Abstract
Background:
Acute kidney injury (AKI) is a common and life-threatening complication following coronary artery bypass graft (CABG). Neutrophil gelatinase-associated lipocalin (NGAL) and Cystatin C have shown to be good predictive factors for AKI. Recently, there has been a growing interest in the use of hypertonic saline in cardiac operations.
The purpose of this study was to evaluate the prophylactic anti-inflammatory effect of hypertonic saline (Group A) infusion versus normal saline (Group B) on serum NGAL and Cystatin C levels as the two biomarkers of AKI in CABG patients.
Methods:
This randomized double-blinded clinical trial recruited 40 patients undergoing CABG in Tehran Heart Center, Tehran, Iran. After applying exclusion criteria, the effects of preoperative hypertonic saline (294 meq Na) versus normal saline (154 meq Na) infusion on serum NGAL and Cystatin C levels were investigated in three intervals: before surgery and 24 and 48 hours postoperatively. The probable intraoperative or postoperative confounders, including pump time, cross-clamp time, heart rate, systolic and diastolic blood pressures, central venous pressure, arterial pH, partial pressure of arterial oxygen, fraction of inspired oxygen, blood sugar, Na, K, Mg, hemoglobins, white blood cells, hematocrits, and platelets, were recorded and compared between the two groups of study.
Results:
The study population comprised 40 patients, including 25 (62.5%) males, at a, mean age ± SD of 61.75 ± 8.13 years. There were no statistically significant differences between the patients’ basic, intraoperative, and postoperative characteristics, including intraoperative and postoperative hemodynamic variables and supports such as inotropic use. Intra-aortic balloon pump use and mortality were not seen in our cases.
Three patients in the normal saline group and one patient in the hypertonic saline group had serum NGAL levels greater than 400 ng/ml. Moreover, 10 patients in Group A and 17 patients in group B showed a rise in serum Cystatin C levels above 1.16 mg/dl.
Patients with AKI had significantly elevated NGAL and Cystatin C levels (p value < 0.001), but there were no significant differences in the decrease in the NGAL level in the hypertonic saline group versus the normal saline group (230.91 ± 92.68 vs. 239.74 ± 116.58 ng/ml, respectively; p value = 0.792), or in the decrease in the Cystatin C level in the hypertonic saline group versus the normal saline group (1.05 ± 0.26 vs. 1.06 ± 0.31, respectively; p value = 0.874).
Conclusion:
Pre-treatment of CABG patients with hypertonic saline had no significant effect on serum NGAL and Cystatin C levels compared to the normal saline-receiving group. Our present data, albeit promising, have yet to fully document outcome differences.
Keywords: Acute, kidney injury; Coronary artery bypass; Cystatin C; Neutrophil gelatinase-associated lipocalin protein, rat; Saline solution, hypertonic
Introduction
Acute renal failure is a known predictor for mortality and morbidity and occurs in 5–7% of patients during their hospitalization.1, 2 The mortality rate is high but it even rises to levels higher than 65% when dialysis is required.3, 4 Acute kidney injury (AKI) imposes a financial burden of up to 10 billion dollars on the US health system annually.2 In cardiac surgery patients, AKI is reported in 5–40% of patients and is responsible for a high mortality rate (up to 80%), lengthened intensive care unit (ICU) stays, increased costs,5, 6 and deterioration in the quality of life.7
The idea of possible anti-inflammatory properties of hypertonic saline during fluid resuscitation has recently been proposed and reported by Bulger8 and Wigginton,9 who suggested that hypertonic saline might be of value in patients with severe circulatory compromise with the advantage of suppressing C-reactive protein, Interleukin 1 (IL1), and Interleukin 6 (IL6). This attribute could be advantageous during and after coronary artery bypass graft (CABG) with a subsequent massive inflammatory response.
Currently, the diagnosis is originally based on a rise in serum creatinine (Sr Cr) concentration, which is believed to be unreliable and a delayed diagnostic means.10 Although there are no early biomarkers in the diagnostic criteria of AKI, many experimental studies seeking to address prophylactic interventions in a clinical setting11 have been unable to draw upon the AKI criteria to present their findings. Furthermore, even the least serious forms of adult postoperative AKI with a 0.3 mg/dl rise in Sr Cr, which is routinely deemed benign clinically, have been associated with a significant increase in mortality.12
The validity of serum Cystatin C, a marker to estimate the glomerular filtration rate (GFR), has been previously approved in other patient groups, including kidney transplant recipients13 and critically ill patients.14 Moreover, recently Cystatin C has been introduced as an early biomarker for post-cardiac surgery AKI in adult patients.15, 16 Cystatin C is a cysteine proteinase inhibitor constantly released from nucleated cells; it has free glomerular filtration and is reabsorbed, but it has no tubular secretion.
Neutrophil gelatinase-associated lipocalin (NGAL) is a small 25-kDa glycoprotein secreted by activated neutrophils. In normal conditions, kidney tubule cells produce and excrete NGAL at low levels (37–106 ng/ml); nevertheless, in inflammatory, infective, ischemic, or nephrotoxic conditions, its plasma and urine levels rise significantly. This elevation occurs as early as two hours after injury.17 The predictive value of increased urinary NGAL concentration in developing AKI has been previously confirmed. Thus, NGAL could be regarded as a noninvasive, useful, and sensitive biomarker for early AKI diagnosis.17, 18
AKI is defined as a greater than 0.3 mg/dl increase in Sr Cr. Be that as it may, serum Cystatin C levels higher than 1.16 mg/dl,15 a significant rise from serum NGAL baseline, or mainly NGAL levels higher than 400 ng/ml are also considered as AKI.19
Recently, it has been posited that early hypertonic saline infusion may be associated with smaller volumes of fluids and have anti-inflammatory properties. This attribute may be advantageous in acutely ill patients or extensive operations like cardiac surgeries. However, to date, data have not fully documented an outcome difference with the use of hypertonic saline.20
We hypothesized that initiating such a fluid might reduce NGAL and Cystatin C levels following CABG. In the present study, therefore, we assessed the effect of hypertonic saline versus normal saline (the routine fluid) on these two markers (NGAL and Cystatin C) in patients undergoing CABG.
Methods
This randomized double-blinded clinical trial recruited 40 patients undergoing on-pump CABG in Tehran Heart Center, Tehran University of Medical Sciences, Tehran, Iran, between December 2010 and March 2011. All the patients had an ejection fraction greater than 35% and their Sr Cr were between 0.5 and 1.5 mg/dl preoperatively. Our research was approved by the Ethics Committee of Tehran University of Medical Sciences, grant was awarded by Pharmaceutical Research Center, Tehran University of Medical Sciences, Tehran, Iran, and written informed consent was obtained from all the patients or their legal guardians before enrollment.
Fluid loading may be a standard treatment during the resuscitation phase of hypovolemia, but rapid fluid replacement with hyperoncotic fluid in thermodynamically stable patients may negatively impact the hemodynamic response. This was initially detected in 2 patients with chest pain. Our protocol was thereafter revised and after re-approval, those 2 patients were excluded from the study. We hypothesized that the rapid changes in the stroke volume and increased afterload and preload might have aggravated the ischemia during volume expansion; this was quickly resolved and revised by expanding the duration of fluid replacement (100 cc over six hours).
The exclusion criteria comprised any concomitant operation, recent myocardial infarction (during the previous six months), emergent surgery, urgent or elective intubation before surgery, unstable hemodynamic or pulmonary situation, recent cerebral vascular accident, neurologic complications or consciousness disorder or significant neurologic defect, uncontrolled diabetes mellitus, cardiac valvular disorder more than moderate, congenital heart disorder, preoperative infection, Sr Cr greater than 1.5 mg/dl or GFR smaller than 50 ml/min, blood transfusion before surgery, Na higher than 150 meq/dl, head trauma, body mass index greater than 40 kg/m2, and chronic obstructive pulmonary disease or abnormal pulmonary function test (respiratory distress, partial carbon dioxide pressure greater than 45 mm Hg and partial oxygen pressure of 60 mm Hg, forced expiratory volume in one second less than 60% or forced vital capacity less than 60%, and vital capacity less than 50%).
All the preparation and treatment measurements were performed in a unified manner based on the hospital protocols by the same physicians, who were blinded to the study. All the surgical operations were performed by the same surgeon. The total fluid requirements were individualized based on continuous hemodynamic monitoring and were adjusted according to clinical, surgical, or anesthetic necessities and/or clinical assessments to achieve the hemodynamic targets in each particular phase of pre, intra, or post operation. Furthermore, ventilation and oxygenation indices were similarly individualized for each patient.
All the patients were randomized to receive 100 ml hypertonic saline (5%) + 400 ml normal saline (294 meq Na) or 500 ml normal saline (0.9%) (154 meq Na) in uniform packages from a peripheral venous line in the upper arm during a six-hour period before surgery.
A 5-ml sample of blood was obtained from each patient before surgery and twenty-four and forty-eight hours postoperatively and was anonymously sent to the local laboratory to be centrifuged. The serum was stored at − 70 °C. The levels of the serum markers of renal injury, namely Cystatin C and NGAL, were measured. Sr Cr was also measured at baseline and was routinely monitored at least daily in the postoperative period. Additionally, heart rate, systolic and diastolic blood pressures, central venous pressure, arterial pH, partial pressure of arterial oxygen, fraction of inspired oxygen, blood sugar, Na, K, Mg, hemoglobins, white blood cells, hematocrits, and platelets were platelets were also measured for each patient at baseline and after surgery.
Serum Cystatin C was measured via the particle-enhanced immune turbidimetric assay, using COBAS INTEGRA 400/800 (Tina-Quant, Roche, Switzerland). Serum NGAL was measured using the NGAL Rapid ELISA kit. The assay is an ELISA performed in micro wells coated with a monoclonal antibody against human NGAL. Bound NGAL was detected with a horseradish peroxidase and was anonymously sent to the local laboratory to be centrifuged and the serum was conjugated with monoclonal antibody. The assay, a rapid two-step procedure, was developed by incubation with a color-forming substrate.
The continuous variables are presented as mean ± SD (standard deviation), while the categorical variables are summarized by absolute frequencies and percentages. The continuous variables were compared using the Student t-test or Mann-Whitney U test whenever the data did not appear to have normal distributions. The categorical variables were compared using the chi-square or the Fisher exact test, as required. The repeated measure ANOVA was employed to evaluate inter-group differences and intra-group changes.
For the statistical analyses, the statistical software SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL) was used. A p value < 0.05 was considered statistically significant.
Results
The study population was comprised of 40 patients, including 25 (62.5%) males, at a mean age of 61.75 ± 8.13 years (between 42 and 78 years old) undergoing CABG in Tehran Heart Center during a four-month period. The patients were divided into two groups: Group A received 100 ml of hypertonic saline (5%) plus 400 ml of normal saline (294 meq Na) six hours before surgery and Group B received 500 cc of normal saline (0.9%) (154 meq Na).
The mean Body Mass Index (BMI) was 26.21 ± 3.97 kg/m2, the mean operation time was 4.29 ± 0.36 hours, and the mean cross-clamp time was 31.09 ± 6.81 minutes.
There were no statistically significant differences between the patients’ basic, intraoperative, and postoperative characteristics, including intraoperative and postoperative hemodynamic variables and supports such as inotropic use. Intra-aortic balloon pump use and mortality were not seen in our cases. (The mean variables are presented in Table 1.)
Table 1.
The patients’ characteristics
| variables | Hypertonic saline | Normal saline | P value |
|---|---|---|---|
| Basic | |||
| Sex | |||
| Male | 14 (70) | 11 (55) | 0. 257 |
| Female | 6 (30) | 9 (45) | |
| Age (y) | 62.15±7.51 | 61.35±8.91 | 0.760 |
| Serum Na concentrations (mEq/L) | 141.45±4.85 | 142.80±3.80 | 0.334 |
| WBC (/μL) | 7143.75±1831.92 | 7200.00±2045.04 | 0.933 |
| Diabetes mellitus | 6 (30) | 10 (50) | 0.197 |
| Hemoglobin (gm/dL) | 13.93±2.02 | 13.44±1.67 | 0.410 |
| During operation | |||
| Pump time (min) | 56.70±18.16 | 61.22±13.61 | 0.397 |
| Cross-clamp duration (min) | 30.36±6.79 | 31.82±7.10 | 0.629 |
| Intubation duration (hr) | 14.08±4.17 | 13.98±3.80 | 0.943 |
| Postoperative | |||
| Inotrope use within the first 24 postoperative hours | 4 (20) | 2 (10) | 0.366 |
| Systolic blood pressure one and three hours after ICU arrival (mm Hg) | 108.84±18.77 | 110.00±10.64 | 0.813 |
| Diastolic blood pressure one and three hours after ICU arrival (mm Hg) | 63.00±14.24 | 66.90±10.16 | 0.329 |
| Diastolic blood pressure falling under 70 mm Hg | 12 (60) | 14 (70) | 0.507 |
| Systolic blood pressure falling under 85 mm Hg | 4 (20) | 5 (25) | 0.999 |
| Central vein pressure one hour after ICU arrival (cm H2O) | 11.25±4.518 | 9.45±4.186 | 0.199 |
| Central vein pressure three hours after ICU arrival (cm H2O) | 11.20±4.50 | 9.88±4.232 | 0.345 |
| Heart rate one hour after ICU arrival (beat/min) | 85.55±14.46 | 88.10±12.03 | 0.548 |
| Heart rate three hours after ICU arrival (beat/min) | 84.90±13.11 | 88.10±10.33 | 0.397 |
Data are represented as mean±SD or n (%)
Postoperative AKI according to NGAL values (> 400 ng/ml) occurred in 4 (10%) patients. Three patients in the normal saline group (Group B) and one patient in the hypertonic saline group (Group A) had serum NGAL levels greater than 400 ng/ml, while 6 patients in Group B and 5 patients in Group A had a significant serum NGAL rise to above 300 ng/ml.
Moreover, 10 patients in Group A and 17 patients in Group B showed a rise in serum Cystatin C levels above 1.16 mg/dl. In comparison, within the first twenty-four postoperative hours, there were 9 patients from Group A and 4 patients from Group B with elevated Sr Cr ≥ 0.3 mg/dl, whereas 7 patients in Group A and 8 patients in Group B had ≥ 0.3 mg/dl rise in Sr Cr during the 48-hour period after surgery.
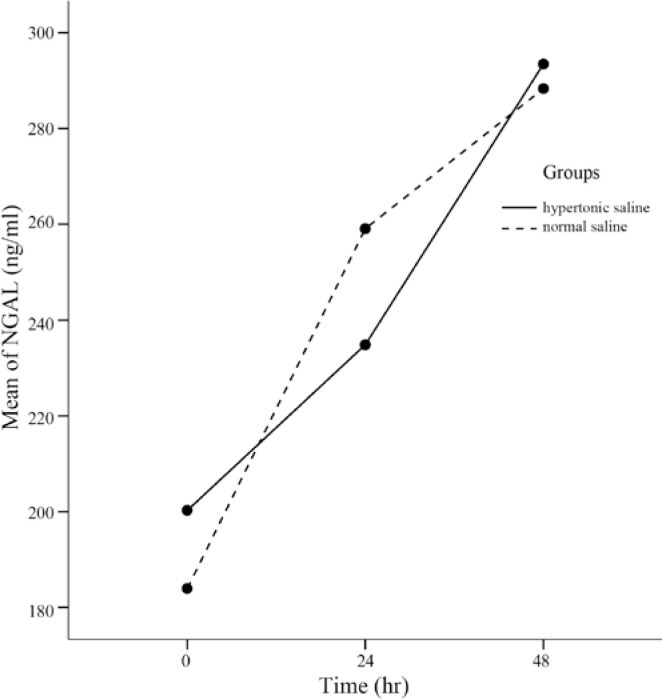
As Figure 1 shows, serum NGAL levels increased significantly during the 48-hour postoperative period (p value < 0.001), while the pattern of NGAL elevation made no difference between the two groups (p value = 0.894). Overall, the mean NGAL concentration levels at each time point (230.91 ± 92.68 ng/ml in the hypertonic saline group vs. 239.74 ± 116.58 ng/ml in the normal saline; p value = 0.792) and Cystatin C levels (1.05 ± 0.26 mg/dl in the hypertonic saline group vs. 1.06 ± 0.31 mg/dl in the normal saline group; p value = 0.874) were similar between the two groups (p value = 0.792).
Figure 1.
Neutrophil gelatinase-associated lipocalin (NGAL) rise during the 48 postoperative hours (adjusted for confounding factors)
Some possible confounders such as Na, white blood cells, and diabetes mellitus were adjusted, and the effects of hypertonic saline versus normal saline infusion on serum NGAL and Cystatin C levels were reassessed. Figure 1 demonstrates that in the presence of the above-mentioned variables, the increase in the NGAL levels was vague as regards the time in the two groups (p value = 0.361).
The mean levels of NGAL, Cystatin C, and Cr right before surgery and twenty-four and forty-eight hours after surgery are reported in Table 2, which shows no significant difference between the hypertonic saline and normal saline groups.
Table 2.
Mean serum NGAL, Cystatin C, and Creatinine concentrations in the two groups
| Variables | Hypertonic saline | Normal saline | P value |
|---|---|---|---|
| Cystatin C concentrations right before surgery (mg/dl) | 1.10±0.27 | 1.12±0.37 | 0.836 |
| Cystatin C concentrations 24 hours postoperatively (mg/dl) | 1.00±0.29 | 1.00±0.34 | 0.976 |
| Cystatin C concentrations 48 hours postoperatively (mg/dl) | 1.05±0.27 | 1.06±0.31 | 0.780 |
| Sr NGAL right before surgery (ng/ml) | 165.74±108.96 | 176.01±96.45 | 0.678 |
| Sr NGAL 24 hours postoperatively (ng/ml) | 246.59±122.02 | 253.44±180.20 | 0.926 |
| Sr NGAL 48 hours postoperatively (ng/ml) | 280.41±121.83 | 283.94±137.85 | 0.836 |
| Sr Cr right before surgery (mg/dl) | 0.85±0.20 | 0.84±0.25 | 0.926 |
| Sr Cr 24 hours postoperatively (mg/dl) | 1.20±0.30 | 0.97±0.20 | 0.583 |
| Sr Cr 48 hours postoperatively (mg/dl) | 1.09±0.35 | 1.06±0.30 | 0.793 |
Sr, Serum, NGAL, Neutrophil gelatinase-associated lipocalin, Cr, Creatinine
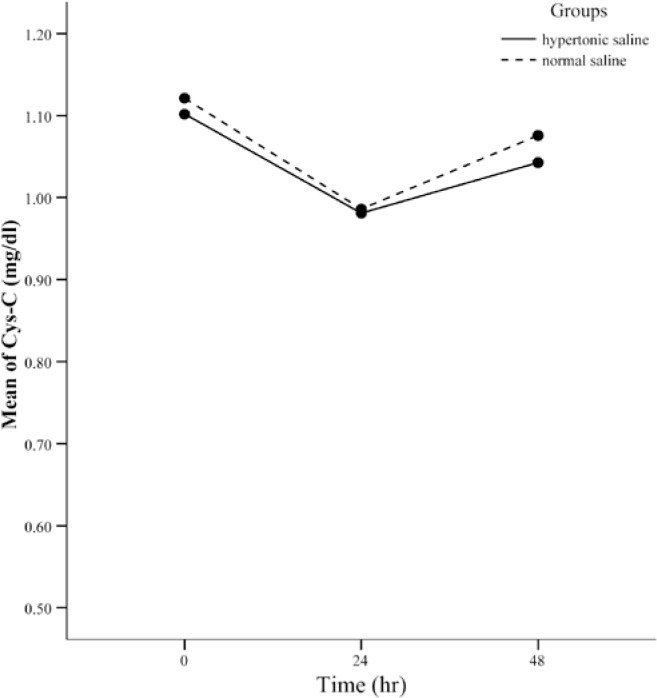
The same process (adjusting the effect of some confounders) was also performed for Cystatin C. Initially, Cystatin C was evaluated at various time points (Figure 2) and then it was reassessed after adjustment for white blood cells and hemoglobins. Cystatin C levels experienced a decline twenty-four hours after surgery and then returned to levels lower than their initial concentrations. The same results were achieved after adjustments.
Figure 2.
Cystatin C (Cys-C) levels during the 48 postoperative hours (adjusted for confounding factors)
Discussion
Inappropriate fluid infusion may lead to a low cardiac output or the inappropriate use of vasopressive or inotropic drugs. We opted to study hypertonic saline as a fluid of choice. In comparison to other fluids such as albumin (with direct antioxidant effect),21, 22 the wide spectrum of the beneficial effects of hypertonic saline along with its cost-effectiveness cannot be ignored. There are many reports addressing the hemodynamic, neurologic, immunologic, and/or prognostic beneficial effects of hypertonic saline in trauma, head injury, shock, and sepsis.8, 9, 23–29
Considering various desired aspects of hypertonic saline, many researchers have tried to address its use in cardiac surgery. Sirieix and colleagues30 demonstrated increased left ventricular pre-load and left ventricular ejection fraction in mitral valve repaired patients, following postoperative hypertonic solution infusion. They reported the beneficial effects of hyperosmolarity to be fluid shift to the vascular bed and plasma expansion, widespread pre-capillary vasodilatory effect in the systemic and pulmonary circulations, positive inotropic effect, reduction in blood viscosity, and other beneficial long-lasting hormonal and immunologic effects. There are a few other reports which have cited the desired hemodynamic31 and diuretic32 effects of hypertonic saline in cardiac surgery. Nonetheless, the value of hypertonic saline in the prevention of AKI remains to be fully elucidated.
In this study, we found no statistically significant beneficial effect for hypertonic saline in the prevention of AKI in terms of NGAL and Cystatin C levels. This can be explained by: 1) the limited extent of our intervention due to the small amount of hypertonic saline utilized, its administration timing, and a lack of supplemental doses; and 2) the huge burden of nephrotoxic insults by a multifactorial etiology, which persisted even on the second postoperative day. We recommend the adjustment of the dose or doses, adjustment of the administration timing, and conjugation with other interventions to minimize the discrepancies in our prophylactic intervention and the nephrotoxic insult in designing the future studies.
As NGAL is a marker for a distressed kidney, it seems to be upregulated in cells under stress, e.g. from infection, inflammation, etc. In the present study, the NGAL levels rose significantly during a 48-hour period after surgery, which may have been due to the presence of a persistent kidney injury even after surgery. A study by Lars Otto Uttenthal33 confirmed NGAL as a potential diagnostic marker for AKI even before a notable rise in Sr Cr. We observed that the patients receiving hypertonic saline had Sr Cr levels higher than those of the normal saline group and their creatinine concentrations after 48 hours had declined to lower levels, whereas the NGAL and Cystatin C concentrations continued to rise unchangeably.
There are various cut-points for NGAL in the literature.19, 34 In the present study, postoperative AKI according to the NGAL values (> 400 ng/ml) occurred in 4 (10%) patients. Three patients in the normal saline group and one patient in the hypertonic saline group had serum NGAL levels higher than 400ng/ml, which means that these patients had already developed AKI,19 while 6 patients in the normal saline group and 5 patients in the hypertonic saline group had a significant serum NGAL rise to more than 300 ng/ml: these patients can be regarded as prone to AKI.19 This finding may be different from that of other reports due to our different cut-off points or our restricted exclusion criteria.
In our study, the changes in NGAL were more pronounced than those in Cystatin C: this supports the fact that NGAL is a direct cell injury marker,35 while Cystatin C is regarded as a renal function indicator.13
For Cystatin C, we used a median above 1.16 mg/L as suggested by Catherine D.Krawczeski.15 Cystatin C changes obeyed the X2 pattern during the study period (p value = 0.01). A similar pattern was observed in both groups (p value = 0.567), and the concentration of Cystatin C at each time point did not vary notably between the two groups (p value = 0.874).
The present study had several strengths. First, we utilized a prospective design of subjects undergoing CABG and employed a rigorous protocol to prospectively collect specimens, followed by a blinded measurement of Cystatin C and NGAL. Second, our subjects (similar age, baseline profile, etc.) had a normal baseline kidney function before surgery.
This study also had important limitations. First, it represents a single-center study of CABG patients. Although this study sought to eliminate confounding variables and co-morbid conditions (such as diastolic dysfunction), it is acknowledged that AKI is often multifactorial, and our results will need to be validated in a larger randomized prospective trial. Second, this was a short-term study of post-CABG AKI. We could not address dose adjustment and were forced to use fixed doses of hypertonic saline for all the patients. We did not follow up the patients to determine the decline in or normalization of Cystatin C and NGAL or the long-term impact of post-CABG AKI. This will be an important future study. Third, the definition of AKI was based on elevations in Sr Cr, NGAL, and Cystatin C in accordance with the definitions in similar articles. If our cut-off points had been something else (in some studies, different patient populations may have different cut-off points), our study might have yielded different results. Fourth, if we had been able to measure the NGAL levels in urine, we could have obtained more significant results. And finally, because NGAL is not a functional marker of kidney damage, it rises much earlier than does Cystatin C (2–6 hours versus 12–24 hours). There was a continuous rise in the NGAL levels; however, we measured it in times other than the expected time of its peak value. This could have affected our results by obscuring the effect of hypertonic saline on the NGAL levels. Further researches are needed to address the question of timing via well-designed randomized control trials.
Conclusion
The infusion of hypertonic saline, as opposed to normal saline, had no significant effect on lowering the NGAL and Cystatin C levels; however, we did not observe any complication regarding its infusion. Because multiple factors affect NGAL and Cystatin C levels, further research seems necessary to determine the probable role of hypertonic saline in the prevention of post-CABG AKI.
Acknowledgments
The authors would like to express their deep appreciation to the staff of Tehran Heart Center, Deputy Research Directorship at Tehran University of Medical Sciences, and Gholhak Laboratory for their sincere collaboration.
References
- 1.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 4.Cho KC, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM. Survival by dialysis modality in critically ill patients with acute kidney injury. J Am Soc Nephrol. 2006;17:3132–3138. doi: 10.1681/ASN.2006030268. [DOI] [PubMed] [Google Scholar]
- 5.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 7.Lok CE, Austin PC, Wang H, Tu JV. Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery. Am Heart J. 2004;148:430–438. doi: 10.1016/j.ahj.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Bulger EM, May S, Brasel KJ, Schreiber M, Kerby JD, Tisherman SA, Newgard C, Slutsky A, Coimbra R, Emerson S, Minei JP, Bardarson B, Kudenchuk P, Baker A, Christenson J, Idris A, Davis D, Fabian TC, Aufderheide TP, Callaway C, Williams C, Banek J, Vaillancourt C, van Heest R, Sopko G, Hata JS, Hoyt DB, ROC Investigators Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304:1455–1464. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigginton JG, Roppolo L, Pepe PE. Advances in resuscitative trauma care. Minerva Anestesiol. 2011;77:993–1002. [PubMed] [Google Scholar]
- 10.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devarajan P. Emerging urinary biomarkers in the diagnosis of acute kidney injury. Expert Opin Med Diagn. 2008;2:387–398. doi: 10.1517/17530059.2.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 13.Böken kamp A, Ozden N, Dieterich C, Schumann G, Ehrich JH, Brodehl J. Cystatin C and creatinine after successful kidney transplantation in children. Clin Nephrol. 1999;52:371–376. [PubMed] [Google Scholar]
- 14.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 15.Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, Griffiths RE, Devarajan P. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–1557. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 18.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuladhar SM, Püntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53:261–266. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]
- 20.Azoubel G, Nascimento B, Ferri M, Rizoli S. Operating room use of hypertonic solutions: a clinical review. Clinics (Sao Paulo) 2008;63:833–840. doi: 10.1590/S1807-59322008000600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan GJ, Mumby S, Martin GS, Bernard GR, Gutteridge JM, Evans TW. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med. 2004;32:755–759. doi: 10.1097/01.ccm.0000114574.18641.5d. [DOI] [PubMed] [Google Scholar]
- 22.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 23.DuBose JJ, Kobayashi L, Lozornio A, Teixeira P, Inaba K, Lam L, Talving P, Branco B, Demetriades D, Rhee P. Clinical experience using 5% hypertonic saline as a safe alternative fluid for use in trauma. J Trauma. 2010;68:1172–1177. doi: 10.1097/TA.0b013e3181d76d40. [DOI] [PubMed] [Google Scholar]
- 24.Kerwin AJ, Schinco MA, Tepas JJ, 3rd, Renfro WH, Vitarbo EA, Muehlberger M. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277–282. doi: 10.1097/TA.0b013e3181acc726. [DOI] [PubMed] [Google Scholar]
- 25.Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL, Kofke WA, Mayer SA, Le Roux PD. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 2009;80:916–920. doi: 10.1136/jnnp.2008.156596. [DOI] [PubMed] [Google Scholar]
- 26.Bulger EM, Tower CM, Warner KJ, Garland T, Cuschieri J, Rizoli S, Rhind S, Junger WG. Increased neutrophil adenosine a3 receptor expression is associated with hemorrhagic shock and injury severity in trauma patients. Shock. 2011;36:435–439. doi: 10.1097/SHK.0b013e318231ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizoli SB, Rhind SG, Shek PN, Inaba K, Filips D, Tien H, Brenneman F, Rotstein O. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243:47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu GC, Quan ZY, Shao YS, Zhao JG, Zhang YT. The study of hypertonic saline and hydroxyethyl starch treating severe sepsis. Zhongguo Wei Zhong Bing JiJiu Yi Xue. 2011;23:150–153. [PubMed] [Google Scholar]
- 29.Vincenzi R, Cepeda LA, Pirani WM, Sannomyia P, Rocha-E-Silva M, Cruz RJ., Jr Small volume resuscitation with 3% hypertonic saline solution decrease inflammatory response and attenuates end organ damage after controlled hemorrhagic shock. Am J Surg. 2009;198:407–414. doi: 10.1016/j.amjsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Sirieix D, Hongnat JM, Delayance S, D’Attellis N, Vicaut E, Bérrébi A, Paris M, Fabiani JN, Carpentier A, Baron JF. Comparison of the acute hemodynamic effects of hypertonic or colloid infusions immediately after mitral valve repair. Crit Care Med. 1999;27:2159–2165. doi: 10.1097/00003246-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Prien T, Thülig B, Wüsten R, Schoofs J, Weyand M, Lawin P. Hypertonic-hyperoncotic volume replacement (7.5% NaCl/10% hydroxyethyl starch 200.000/0.5) in patients with coronary artery stenoses. ZentralblChir. 1993;118:257–263. [PubMed] [Google Scholar]
- 32.Järvelä K, Kaukinen S. Hypertonic saline (7.5%) after coronary artery bypass grafting. Eur J Anaesthesiol. 2001;18:100–107. doi: 10.1046/j.0265-0215.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- 33.Uttenthal L. NGAL: a marker molecule for the distressed kidney. Clin Lab Internat. 2005;29:39–41. http://www.cli-online.com/fileadmin/artimg/ngal-a-marker-molecule-for-the-distressed-kidney.pdf (20 February 2012) [Google Scholar]
- 34.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Uttenthal L. NGAL: how useful is the new marker of kidney damage? Clin Lab Internat. 2007;32:36–40. http://www.cli-online.com/fileadmin/artimg/ngal-how-useful-is-the-new-marker-of-kidney-damage.pdf (20 February 2012) [Google Scholar]