Abstract
Background:
Despite major advances in percutaneous coronary intervention (PCI), in-stent restenosis (ISR) remains a therapeutic challenge. We sought to compare the mid-term clinical outcomes after treatment with repeat drug-eluting stent (DES) implantation (“DES sandwich” technique) with DES placement in the bare-metal stent (DES-in-BMS) in a “real world” setting.
Methods:
We retrospectively identified and analyzed clinical and angiographic data on 194 patients previously treated with the DES who underwent repeat PCI for ISR with a DES or a BMS. ISR was defined, by visual assessment, as a luminal stenosis greater than 50% within the stent or within 5 mm of its edges. We recorded the occurrence of major adverse cardiac events (MACE), defined as cardiac death, non-fatal myocardial infarction, and the need for target vessel revascularization (TVR).
Results:
Of the 194 study participants, 130 were men (67.0%) and the mean ± SD of age was 57.0 ± 10.4 years, ranging from 37 to 80 years. In-hospital events (death and Q-wave myocardial infarction) occurred at a similar frequency in both groups. Outcomes at twelve months were also similar between the groups with cumulative clinical MACE at one-year follow-up of 9.6% and 11.3% in the DES-in-BMS and the DES-in-DES groups, respectively (p value = 0.702). Although not significant, there was a trend toward a higher TVR rate in the intra-DES ISR group as compared to the intra-BMS ISR group (0.9% BMS vs. 5.2% DES; p value = 0.16).
Conclusion:
Our study suggests that the outcome of the patients presenting with ISR did not seem to be different between the two groups of DES-in-DES and DES-in-BMS at one-year follow-up, except for a trend toward more frequent TVR in the DES-in-DES group. Repeat DES implantation for DES restenosis could be feasible and safe with a relatively low incidence of MACE at mid-term follow-up.
Keywords: Angioplasty; Drug-eluting stent; Treatment outcome; Graft occlusion, vascular; Prognosis
Introduction
In-stent restenosis (ISR) remains one of the most difficult challenges in coronary interventional therapy since patients with ISR are at a higher risk for the recurrence of restenosis.1, 2 The drug-eluting stent (DES), as compared to the bare-metal stent (BMS), has shown impressive results for the prevention of restenosis in primary lesions. Nevertheless, due to the increased use of the DES, the rate of ISR after DES implantation is becoming increasingly prevalent.3–7 Although in two recent controlled randomized trials, the DES was demonstrated to be superior to conventional brachytherapy in treating patients with intra-BMS ISR,8, 9 data regarding the role of the DES in treating intra-DES ISR are limited.
The use of the DES for the treatment of intra-BMS ISR and intra-DES ISR, both, has risen as a result of the simplicity of this approach and its effectiveness.10–12 Be that as it may, little is known about the mid- and long-term outcomes with this procedure, particularly for DES-in-DES stenting. The aim of this study was, therefore, to compare the one-year clinical outcomes of DES stenting in patients with ISR after the BMS versus DES implantation.
Methods
Baseline clinical and angiographic data were obtained from a computerized database of prospectively recorded clinical and procedural information on standardized forms during the in-hospital period and at follow-up. After the exclusion of primary PCI patients (410), a total of 11,249 consecutive patients with 14,898 lesions underwent stenting at Tehran Heart Center between February 2004 and March 2010. Of these patients, those with a first episode of ISR in whom a single DES was implanted were regarded as the study population. For ISR treatment, 131 (67.5%) first-generation stents and 63 (32.5%) second-generation stents were implanted. Patients in whom the operators were unable to place a stent and those who received a minimum of two stents were excluded from the study.
A comparison was made between 114 consecutive patients (114 lesions) with the ISR of the initial BMS treated with the DES and 80 consecutive patients (with 80 lesions) with the ISR of the initial DES treated with the DES.
Informed consent was obtained from all the patients, and the study protocol was approved by the local Ethics Committee.
Using standard percutaneous techniques, percutaneous coronary intervention (PCI) and intracoronary stent implantation were performed by operators, who relied on their own judgment to assess stent expansion. All the patients were on Aspirin and received a heparin bolus of 7500–10000 IU. The patients also received oral Clopidogrel (75 mg) once daily at least 3–5 days before the procedure or a 300–600 mg oral loading dose before catheterization at the discretion of the operator, followed by a daily oral administration of 75 mg of the drug. The patients were encouraged to continue Clopidogrel therapy for a minimum of 6 months between 2003 and 2004 and a minimum of 12 months from 2005 onwards. Platelet GP IIb/IIIa inhibitors were administered at the operator’s discretion.
Upon the completion of the procedure, the patients were transferred to a monitored unit, where vascular access sheaths, if still in place, were removed.
In-hospital mortality was defined as death within the same hospital admission regardless of the cause after PCI. For all the patients, 12-lead electrocardiography was obtained prior and following intervention to detect procedure-related ischemic changes and/or the appearance of a new pathologic Q wave on the surface electrocardiogram. After the procedure, all the patients were checked for creatine kinase MB fraction enzyme sampling at 8 and 16 hours (normal values up to 35 IU/L). The diagnosis of non-Q wave myocardial infarction (MI) was considered as creatine kinase MB fraction elevation greater than three times of the normal values in the absence of new pathologic Q-waves on electrocardiograms following intervention. Emergency coronary artery bypass grafting (CABG) was defined as CABG performed within 24 hours after the index percutaneous procedure. Target lesion revascularization (TLR) was defined as clinically indicated percutaneous or surgical revascularization of the index lesion during the follow-up, and target vessel revascularization (TVR) was defined as the revascularization of the vessel formerly treated via PCI during the index hospitalization through a repeat percutaneous intervention or bypass surgery.
The primary end-points of this study were late major adverse cardiac events (MACE), defined as death, non-fatal MI, and the need for TVR. Cumulative MACE was defined as in-hospital and one-year follow-up MACE.
The data on the early outcomes and occurrence of death, new non-fatal MI, need for CABG, and subsequent need for repeat PCI in both groups were recorded. Follow-up was scheduled at one month, 6 months, and 12 months and was conducted by clinic visits. If the patients were unable to return to the clinics, follow-up was performed using telephone interviews, mailing, and reviewing hospital records. All the patients had at least data on a one-year follow-up.
Angiographic analysis was conducted based on the consensus opinion of two experienced interventionists. ISR was defined as a luminal narrowing of more than 50% inside the stent or a segment at 5 mm within its vicinity. Angiographic ISR was classified according to the previously reported Mehran classification.1
For the statistical analyses, statistical software SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL) was used. All the p values were two-tailed, with statistical significance defined as a p value ≤ 0.05.
The continuous variables are expressed as mean ± standard deviation (SD), and the categorical data are presented as absolute frequencies and percentages. The continuous variables were compared using the Student t-test, and the categorical variables were compared using the chi-square (or the Fisher exact test, as required).
The authors of this manuscript hereby certify that they have fully complied with the principles of the European Association of Science Editors (EASE) guidelines for authors and translators of scientific articles.13
Results
The study population’s baseline demographic and clinical characteristics are shown in Table 1, and the angiographic and procedural characteristics are depicted in Table 2. No significant differences in the baseline demographic and clinical characteristics were found between the “DES-sandwich” group and the DES-in-BMS group, except that the patients with BMS ISR had a significantly higher level of creatinine (p value = 0.03). In both groups, there was a high prevalence of hyperlipidemia (75.4% and 70.0% in the intra-BMS ISR and in the “DES-sandwich” group, respectively; p value = 0.90), denoting that hyperlipidemia was a common risk factor in these groups of patients. Clinical presentation was found to be acute MI in 16% of the patients with a non-significant increase in the patients with DES ISR. Unstable angina and stable angina were the presenting syndromes in 38% and 37% of the patients, respectively; they were similar between the BMS and DES groups.
Table 1.
Baseline clinical characteristics*
| All patients (n=194) | Intra-BMS ISR (n=114) | Intra-DES ISR (n=80) | P values** | |
|---|---|---|---|---|
| Age (y) | 57.04±10.36 | 57.50±9.88 | 56.38±11.03 | 0.458 |
| Male sex | 130 (67.0) | 77 (67.5) | 53 (66.3) | 0.850 |
| LVEF (%) | 51.60±8.86 | 51.71±8.69 | 51.44±9.15 | 0.892 |
| Creatinine (mg/dl) | 1.13±0.28 | 1.17±0.30 | 1.08±0.24 | 0.034 |
| Diabetes mellitus | 50 (25.8) | 29 (25.4) | 21 (26.3) | 0.899 |
| Hypertension | 89 (45.9) | 55 (48.2) | 34 (42.5) | 0.429 |
| Hyperlipidemia | 142 (73.2) | 86 (75.4) | 56 (70.0) | 0.400 |
| Current cigarette smoking | 44 (22.7) | 25 (21.9) | 19 (23.8) | 0.766 |
| Family history of CAD | 60 (30.9) | 37 (32.5) | 23 (28.8) | 0.582 |
| Previous myocardial infarction | 73 (37.6) | 39 (34.2) | 34 (42.5) | 0.241 |
| Previous CABG | 8 (4.1) | 2 (1.8) | 6 (7.5) | 0.067 |
| Previous stroke | 3 (1.5) | 1 (0.9) | 2 (2.5) | 0.570 |
| Presenting symptom | 0.606 | |||
| Stable angina | 89 (45.9) | 56 (49.1) | 33 (41.3) | |
| Unstable angina | 74 (38.1) | 42 (36.8) | 32 (40.0) | |
| Non-STEMI | 21 (10.8) | 10 (8.8) | 11 (13.8) | |
| STEMI | 10 (5.2) | 6 (5.3) | 4 (5.0) |
Data are presented as mean±SD or n (%)
P values for intra-BMS ISR versus intra-DES ISR
LVEF, Left ventricular ejection fraction; CAD, Coronary artery disease; CABG, Coronary artery bypass grafting; STEMI, ST-segment elevation; MI, Myocardial infarction; BMS, Bare metal stent; DES, Drug-eluting stent; ISR, In-stent restenosis
Table 2.
Baseline lesion and procedural characteristics of the study population*
| Intra-BMS ISR (n=114) | Intra-DES ISR (n=80) | P value | |
|---|---|---|---|
| Target vessel | 0.008 | ||
| Left anterior descending | 68 (59.6) | 56 (70.0) | |
| Left circumflex | 13 (11.4) | 9 (11.3) | |
| Right coronary artery | 33 (28.9) | 11 (13.8) | |
| Saphenous vein graft | 0 | 4 (5.0) | |
| Type B2+C lesion | 100 (87.7) | 65 (81.3) | 0.507 |
| Lesion length (mm) | 25.62±10.60 | 23.54±11.98 | 0.203 |
| Reference vessel diameter (mm) | 3.11±0.42 | 3.10±0.44 | 0.800 |
| Chronic total occlusion | 11 (9.6) | 6 (7.5) | 0.602 |
| Bifurcations | 11 (9.6) | 2 (2.5) | 0.050 |
| Proximal lesion treated | 49 (43.0) | 41 (51.3) | 0.256 |
| Diffuse lesion | 87 (76.3) | 49 (61.3) | 0.024 |
| Long tubular lesion | 14 (12.3) | 17 (21.3) | 0.093 |
| Ostial lesion | 8 (7.0) | 5 (6.3) | 0.833 |
| Mehran Type ISR | 0.103 | ||
| I | 31 (27.2) | 21 (26.3) | |
| II | 25 (48.2) | 49 (61.3) | |
| III | 20 (17.5) | 5 (6.3) | |
| IV | 8 (7.0) | 5 (6.3) | |
| Stent length (mm) | 28.48±6.47 | 27.31±7.70 | 0.522 |
| Stent diameter (mm) | 3.01±0.36 | 3.05±0.39 | 0.470 |
| Stent overlapping | 8 (7.0) | 6 (7.5) | 0.898 |
| Residual stenosis | 5 (4.4) | 7 (8.8) | 0.238 |
Data are presented as mean ± SD or n (%)
BMS, Bare metal stent; DES, Drug-eluting stent; ISR, In-stent restenosis
The target vessel was predominantly the left anterior descending artery (LAD), followed by the right coronary artery (RCA) and the left circumflex artery (LCX) in both groups, but the distribution pattern was significantly different between the two groups (p value = 0.008) (Table 2). The ISR target lesion was a chronic total occlusion in 9% of the patients and a bifurcation lesion in 7% of the patients. Bifurcations and diffuse lesions were more prevalent in the intra-BMS ISR group than in the intra-DES ISR group (p value = 0.05 and p value = 0.02, respectively). Calcification, ostial location, and tortuous lesions were seen similarly in both groups. Lesion length was similar in both groups with a similar reference vessel diameter and baseline percent diameter stenosis.
Post-procedural coronary angiography demonstrated similar residual stenosis in both groups with a non-significant final lumen area higher in the intra-BMS ISR group (p value = 0.24). Both groups presented predominantly with a type II diffuse pattern of restenosis according to the Mehran classification1, and there was no statistically significant difference between the groups with regard to the pattern of restenosis.
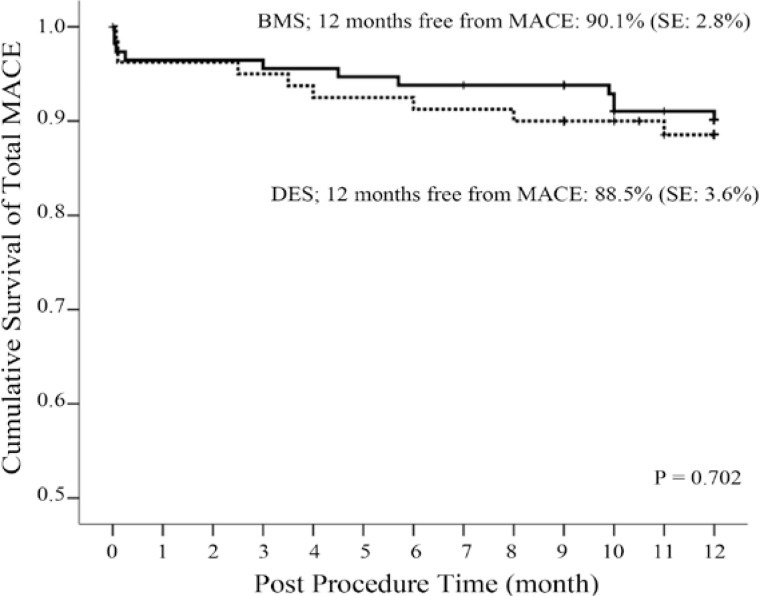
As is shown in Table 3, in-hospital events (death and Q-wave MI) occurred with a similar frequency in both groups. Outcomes at twelve months were also similar between the groups with respect to death, Q-wave MI, TVR, TLR, and overall MACE (Table 4). Although not significant, there was a trend toward a higher TVR rate in the intra-DES ISR group as compared to the intra-BMS group (0.9% BMS vs. 5.2% DES; p value = 0.16). The Kaplan-Meier curve of MACE-free survival is illustrated in Figure 1. Cumulative clinical MACE at one-year follow-up was 9.6% and 11.3% in the DES-in-BMS and DES-in-DES groups, respectively (p value = 0.702).
Table 3.
In-hospital clinical outcomes of the study population*
| Intra-BMS ISR (n=114) | Intra-DES ISR (n=80) | P value | |
|---|---|---|---|
| Cardiac mortality | 1 (0.9) | 0 | 0.999 |
| Myocardial infarction | 4 (3.5) | 3 (3.8) | 0.999 |
| Overall events | 5 (4.4) | 3 (3.8) | 0.806 |
Data are presented as n (%)
BMS, Bare metal stent; DES, Drug-eluting stent; ISR, In-stent restenosis
Table 4.
Clinical events at one-year follow-up*
| Intra-BMS ISR (n=109) | Intra-DES ISR (n=77) | P value | |
|---|---|---|---|
| Cardiac mortality | 0 | 0 | - |
| Target vessel revascularization | 1 (0.9) | 4 (5.2) | 0.162 |
| Target lesion revascularization | 3 (2.8) | 1 (1.3) | 0.642 |
| Non-fatal myocardial infarction | 2 (1.8) | 1 (1.3) | 0.999 |
Data are presented as n (%)
BMS, Bare metal stent; DES, Drug-eluting stent; ISR, In-stent restenosis
Figure 1.
Kaplan-Meier estimations of total major adverse cardiac events-free survival MACE, Major adverse cardiac events; BMS, Bare-metal stent; DES, Drug-eluting stent
Discussion
The main finding of the present study is that the outcome in the patients presenting with ISR was not different between the two groups of DES-in-DES and DES-in-BMS at one-year follow-up, except for a trend toward more frequent TVR in the DES-in-DES group. Moreover, regardless of the type of the initial stent (DES or BMS), the patients with ISR more commonly presented with acute coronary syndrome. This latter finding confirms the results of several previous studies reporting that intra-BMS ISR may not be as benign as once believed.14–16
Even in the DES era, restenosis remains the Achilles’ heel of PCI. The use of the DES during PCI may have failed to eradicate the incidence of restenosis, but it has achieved a dramatic decrease in this complication, which is the main drawback of the BMS implantation in patients with de novo lesions.17 Two landmark randomized trials,8, 9 the SISR (Sirolimus in-stent restenosis) and the TAXUS-V in-stent restenosis (ISR), compared the DES with vascular brachytherapy in patients with post-BMS restenosis and recommended the DES as the preferred modality for the treatment of patients with ISR. A meta-analysis of randomized trials also demonstrated that the use of the DES effectively reduced the risk of ISR recurrence after the implantation of the BMS and was associated with superior results as compared to plain balloon angioplasty and vascular brachytherapy. Thus, the DES should be recommended as the treatment of choice for intra-BMS restenosis.18
Although the use of the DES is associated with low rates of TVR in patients with intra-BMS ISR and is considered to be standard therapy in this group of patients,8, 9, 18, 19 outcomes are less known in patients with intra-DES ISR treated with repeat DES implantation (“DES sandwich” technique).19 To date, only one randomized clinical trial, the ISAR-DESIRE (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis)20 has been published. This trial examined the treatment of intra-DES ISR with a similar DES (homo-DES) versus a different DES (hetero-DES) and showed no statistically significant differences regarding the one-year clinical end-points of TLR (17% vs. 15%), death/MI (6.1% vs. 5.8%), or stent thrombosis (0.4% in both groups). The final results of the CRISTAL study, a multi-center randomized clinical trial comparing the homo- and hetero-DES with balloon re-angioplasty for the treatment of intra-DES restenosis and a comparator group with intra-BMS restenosis, have not yet been published in full. That study, however, hypothesized that the DES re-stenting would demonstrate a similar outcome in both intra-DES ISR and intra-BMS ISR, and the preliminary results showed that the post-procedural and follow-up minimal luminal diameter (MLD) rates were higher in the BMS group than in the DES groups. As a result, performance of the Sirolimus-eluting stent seems superior in the BMS restenosis than in the DES restenosis, with the limitation of differences in baseline characteristics including pre-procedural MLD.17
A number of researchers have investigated the DES implantation for patients with intra-DES ISR. Lemos et al.21 examined 24 patients treated with DES-in-DES placement for intra-DES ISR and reported a recurrence rate of 18.2%, suggesting relatively better results than those obtained with other modalities. Cosgrave et al.12 compared outcomes in 174 patients with 201 lesions following similar DES versus different DES placement for intra-DES ISR and reported similar rates of TVR (15.9% vs. 16.0%) at follow-up. Garg et al.22 reported a striking 28.8% rate of one-year TVR after the treatment of 116 patients with intra-DES ISR using repeat DES implantation. Notably, 19.2% of the patients in this study had been previously treated for ISR, representing a higher risk cohort than that in previous reports. Park et al.23 reported a high rate (53.6%) of recurrent restenosis following the treatment of intra-DES ISR with the DES, while Solinas et al.24 observed the focal pattern of restenosis in 69.5% of the intra-DES ISR lesions, and the TVR rate in their series was 8% at one year. More recently, Steinberg et al.19 reported outcome differences between intra-BMS ISR and intra-DES ISR following the treatment of 238 patients using the DES. They observed significantly higher rates of TVR in the patients with intra-DES ISR than in those with intra-BMS ISR (22.2% vs. 10.3%; p value = 0.01), with a trend toward higher overall MACE (16% vs. 25.2%; p value = 0.08). In our study, although there was a non-significant trend toward increases in TVR in the intra-DES ISR group, TVR and angiographic recurrent restenosis were not only similar between intra-BMS ISR and intra-DES ISR but were also lower than the rates published in the literature. This difference could not be easily explained; it may, however, be because of the different baseline characteristics and immeasurable differences in patient treatment in our study as compared to the above-mentioned studies.
Our study has several limitations. First, quantitative coronary angiographic or volumetric ultrasound was not conducted. Using angiography alone precluded an analysis of the differences in the patterns of restenosis or neointimal plaque morphologic characteristics, which may have confounded the present findings. Second, this study was retrospective in its nature and may be subject to selection bias. Finally, the study involved a small group in total and particularly the number of the patients in the intra-DES ISR group was too small to compare the outcome within the different types of the DES.
Conclusion
Our results suggest that, regardless of the type of the initial stent (DES or BMS), the patients with ISR more commonly presented with acute coronary syndrome. This finding confirms the results of several previous studies reporting that intra-BMS ISR may not be as benign as once believed. We also found that the outcome of the patients presenting with ISR was not different between the two groups of DES-in-DES and DES-in-BMS at one-year follow-up, except that there was a trend toward more frequent TVR in the DES-in-DES group. DES-in- DES implantation for restenosis seems feasible and safe with a relatively low incidence of MACE at one-year follow-up.
Acknowledgments
This study was supported by Tehran Heart Center, Tehran University of Medical Sciences. We gratefully thank Dr. Arash Jalali for statistical analyses.
References
- 1.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 2.Radke PW, Kaiser A, Frost C, Sigwart U. Outcome after treatment of coronary in-stent restenosis; results from a systematic review using meta-analysis techniques. Eur Heart J. 2003;24:266–273. doi: 10.1016/s0195-668x(02)00202-6. [DOI] [PubMed] [Google Scholar]
- 3.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Saia F, Lemos PA, Arampatzis CA, Hoye A, Degertekin M, Tanabe K, Sianos G, Smits PC, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW. Routine sirolimus eluting stent implantation for unselected in-stent restenosis: insights from the Rapamycin Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry. Heart. 2004;90:1183–1188. doi: 10.1136/hrt.2003.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibra A, Kastrati A, Mehilli J, Pache J, Schuhlen H, von Beckerath N, Ulm K, Wessely R, Dirschinger J, Schomig A. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–670. doi: 10.1056/NEJMoa044372. [DOI] [PubMed] [Google Scholar]
- 6.Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schuhlen H, Schmitt C, Dirschinger J, Schomig A. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293:165–171. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O’Shaughnessy CD, DeMaio S, Hall P, Popma JJ, Koglin J, Russell ME. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 8.Holmes DR, Jr, Teirstein P, Satler L, Sketch M, O’Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, Cohen SA. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295:1264–1273. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Ellis SG, O’Shaughnessy CD, Martin SL, Satler L, McGarry T, Turco MA, Kereiakes DJ, Kelley L, Popma JJ, Russell ME. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295:1253–1263. doi: 10.1001/jama.295.11.1253. [DOI] [PubMed] [Google Scholar]
- 10.Torguson R, Sabate M, Deible R, Smith K, Chu WW, Kent KM, Pichard AD, Suddath WO, Satler LF, Waksman R. Intravascular brachytherapy versus drug-eluting stents for the treatment of patients with drug-eluting stent restenosis. Am J Cardiol. 2006;98:1340–1344. doi: 10.1016/j.amjcard.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Lee BK, Park DW, Park KH, Choi BR, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Comparison with conventional therapies of repeated sirolimus-eluting stent implantation for the treatment of drug-eluting coronary stent restenosis. Am J Cardiol. 2006;98:1451–1454. doi: 10.1016/j.amjcard.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrave J, Melzi G, Corbett S, Biondi-Zoccai GG, Babic R, Airoldi F, Chieffo A, Sangiorgi GM, Montorfano M, Michev I, Carlino M, Colombo A. Repeated drug-eluting stent implantation for drug-eluting stent restenosis: the same or a different stent. Am Heart J. 2007;153:354–359. doi: 10.1016/j.ahj.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Ufnalska SB. Free help for Scientists and Translators. J Teh Univ Heart Ctr. 2011;6:206–210. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Doyle B, Rihal CS, O’Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, Bresnahan J, Holmes DR., Jr Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116:2391–2398. doi: 10.1161/CIRCULATIONAHA.107.707331. [DOI] [PubMed] [Google Scholar]
- 16.De Labriolle A, Bonello L, Lemesle G, Steinberg DH, Roy P, Xue Z, Kaneshige K, Suddath WO, Satler LF, Kent KM, Pichard AD, Lindsay J, Waksman R. Clinical presentation and outcome of patients hospitalized for symptomatic in-stent restenosis treated by percutaneous coronary intervention: comparison between drug-eluting stents and bare-metal stents. Arch Cardiovasc Dis. 2009;102:209–217. doi: 10.1016/j.acvd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Tsigkas GG, Karantalis V, Hahalis G, Alexopoulos D. Stent restenosis, pathophysiology and treatment options: a 2010 update. Hellenic J Cardiol. 2011;52:149–157. [PubMed] [Google Scholar]
- 18.Dibra A, Kastrati A, Alfonso F, Seyfarth M, Perez-Vizcayno MJ, Mehilli J, Schomig A. Effectiveness of drug-eluting stents in patients with bare-metal in-stent restenosis: meta-analysis of randomized trials. J Am Coll Cardiol. 2007;49:616–623. doi: 10.1016/j.jacc.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg DH, Gaglia MA, Jr, Pinto Slottow TL, Roy P, Bonello L, De Labriolle A, Lemesle G, Torguson R, Kineshige K, Xue Z, Suddath WO, Kent KM, Satler LF, Pichard AD, Lindsay J, Waksman R. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. Am J Cardiol. 2009;103:491–495. doi: 10.1016/j.amjcard.2008.09.107. [DOI] [PubMed] [Google Scholar]
- 20.Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schomig A, Kastrati A. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol. 2010;55:2710–2716. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Lemos PA, van Mieghem CA, Arampatzis CA, Hoye A, Ong AT, McFadden E, Sianos G, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW. Post-sirolimus-eluting stent restenosis treated with repeat percutaneous intervention: late angiographic and clinical outcomes. Circulation. 2004;109:2500–2502. doi: 10.1161/01.CIR.0000130173.63105.4E. [DOI] [PubMed] [Google Scholar]
- 22.Garg S, Smith K, Torguson R, Okabe T, Slottow TL, Steinberg DH, Roy P, Xue Z, Gevorkian N, Satler LF, Kent KM, Suddath WO, Pichard AD, Waksman R. Treatment of drug-eluting stent restenosis with the same versus different drug-eluting stent. Catheter Cardiovasc Interv. 2007;70:9–14. doi: 10.1002/ccd.21106. [DOI] [PubMed] [Google Scholar]
- 23.Park CB, Hong MK, Kim YH, Park DW, Han KH, Lee CW, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Comparison of angiographic patterns of in-stent restenosis between sirolimus- and paclitaxel-eluting stent. Int J Cardiol. 2007;120:387–390. doi: 10.1016/j.ijcard.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Solinas E, Dangas G, Kirtane AJ, Lansky AJ, Franklin-Bond T, Boland P, Syros G, Kim YH, Gupta A, Mintz G, Fahy M, Collins M, Kodali S, Stone GW, Moses JW, Leon MB, Mehran R. Angiographic patterns of drug-eluting stent restenosis and one-year outcomes after treatment with repeated percutaneous coronary intervention. Am J Cardiol. 2008;102:311–315. doi: 10.1016/j.amjcard.2008.03.060. [DOI] [PubMed] [Google Scholar]