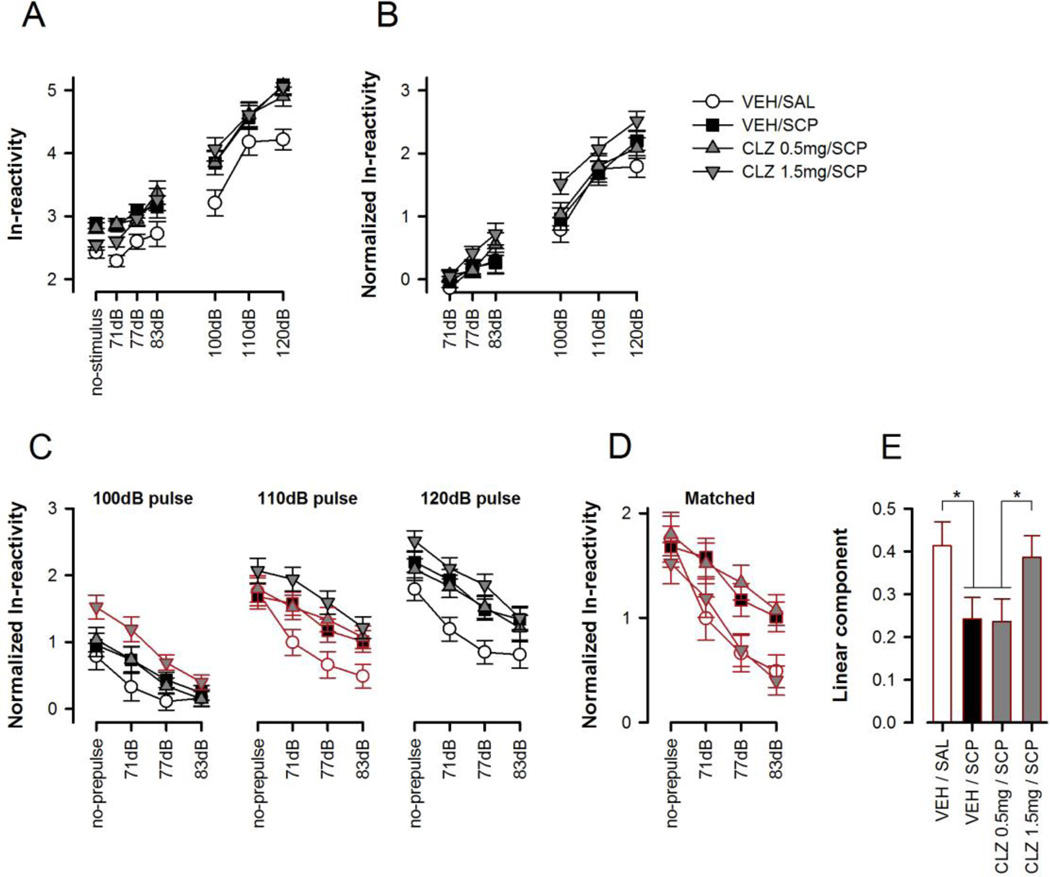
The baseline spontaneous activity on no-stimulus trials was again found to be increased by scopolamine, and this effect was antagonized by clozapine pretreatment at 1.5 mg/kg but not 0.5 mg/kg (Figure 3A). Consistent with this impression, a one-way ANOVA of baseline reactivity revealed a significant main effect of treatment [F(3,33)=7.17, p<0.001]. Student-Newman-Keuls post-hoc comparisons identified one homogeneous subset consisting of the VEH/SAL and CLZ 1.5mg/SCP groups that significantly differed (p<0.05) from the other subset comprising the VEH/SCP and CLZ 0.5mg/SCP groups.
Figure 3.
Summary of the results obtained in the clozapine experiment. The following data sets were derived and separately analyzed: (A) ln-transformed reactivity on single stimulus trials including spontaneous activity obtained in no-stimulus trials, (B) Normalized reactivity scores on single stimulus trials, (C) PPI as indexed by the reduction in startle reaction as a function of increasing prepulse intensity, which is depicted separately for each of the three pulse intensities (as indicated at the top). The different prepulse intensities are denoted along the abscissa in dB units. Pulse-alone trials are denoted as ‘no-prepulse’. The respective pulse conditions that are explicitly compared in the cross-pulse analysis are highlighted in red and are re-plotted in (D). (E) Represents the linear component (slope) of the reactivity curve plotted in (D). * Denotes a significant group difference (p<0.05) based on Fisher’s LSD post hoc comparison following the emergence of significant drug effect in the one-way ANOVA. All values refer to mean values ± SE.
A 4 × 7 (Treatment × Trial-type) ANOVA including no-stimulus, prepulse-alone and pulse-alone trials yielded a significant main effect of treatment [F(3,33)=5.66, p<0.005] and trial-type [F(6,198)=236.41, p<0.001]. Student-Newman-Keuls post-hoc comparisons attributed the main effect of treatment solely to the general elevated response in all drug treated groups relative to VEH/SAL control. However, the treatment effect was no longer statistically significant when prepulse- and pulse-elicited reactions were normalized with respect to baseline (no-stimulus) spontaneous activity. A 4 × 6 (Treatment × Trial-type) ANOVA of the normalized reactivity data no longer yielded any significant effect of treatment (Figure 3B). To correct for the non-specific baseline differences subsequent analysis of PPI was therefore performed on the normalized reactivity data.
3.2.2. Effects on PPI
CLZ pre-treatment with 0.5 mg/kg did not modify the response to the subsequent SCP challenge (Figure 3C). At the higher dose of 1.5 mg/kg, CLZ strengthened PPI in SCP-treated mice, but this was coupled with a general increasing effect on reactivity. A dose-dependent antagonism of SCP-induced PPI disruption by CLZ was revealed (Figure 3D) when the divergence on startle reactivity (on pulse-alone trials) was balanced ad hoc between groups.
The normalized reactivity data were first subjected to a 4 × 3 × 4 (Treatment × Pulse intensity × Prepulse intensity) ANOVA. As expected, the general effect of pulse intensity was highly significant [F(2,66)=137.32, p<0.001]. The overall presence of PPI was evident by a significant decrease in reactivity with increasing prepulse intensity [F(3,99)=197.95, p<0.001]. The emergence of a significant treatment × prepulse intensity interaction [F(9,99)=3.16, p<0.005] indicated that PPI was modified by the drug treatment. This interaction stemmed solely from the PPI-disruptive effect of scopolamine, because it was no longer significant in a restricted analysis excluding the VEH/SAL group, a finding that might at first sight suggest the lack of an effect by clozapine to modify PPI expression in SCP-treated animals. However, interpretation was further complicated by the presence of a 3-way interaction in the overall analysis [F(18,198)=1.77, p<0.05], suggesting that the factor pulse needed to be taken into account. To achieve this, the PPI profile was balanced with respect to the pulse-alone condition among all four treatment conditions, indicated by the red line, as shown in Figure 3D. The matching comprised the 100-dB pulse condition for the ‘CLZ 1.5mg + scopolamine group’ and the 110 dB-pulse condition for the others three groups (i.e., ‘CLZ 0.5mg/kg + scopolamine’, ‘vehicle/saline’, ‘vehicle/scopolamine’), and the analysis of the matched data set considerably clarified the interpretation of the drug effects on PPI. The 4 × 4 (Treatment × Prepulse intensity) ANOVA of the startle-matched data again yielded a highly significant treatment × prepulse intensity interaction [F(9,99)=3.07, p<0.005], which was largely accounted for by the linear trend of prepulse intensity (p<0.05). Post-hoc comparisons of the linear component (i.e. the slope of the reactivity curve), which represents the strength of the PPI effect, further supported the interpretation that clozapine dose-dependently antagonized the PPI disruptive effect of scopolamine (Figure 3E).
4. DISCUSSION
The present study yielded three major findings. First, scopolamine dose-dependently impaired PPI in C57BL/6 mice and this was solely attributed to the central action of the drug – as validated here for the first time in mice, in agreement with the findings in rats (Jones and Shanon, 2000b; Stanhope et al. 2001). Second, scopolamine consistently increased baseline spontaneous activity in no-stimulus trials which might partly explain the apparent startle-enhancing effect of scopolamine previously reported (see Table 1). Third, we demonstrated for the first time that the PPI-disruptive effect of scopolamine was reversible by the atypical antipsychotic clozapine. Since the disruption is also prevented by the typical antipsychotic haloperidol (Jones et al. 2005), one may conclude that the anti-muscarinic PPI model possesses predictive validity for both classes of antipsychotic drugs.
4.1 Characteristics of the PPI-disruptive effect of scopolamine
Throughout the three experiments it is obvious that the PPI disruptive effect of scopolamine was most pronounced at the lowest prepulse intensity of 71 dB corresponding to 6 dB units above the background noise level. Indeed, when restricting the PPI analysis to the lowest prepulse intensity, the PPI-disruptive effect produced by 1 mg/kg of scopolamine would yield sufficient statistical power to demonstrate a significant PPI disruption. A similar impression has been reported by Jones and Shannon (2000b) who showed that the PPI disruption produced by scopolamine in rats could be overcome by increasing the prepulse intensity to 30 dB units above the background noise level. The authors interpreted this as evidence for a reduction in the signal-to-noise ratio to detect the prepulse stimulus. This interpretation is consistent with suggestions that cholinergic activation decreases processing of background noise and thereby increases the signal-to-noise ratio (Drachman and Sahakian, 1979; Everitt and Robbins, 1997; Robbins, 1997). Here, we did not find clear evidence that scopolamine had reduced the direct reaction to the prepulse stimulus. However, this is likely due to the fact that the measurement of whole-body movement may not be sufficiently sensitive to detect a motor response to weak prepulse stimuli, especially in the presence of increased baseline reactivity (see also Yee and Feldon, 2009). Thus, the present findings are in line with the hypothesis that muscarinic cholinergic control of PPI may involve the modulation of the signal-to-noise ratio.
Barak (2009) suggests that scopolamine-induced PPI disruption stems mainly from a blockade of inhibitory muscarinic autoreceptors in the midbrain leading to an increase in cholinergic activity in dopaminergic cells of the ventral tegmental area (VTA) and substantia nigra (SN) (Chapman et al. 1997; Tzavara et al. 2004; Yeomans, 1995). Enhanced excitation of the VTA and SN via cholinergic afferents from the laterodorsal tegmental nucleus and tegmental pedunculopontine nucleus (Chapman et al. 1997; Mesulam et al. 1983; Woolf and Butcher, 1986) could then result in increased dopamine efflux in the striatum which has been closely associated with disruption of PPI (Swerdlow et al. 2001). This is consistent with the reported efficacy of muscarinic agonists to reverse PPI disruption induced by the direct dopamine receptor agonist, apomorphine, which is likely to activate directly dopamine receptors in the nucleus accumbens (Jones et al. 2005). However, the PPI disruption produced by apomorphine could not be eliminated by prepulse of stronger intensity in rats (Jones and Shannon, 2000b; Swerdlow et al. 1991; Swerdlow and Geyer, 1993, but see Davis et al. 1990) and was accompanied by an increase in prepulse-elicited reaction in C57BL/6 mice without compromising the dependency of PPI on the prepulse intensity (Yee et al. 2004b). Hence, these findings suggest that apomorphine does not impair PPI by reducing the signal-to-noise ratio of the prepulse stimulus, implying that scopolamine and apomorphine may disrupt PPI via distinct mechanisms. Of note here is the observation that, at least in C57BL/6 mice, the PPI-disruptive effect exhibited by apomorphine or the NMDAR antagonists MK-801 and phencyclidine is dissociable by their opposite effects on prepulse-elicited reactivity (Yee and Feldon, 2009). Thus, prepulse-elicited reaction was increased by apomorphine (Yee et al. 2004b) but reduced by high doses of phencyclidine (Yee et al. 2004a). Here we found that scopolamine impaired PPI without affecting prepulse-elicited reactivity as measured by whole-body movement indicating that manipulations of the dopaminergic, glutamatergic and cholinergic systems differentially affect the motor response to the prepulse-stimulus in the C57BL/6 mouse strain. Although, it must be mentioned that the observed effects on PPI and prepulse-elicited reactivity are not necessarily causally related (see also Yee et al. 2004a).
4.2. Does scopolamine enhance startle reaction?
In all experiments reported here, scopolamine robustly increased baseline spontaneous activity recorded on no-stimulus trials. Likewise, scopolamine also stimulates spontaneous motor activity (Sanberg et al. 1987; Shannon and Peters, 1990; Sipos et al. 1999). In agreement with our data, this motor effect has been solely attributed to the central action of scopolamine because peripheral blockade of muscarinic receptors by methylscopolamine is devoid of such effects (Bond, 1986). Interestingly, this non-specific shift in baseline reactivity was also responsive to clozapine pre-treatment at the corresponding dose that was effective against PPI disruption. Thus, it follows that the motor effect of scopolamine might have been over-interpreted as a startle-enhancing effect in some studies (see Table 1), and the normalization procedure adopted here has been instrumental in minimizing such confounding in the evaluation of PPI. Our approach of balancing pulse-alone reactivity between groups had further removed any residual confounding differences that might exist and clarified the action of scopolamine and clozapine on PPI by further diminishing confounding effects on startle reactivity (c.f., Singer et al. 2009).
4.3. Scopolamine-induced PPI disruption predicts typical and atypical antipsychotic activity
Jones et al. (2005) reported that the PPI-disruptive effect of scopolamine was almost completely reversed by the typical antipsychotic haloperidol. Here, we demonstrated for the first time that scopolamine-induced PPI disruption was similarly sensitive to atypical antipsychotics by showing a dose-dependent reversal by clozapine, the prototypical atypical antipsychotic drug. At a dose of 0.5 mg/kg, clozapine pre-treatment essentially had no impact, whereas increasing the dose to 1.5 mg/kg achieved a near-complete restoration of PPI (Figures 3D and 3E). In this context, the unique affinity of clozapine for muscarinic receptors (Davies et al. 2005; Olianas et al. 1999; Weiner et al. 2004) is noteworthy. Although recent knockout mouse models do not support an involvement of M1/M4 muscarinic receptors in the PPI-enhancing effect of clozapine (Thomsen et al. 2010), clozapine is known to act as a weak muscarinic receptor agonist, particularly at M4 receptors (Bymaster et al., 2003; Zorn et al., 1994). Affinity for M1, M2 and M3 muscarinic receptors has also been observed in vitro cell culture preparation (e.g., Oianas et al., 1999; Zorn et al., 1994). Here, clozapine was tested specifically in an anti-muscarinic environment due to scopolamine co-treatment, which may favour expression of the agonistic effect of clozapine on muscarinic receptors. The possibility that clozapine-induced muscarinic receptor agonism countered the functional blockade of muscarinic receptors by scopolamine therefore cannot be entirely eliminated. In this regards, it is worth noting that clozapine is highly effective in enhancing extracellular acetylcholine amongst atypical anti-psychotic drugs (see Bywater et al. 2003). Thomson et al. (2007) have also shown that the M5 receptor is not necessary for clozapine to enhance PPI but its genetic inactivation appears to potentiate the PPI-enhancing effect of clozapine but not haloperidol which may have some therapeutic relevance given that the M5 receptor gene has been implicated in susceptibility to schizophrenia in humans (De Luca et al. 2004). However, the underlying mechanism by which disruption of M5 receptor function enhances the antipsychotic efficacy of clozapine remains to be elucidated. Nonetheless, such functional synergism may provide a functional link between muscarinic antagonism by clozapine and the drug’s anti-psychotic efficacy (see Bywater et al. 2003). Receptor subtypes dependent mechanisms may hold the key to a meaningful integration of clozapine’s pharmacological property as a partial agonist at muscarinic receptors with dual agonistic and antagonistic actions, and its delineation may provide novel impetus in the search for anti-psychotic drugs with novel mechanisms.
Reversibility by atypical antipsychotic drugs is considered a crucial feature of a PPI deficiency model to identify drugs that may exert efficacy against the cognitive and negative symptoms of schizophrenia that are largely resistant to typical antipsychotics, such as haloperidol (Geyer et al. 2001, 2003). This is mainly based on the finding that PPI disruption induced by NMDAR antagonists is reversible by clozapine but not haloperidol (Geyer et al. 2003) – a pattern that is consistent with the suggestion that NMDAR hypofunction primarily underlies the negative and cognitive symptoms of schizophrenia (Geyer et al. 2003). By contrast, clozapine lacks efficacy against apomorphine-induced PPI disruption in C57BL/6 mice, which is readily restored by haloperidol (Russig et al. 2004; Yee et al. 2004b). Hence, the PPI disruption produced by scopolamine in the C57BL/6 mouse strain can be distinguished from that induced by NMDAR antagonists as well as dopamine receptor agonists by its unique sensitivity to both classes of antipsychotic drugs. The three pharmacologically distinct drug-induced PPI disruption models form a complementary triad instrumental in the preclinical screening of potential antipsychotic drugs.
5. Conclusions
The present study suggests that scopolamine-induced PPI disruption in C57BL6 mice might represent a pharmacologic model for positive as well as cognitive/negative schizophrenia symptoms providing further justification for the development of anti-cholinergic PPI disruption mouse models. This is also in line with recent data suggesting a direct involvement of central cholinergic dysfunction in the pathophysiology of both positive and cognitive symptoms of schizophrenia (e.g., Barak, 2009; Bymaster et al. 2002; Dean et al. 2003; Raedler et al. 2007; Scarr and Dean, 2008).
Highlights.
-
►
Prepulse inhibition is disrupted by muscarinic cholinergic blocker in mice
-
►
The effect is centrally mediated & independent of its effect on startle reactivity
-
►
Clozapine antagonizes the PPI-disruptive effect of scopolamine
-
►
A drug-induced PPI deficit model uniquely responsive to typical/atypical neuroleptics
ACKNOWLEDGEMENTS
The present study was supported by the Swiss Federal Institute of Technology Zurich, with additional support from the Swiss National Science Foundation (#3100A0-116719) and the NIH (#MH083973). We are also grateful to Peter Schmid for his technical support, Daniel Blaser, Ruedi Blersch and Michèle Bürkli for their assistance in animal husbandry, and Joram Feldon for providing access to the necessary equipment, provision of drugs and animals in the reported experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The authors declare no conflicts of interests.
REFERENCES
- Andrus AK, Marable BR, Dunbar GL, Reilly MP, Maurissen JP. Effects of intensity and type of prepulse stimulus on prepulse inhibition in scopolamine treated rats. Pharmacol Biochem Behav. 2007;87:481–488. doi: 10.1016/j.pbb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Barak S. Modeling cholinergic aspects of schizophrenia, Focus on the antimuscarinic syndrome. Behav Brain Res. 2009;204:335–351. doi: 10.1016/j.bbr.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Bond NW. Prenatal alcohol exposure and offspring hyperactivity, effects of scopolamine and methylscopolamine. Neurobehav Toxicol Teratol. 1986;8:287–292. [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia, human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Ahmed S, McKinzie DL. Muscarinic receptors as a target for drugs treating schizophrenia. Curr Drug Targets CNS Neurol Disord. 2002;1:163–181. doi: 10.2174/1568007024606249. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76:177–186. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Quednow BB, Stadler RR, Feldon J, Vollenweider FX. The monotonic dependency of prepulse inhibition of the acoustic startle reflex on the intensity of the startle-eliciting stimulus. Behav Brain Res. 2006;174:143–150. doi: 10.1016/j.bbr.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Davies MA, Compton-Toth BA, Hufeisen SJ, Meltzer HY, Roth BL. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a pre-requisite for mimicking clozapine's actions? Psychopharmacology (Berl) 2005;178:451–460. doi: 10.1007/s00213-004-2017-1. [DOI] [PubMed] [Google Scholar]
- Davis KL, Hollister LE, Berger PA, Barchas JD. Cholinergic imbalance hypothesis of psychoses and movement disorders, strategies for evaluation. Psychopharmacol Commun. 1975;1:533–543. [PubMed] [Google Scholar]
- Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology (Berl) 1990;1021:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- De Luca V, Wang H, Squassina A, Wong GW, Yeomans J, Kennedy JL. Linkage of M5 muscarinic and alpha7-nicotinic receptor genes on 15q13 to schizophrenia. Neuropsychobiology. 2004;50:124–127. doi: 10.1159/000079102. [DOI] [PubMed] [Google Scholar]
- Dean B, Bymaster FP, Scarr E. Muscarinic receptors in schizophrenia. Curr Mol Med. 2003;3:419–426. doi: 10.2174/1566524033479654. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- Drachman DR, Sahakian BJ. The effects of cholinergic agents on human learning and memory. In: Barbeau A, Growden JH, Wurtman RJ, editors. Nutrition in the Brain. New York: Raven; 1979. pp. 351–366. [Google Scholar]
- Eleore L, López-Ramos JC, Yi PJ, Delgado-García JM. The cognitive enhancer T-588 partially compensates the motor associative learning impairments induced by scopolamine injection in mice. Behav Neurosci. 2007;121:1203–1214. doi: 10.1037/0735-7044.121.6.1203. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Clin Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M. Cholinergic modulation of the acoustic startle response in the caudal pontine reticular nucleus of the rat. Eur J Pharmacol. 1999;370:101–207. doi: 10.1016/s0014-2999(99)00156-9. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychol. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia. A decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hohnadel E, Bouchard K, Terry AV., Jr Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology. 2007;52:542–551. doi: 10.1016/j.neuropharm.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE. Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J Pharmacol Exp Ther. 2005;312:1055–1063. doi: 10.1124/jpet.104.075887. [DOI] [PubMed] [Google Scholar]
- Jones CK, Shannon HE. Effects of scopolamine in comparison with apomorphine and phencyclidine on prepulse inhibition in rats. Eur J Pharmacol. 2000a;391:105–112. doi: 10.1016/s0014-2999(00)00055-8. [DOI] [PubMed] [Google Scholar]
- Jones CK, Shannon HE. Muscarinic cholinergic modulation of prepulse inhibition of the acoustic startle reflex. J Pharmacol Exp Ther. 2000b;294:1017–1023. [PubMed] [Google Scholar]
- Marchlewski MB. Anticholinergic syndrome, avoiding misdiagnosis. J Psychosoc Nurs Ment Health Serv. 1994;32:22–24. doi: 10.3928/0279-3695-19940901-09. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptor antagonist reversal of emotional learning and prepulse inhibition deficits induced by apomorphine or scopolamine. Pharmacol Biochem Behav. 2008;88:291–298. doi: 10.1016/j.pbb.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas MC, Maullu C, Onali P. Mixed agonist–antagonist properties of clozapine at different human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary cells. Neuropsychopharmacology. 1999;20:263–270. doi: 10.1016/S0893-133X(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Jenck F, Moreau JL. Drug–induced potentiation of prepulse inhibition of acoustic startle reflex in mice, a model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH. Acetylcholine and hallucinations, disease-related compared to drug–induced alterations in human consciousness. Brain Cogn. 1995;28:240–258. doi: 10.1006/brcg.1995.1255. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Wilding DC, Juhl RP. Anticholinergic psychosis. Am J Hosp Pharm. 1978;35:725–728. [PubMed] [Google Scholar]
- Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice, preferential vulnerability in the male sex. Psychopharmacology (Berl) 2008;197:613–628. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Russig H, Spooren W, Durkin S, Feldon J, Yee BK. Apomorphine-induced disruption of prepulse inhibition that can be normalised by systemic haloperidol is insensitive to clozapine pretreatment. Psychopharmacology (Berl) 2004;175:143–147. doi: 10.1007/s00213-004-1810-1. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Henault MA, Hagenmeyer-Houser SH, Russell KH. The topography of amphetamine and scopolamine-induced hyperactivity, toward an activity print. Behav Neurosci. 1987;101:131–133. doi: 10.1037//0735-7044.101.1.131. [DOI] [PubMed] [Google Scholar]
- Scarr E, Dean B. Muscarinic receptors, do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–1195. doi: 10.1111/j.1471-4159.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine–induced hyperactivity in mice. J Pharmacol Exp Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- Singer P, Feldon J, Benjamin K, Yee BK. Are DBA/2 mice associated with schizophrenia–like endophenotypes? A behavioural contrast with C57BL/6 mice. Psychopharmacology (Berl) 2009;206:677–698. doi: 10.1007/s00213-009-1568-6. [DOI] [PubMed] [Google Scholar]
- Sipos ML, Burchnell V, Galbicka G. Dose-response curves and time-course effects of selected anticholinergics on locomotor activity in rats. Psychopharmacology (Berl) 1999;147:250–256. doi: 10.1007/s002130051164. [DOI] [PubMed] [Google Scholar]
- Sipos ML, Burchnell V, Galbicka G. Effects of selected anticholinergics on acoustic startle response in rats. Fundam Appl Toxicol. 2001;21:95–101. doi: 10.1002/jat.821. [DOI] [PubMed] [Google Scholar]
- Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, Kennett GA, Lightowler S, Sheardown MJ, Syed R, Upton RL, Wadsworth G, Weiss SM, Wyatt A. The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther. 2001;299:782–792. [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating, what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–115. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Geyer MA. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav Neurosci. 1993;107:104–117. doi: 10.1037//0735-7044.107.1.104. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J. Pharmacol Exp Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- Tadros MG, Mohamed MR, Youssef AM, Sabry GM, Sabry NA, Khalifa AE. Proapoptotic and prepulse inhibition (PPI) disrupting effects of Hypericum perforatum in rats. Journal of Ethnopharmacology. 2009;122:561–566. doi: 10.1016/j.jep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Wess J, Fulton BS, Fink-Jensen A, Caine SB. Modulation of prepulse inhibition through both M(1) and M(4) muscarinic receptors in mice. Psychopharmacology (Berl) 2010;208:401–416. doi: 10.1007/s00213-009-1740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Wortwein G, Fink-Jensen A, Woldbye DP, Wess J, Caine SB. Decreased prepulse inhibition and increased sensitivity to muscarinic, but not dopaminergic drugs in M5 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 2007;192:97–110. doi: 10.1007/s00213-006-0682-y. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Ukai M, Okuda A, Mamiya T. Effects of anticholinergic drugs selective for muscarinic receptor subtypes on prepulse inhibition in mice. Eur J Clin Pharmacol. 2004;492:183–187. doi: 10.1016/j.ejphar.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, Vanover KE, Olsson R, Jayathilake K, Lee M, Levey AI, Hacksell U, Burstein ES, Davis RE, Brann MR. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology (Berl) 2004;177:207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems of the rat brain. III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Wu MF, Jenden DJ, Fairchild MD, Siegel JM. Cholinergic mechanisms in startle and prepulse inhibition, effects of the false cholinergic precursor N-aminodeanol. Behav Neurosci. 1993;107:306–316. doi: 10.1037//0735-7044.107.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Chang DL, Feldon J. The Effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology. 2004a;29:1865–1877. doi: 10.1038/sj.npp.1300480. [DOI] [PubMed] [Google Scholar]
- Yee BK, Chang T, Pietropaolo S, Feldon J. The expression of prepulse inhibition of the acoustic startle reflex as a function of three pulse stimulus intensities, three prepulse stimulus intensities, and three levels of startle responsiveness in C57BL6/J mice. Behav Brain Res. 2005;163:265–276. doi: 10.1016/j.bbr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Yee BK, Feldon J. Distinct forms of prepulse inhibition disruption distinguishable by the associated changes in prepulse-elicited reaction. Behav Brain Res. 2009;204:387–395. doi: 10.1016/j.bbr.2008.11.049. [DOI] [PubMed] [Google Scholar]
- Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004b;29:240–248. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology. 1995;12:3–16. doi: 10.1038/sj.npp.1380235. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Bosch D, Alves N, Daros A, Ure RJ, Schmid S. GABA receptors and prepulse inhibition of acoustic startle in mice and rats. Eur J Neurosci. 2010;31:2053–2061. doi: 10.1111/j.1460-9568.2010.07236.x. [DOI] [PubMed] [Google Scholar]
- Zorn SH, Jones SB, Ward KM, Liston DR. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994;269:R1–R2. doi: 10.1016/0922-4106(94)90047-7. [DOI] [PubMed] [Google Scholar]