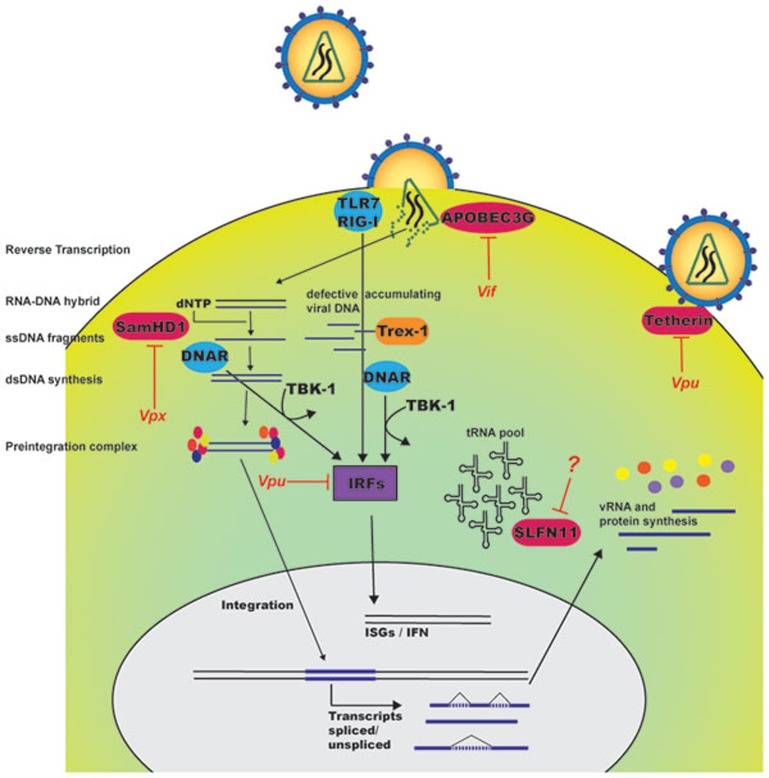
Human immunodeficiency virus type 1 (HIV-1) is an RNA virus that infects human T-cells, macrophages and dendritic cells. A part of the viral replication cycle is to transcribe the RNA genome into a proviral DNA copy that is actively integrated into the host cell genome. Subsequently, the cell produces spliced viral mRNAs that are transcribed into viral proteins using the host machinery. In a recent paper published in Nature, Li et al.1 describe schlafen (SLFN) 11 as a restriction factor for HIV, acting by regulating viral protein synthesis (Figure 1). Expression of SLFN genes was found to be induced by RNA, DNA, and type I interferon (IFN), hence classifying the SLFN proteins as interferon-stimulated genes (ISGs). The ISGs are important for innate antiviral defence and are regulated by transcription factors of the IFN regulatory factor (IRF) family, with IRF3 and 7 being activated by pattern recognition receptors (PRRs) and IRF9 being an integral part of the IFN-activated transcription factor interferon-stimulated gene factor 3 (ISGF3). IRF3 and 7 become activated through phosphorylation by tank-binding kinase 1 (Figure 1) and IκB kinase ε and translocates into the nucleus for binding to IFN-stimulated response elements, hence inducing expression of IFNs and ISGs2. This pathway is induced by host PRRs, among which nucleic acid sensors are known to play particularly important roles2. In contrast to the typical scenario, HIV-1 infection of T-cells and macrophages does not stimulate IRF3 activation and fails to induce IFN expression, but does lead to expression of a subset of ISGs3,4,5. However, it has been reported that HIV-1 genomic RNA can induce IFN responses in specific cell culture systems via toll-like receptor 7 and retinoid-acid inducible gene I6,7,8. Moreover, it has become clear that a potent type I IFN response can be induced by HIV-1 infection under conditions where the cellular DNA exonuclease Trex-1 is depleted9. The role of Trex-1 seems to be degradation of DNA originating from endogenous retroviruses or HIV, which can be sensed by an unknown sensor of HIV reverse transcription products, leading to induction of ISG expression.
Figure 1.
Current knowledge on cell-intrinsic restriction of HIV replication by ISGs. HIV nucleic acids can be sensed by host pattern recognition receptors (PRRs), which signal through interferon (IFN) regulatory factors to induce expression of IFN-stimulatory genes (ISGs). The figure illustrates the current knowledge on how ISGs target different steps in the HIV replication cycle and how specific HIV proteins counteract this antiviral response. SLFN11 identified in the article by Li et al.1, exerts antiviral activity through a novel mechanism by targeting tRNAs and reducing their cellular abundance, thus interfering with mRNA translation. TLR7, toll-like receptor 7; RIG-1, retinoid-acid inducible gene 1; TBK-1, tank-binding kinase 1; DNAR, DNA receptors.
Accumulating data suggest that ISGs play a key role in restricting HIV replication (Figure 1). One of the first anti-HIV ISGs described was Tetherin10, a protein expressed in the trans-Golgi network and the plasma membrane, which functions to restrict budding of progeny virus particles. However, HIV-1 counteracts the function of Tetherin via the viral protein Vpu10. Similarly, the ISG APOBEC3G that restricts HIV replication by interfering with reverse transcription through its activation-induced deaminase activity is targeted by the viral protein Vif11. Another more recently described ISG is the myeloid-specific restriction factor (SAMHD1), which decreases the pool of dNTPs, thereby counteracting the viral capacity to produce DNA proviruses12. The Vpx protein expressed by HIV-2 and sooty mangabey simian immunodeficiency virus, but not HIV-1, degrades SAMHD1. Expressing vpx together with HIV-1 has been demonstrated to increase viral production extensively. The results from Li et al.1 add new evidence of an ISG, namely SLFN11, that controls HIV replication. Interestingly, the major function of SLFN11 is proposed to down-modulate HIV-1 mRNA translation.
Six human SLFN genes exist, which encode proteins originally described to regulate cell growth and T-cell development. Li et al. now show that SLFN11 also has antiviral functions. They first screened for induction of SLFNs in human fibroblasts by type I IFN and observed upregulation of SLFN5 and 11. The authors took an interesting turn by deploying 293T cells, the cell line most commonly used to produce HIV virions, to decipher the function of SLFN proteins in the anti-HIV response. Stable cell lines depleted of SLFN 11 were generated by short hairpin RNA interference and the authors found that virus-encoded luciferase expression was not affected by SLFN11 knockdown. However, production of new virus particles was significantly increased in SLFN11-depleted 293T cells. Independently of the scope of the paper, this finding will potentially have impact on HIV-1 propagation procedures in the future, as higher viral titers can be obtained in 293T/SLFN11-knockdown cells.
The authors then searched for the mechanism of action of SLFN11 in anti-HIV defences. They found that viral RNA levels were unaffected by the expression of SLFN11, thus suggesting restriction to occur at a late stage of replication, which was found to be independent of Tetherin. The restricting function of SLNF11 was unravelled when the authors compared the profile of viral proteins expressed in the 293T cell system, where expression of viral proteins but not host proteins was highly elevated in SLNF11-knockdown cells. One question that needs to be addressed in the future will be to define whether increased viral protein synthesis results in an increase of functional virus particles or whether they are non-functional due to uncontrolled protein expression and particle modulation. One could speculate that APOBEC3G that is normally inhibited by HIV-1 vif to be packed in virions, could suddenly be included as a result of an uncontrolled viral production and budding.
One important difference between retroviral and mammalian mRNA translation lies in the different frequencies in use of codons in the coding regions. Many viruses, including retroviruses, have a bias toward an adenosine at the third position of the codon. This may lead to a slow down of protein synthesis, as tRNA decoding A-ending codons is the limiting factor. The authors found that HIV could trigger elevation of the levels of tRNAs in cells depleted of SLFN11, but not in cells expressing the protein. Together with the finding that SLFN11 directly bound tRNAs, it was concluded that SLFN11 directly binds to tRNA, thereby limiting the availability of tRNAs for HIV protein synthesis. However, it still needs to be addressed whether this phenomenon is being counteracted by HIV-1.
In summary, the study by Li et al.1 has identified a new antiviral ISG, which should be included in the rapidly expanding list of specific innate immune factors restricting HIV replication. Another key question yet to be answered relates to the importance of SLFN11 in restriction of HIV-1 and other retroviruses in primary cells, and also the physiological importance of SLFN proteins in antiviral defences in general. With the growing list of ISGs reported to have anti-HIV functions, there is now a need to understand how these proteins act together to protect cells against infection and also to identify which PRRs sense HIV in different cell types to induce ISG expression. The publication by Li et al. identifies an important novel mechanism of innate anti-HIV defence, which the virus needs to target or exploit in order to replicate.
References
- Li M, Kao E, Gao X, et al. Nature. 2012. pp. 125–128. [DOI] [PMC free article] [PubMed]
- Akira S, Uematsu S, Takeuchi O. Cell. 2006. pp. 783–801. [DOI] [PubMed]
- Harman AN, Lai J, Turville S, et al. Blood. 2011. pp. 298–308. [DOI] [PMC free article] [PubMed]
- Nasr N, Maddocks S, Turville SG, et al. Blood. 2012. pp. 778–788. [DOI] [PubMed]
- Doehle BP, Chang K, Fleming L, et al. J Virol. 2012. pp. 8499–8506. [DOI] [PMC free article] [PubMed]
- Heil F, Hemmi H, Hochrein H, et al. Science. 2004. pp. 1526–1529. [DOI] [PubMed]
- Berg RK, Melchjorsen J, Rintahaka J, et al. PloS One. 2012. p. e29291. [DOI] [PMC free article] [PubMed]
- Solis M, Nakhaei P, Jalalirad M, et al. J Virol. 2011. pp. 1224–1236. [DOI] [PMC free article] [PubMed]
- Yan N, Regalado-Magdos AD, Stiggelbout B, et al. Nat Immunol. 2010. pp. 1005–1013. [DOI] [PMC free article] [PubMed]
- Neil SJ, Zang T, Bieniasz PD. Nature. 2008. pp. 425–430. [DOI] [PubMed]
- Sheehy AM, Gaddis NC, Choi JD, et al. Nature. 2002. pp. 646–650. [DOI] [PubMed]
- Lahouassa H, Daddacha W, Hofmann H, et al. Nat Immunol. 2012. pp. 223–228. [DOI] [PMC free article] [PubMed]