Abstract
Although data are mixed, asthma and rheumatologic conditions may be associated with cognitive impairment. Medications may play a role because corticosteroids are associated with memory impairment. Therefore, an easily administered assessment of cognition would be useful in these patients. We assessed relationships between self-rated and clinician-rated cognitive performance and mood in patients with asthma and rheumatologic diseases. Participants included 31adults treated for asthma or rheumatologic disorders (17 receiving chronic prednisone therapy, and 14 not receiving prednisone). An objective assessment of a variety of cognitive domains was administered through clinician and patient-rated assessments of cognition. Composite scores for the objective (Global Clinical Rating [GCR]) and subjective (Neuropsychological Impairment Scale: Global Measure of Impairment [GMI]) measures of cognition were derived. Depression was assessed with the 17-item Hamilton Rating Scale for Depression (HRSD-17). A linear regression was conducted with GMI scores as dependent variable and GCR, HRSD-17 scores, and prednisone-use status, as independent variables. Significant differences between prednisone-treated patients and other patients were observed on the GCR, GMI, and HRSD-17. In the regression analysis, HRSD-17 scores, but not GCR scores, significantly predicted GMI scores. Prednisone-treated patients had higher levels of depressive symptoms and subjective and objective cognitive deficits than those not taking prednisone. In the combined patient groups, subjective cognitive assessment was more strongly related to depressive symptoms than objective cognition. Findings suggest physicians should be aware of the potential for cognitive deficits in patients taking corticosteroids and, when appropriate, should consider the use of objective neurocognitive tests or neuropsychology consultation to better characterize its presence and severity.
Keywords: Asthma, cognition, corticosteroid, depression, impairment, memory, objective, prednisone, rheumatoid arthritis, subjective
Cognitive deficits have been reported in some1–3 but not all4,5 studies in patients with asthma or rheumatologic diseases. The reasons for poor cognition in these disorders are not well understood but could be related to direct central nervous system involvement in some rheumatic conditions and, possibly, medication effects and brief periods of hypoxia in asthma. In patients with more severe illnesses the use of oral corticosteroids may also impact cognition. Acute high-dose (40–60 mg/day of prednisone equivalents) corticosteroid “bursts” for asthma symptom exacerbations is associated with impairment in memory.5–9 Chronic corticosteroid therapy at more modest doses (10–20 mg/day) is also associated with poorer memory, as well as depressive symptoms, smaller hippocampal volume, and lower temporal lobe levels of N-acetyl aspartate (a metabolite associated with healthy neurons).10 Decline in memory and increases in mood symptoms are generally reversible on discontinuation of brief courses of corticosteroids11,12 but the degree of reversibility of the effects of chronic corticosteroid therapy on the brain is not known. In light of the potential cognitive effects of asthma and other medical illnesses as well as the effects of corticosteroid therapy on cognition, screening patients for cognitive impairment is of clinical importance because cognitive impairment is associated with poor adherence to medications.13
Although objective, observer-rated, cognitive tasks such as in formal neuropsychological evaluations are the “gold standard” for assessing cognitive performance, these tests are time-consuming to administer and require special training. Subjective scales, which measure patient-rated cognitive complaints through self-report, are relatively simple tools to screen for cognitive impairment that can be completed by the patient in the waiting room. Patients with findings outside of expectations could then be referred for more extensive neuropsychological assessment. The current report examines the relationship between self-rated and clinician-rated cognitive assessments in patients with asthma or rheumatic disorders, some of whom were taking oral corticosteroids, with current mood status.
MATERIALS AND METHODS
Participants in this study included 31 outpatients with either asthma or rheumatic diseases (e.g., rheumatoid arthritis) between 18 and 65 years of age. Patients were diagnosed with asthma by board-certified allergy and immunology specialists according to clinical characteristics and/or spirometric criteria.
Of these, 17 were receiving chronic prednisone therapy (≥10 mg/day for ≥6 months) and 14 had minimal lifetime corticosteroid exposure (none in the past 6 months, no course longer than 4 weeks, and <6 months lifetime total). Participants also had some high school or greater education and no known history of major psychiatric, neurological, substance abuse, or developmental/learning disorders. Prednisone and no-prednisone groups did not significantly differ on characteristics of age, education, gender, marital status, ethnicity, or estimated intelligence. The primary analysis from this study examining mood, cognition, structural magnetic resonance imaging, and spectroscopy was previously published and contains detailed information about experimental procedures and participants.6 A written informed consent process, approved by the University of Texas Southwestern Institutional Review Board, was completed by all participants. Y. Getahun and M. Pacheco contributed equally to this work.
In the current study a linear regression approach was used with multiple predictor variables entered in a single step. The predictor variables were prednisone group (taking prednisone or not taking prednisone), depression score (17-item Hamilton Rating Scale for Depression 14 [HRSD-17]) and an objective composite cognition score (Global Clinical Rating [GCR]). The GCR score was derived using traditional neuropsychological measures reflecting four cognitive domains and administered by standard instructions. The premorbid estimate of intellect was obtained by reading single words from the National Adult Reading Test Revised15 and the Vocabulary Subtest of the Wechsler Adult Intelligence Scale 3.16 Attention domain was derived from the Trail Making Test, Part A,17 and the Stroop Test (Victoria version), Color Trial.18 Learning was derived from immediate passage recall of the Wechsler Memory Scale 3, Logical Memory I, and total words for learning trials of a 15-item word list (Rey Auditory Verbal Learning Test).19 Memory was derived from delayed passage recall (Wechsler Memory Scale 3, Logical Memory II) and delayed word list recall of the Rey Auditory Verbal Learning Test Executive Function was assessed using the Trail Making Test, Part B,17 and Letter Fluency, FAS.20 Standard scores were obtained from appropriate norms for each test measure and transformed into a deficit score (range, 0 = normal to 5 = severely impaired) based on T-scores for each test. A clinical rating score was subsequently assigned to each of the four cognitive domains (attention, learning, memory, and executive function) with a range of 1 (above average) to 9 (severe impairment) as described in guidelines from Woods et al.'s reliability article on human immunodeficiency virus cognitive diagnostic considerations.21 These clinical ratings were informed by subject's premorbid functioning consideration of reading (NART-R) and vocabulary (WAIS-3) scores as well as ethnicity, education, and occupation.
The dependent variable in the linear regression was the subjective composite for cognitive complaints (Global Measure of Impairment [GMI]) from the Neuropsychological Impairment Scale,22 95-item self-report measure of cognitive and affective status. SPSS software Version 12 (SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
Demographic information on the participants is presented in Table 1. The prednisone-treated and nonprednisone-treated groups were similar in terms of medical illnesses, gender, education, race/ethnicity, and overall estimated premorbid intelligence. Other medical conditions included hypertension (n = 2), a history of Grave's disease (n = 1; corticosteroid group), and hypertension (n = 2), diabetes (n = 1), degenerative disk disease (n = 1,) and valvular heart disease (n = 1; controls). None had a diagnosis of chronic obstructive pulmonary disease. The mean current prednisone dose in the group receiving corticosteroids was 15.6 ± 10.6 with a mean duration of ∼8 years.6 Controls either had no lifetime prednisone exposure or minimal exposure (lifetime range, 4 days to 2.5 months with no use in over 2 years). One participant in the control group reported that their daughter had been diagnosed with bipolar disorder and attention deficit hyperactivity disorder.
Table 1.
Demographic information and clinical characteristics of the study participants
Figure 1 shows the percentages of patients in the two groups with impairment/symptomatology on the GCR (objective cognition), GMI (subjective cognitive complaints), and HRSD-17 (clinician-rated depressive symptoms). Prednisone-treated patients were significantly more likely to have elevated depressive symptoms (total score ≥8 on HRSD-17 consistent with the presence of at least mild depressive symptoms), impaired scores on the GCR (global cognition borderline or below), and greater subjective cognitive complaints (GMI scores falling at or >1 SD above the mean).
Figure 1.
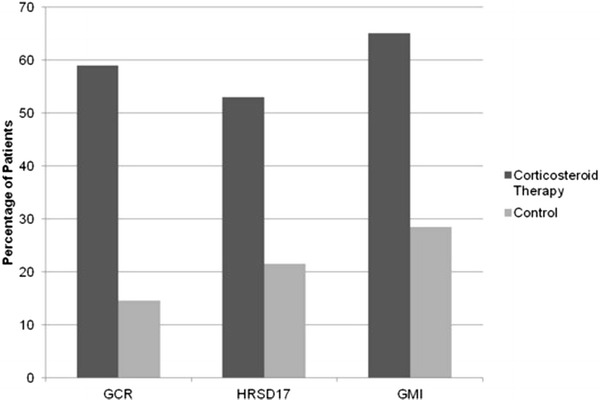
Cognitive impairment, depression, and neuropsychological function in controls and corticosteroid patients. Left bars indicate percentage of patients with objective cognitive impairment (borderline/impaired); middle bars indicate percentage of patients with depression (17-item Hamilton Rating Scale for Depression [HRSD-17] scores ≥8); right bars indicate percentage of patients with subjective cognitive impairment (Global Measure of Impairment [GMI] scores >1 SD above the mean).
In the regression analysis, HRSD-17 scores, but not prednisone treatment or GCR scores, were significantly related to GMI scores (p < 0.001). A scatterplot of HRSD-17 and GCR scores is presented in Fig. 2.
Figure 2.
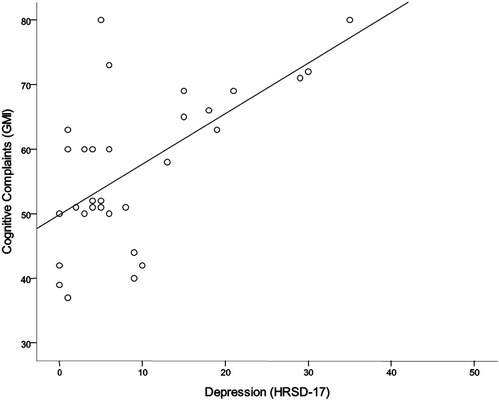
Scatterplot of Global Measure of Impairment (GMI) scores and 17-item Hamilton Rating Scale for Depression (HRSD-17) scores.
DISCUSSION
Corticosteroids are associated with a variety of brain effects including mood23,24 and memory changes,5–9 and with chronic exposure, hippocampal atrophy.10 In animal models corticosteroids are associated with increases in glutamate in the hippocampus,25 and histological changes (e.g., dendritic remodeling and even neuronal death) in the hippocampus can be prevented with agents that decrease glutamate release26 or block the N-methyl-d-aspartate receptor.27 Drugs of these same classes appear to attenuate the cognitive effects of chronic corticosteroid therapy in humans.28,29
The current study had two findings. First, patients taking prednisone were more likely to have depressive symptoms, cognitive complaints, and objective cognitive impairment. Our previous report from the same patient sample observed greater mean HRSD-17 scores and history of major depressive disorder in those taking prednisone.6,23 The current report observes greater frequency of depressive symptoms using categorical scores. We also previously reported significantly poorer performance on the domains of memory and executive functioning but not psychomotor speed, attention, and intelligence. The current report extends these findings by observing greater overall cognitive impairment in the prednisone group using a composite score derived from a cognitive battery. This current study examined the relationship between objective cognitive findings and subjective cognitive complaints with findings of prednisone-treated patients having greater cognitive complaints than those not taking prednisone. Thus, the findings add to the prior literature suggesting that prednisone is associated with changes in cognition and extends this literature by suggesting that these patients also report greater subjective cognitive complaints.
The second finding was that patient subjective cognitive assessment was associated more with depressive symptoms than objective cognitive status. Prior research comparing subjective and objective cognitive assessments have often produced similar findings. Moore et al. found a relationship between self-reported cognition and mood but not with objective cognition, in human immunodeficiency virus–positive patients.30 However, Vance et al. reported that self-rated cognitive ability was significantly related both to performance on neuropsychological tests and depression.31 To our knowledge, no previous reports have compared subjective and objective cognitive assessments in patients with asthma and rheumatologic diseases.
Patients with chronic diseases suffer from depression and psychological distress, including patients with asthma32,33 and rheumatoid arthritis.34–36 Our findings suggest that self-rated cognitive complaints may provide a short screening with possible implications for presence of depression in these patients. However, many brief, self-rated depression scales are readily available including the Beck Depression Inventory-II,37 Quick Inventory of Depressive Symptomatology-Self-Report,38 and Hospital Anxiety and Depression Scale.39 These would provide more direct assessment of depressive symptomatology and could be administered in the waiting room and quickly scored by clinic staff.
Although easy to administer, self-assessment of cognitive performance may be of limited value in determining which patients need further neuropsychological testing in the patient sample we used. One alternative is use of informant-rated questionnaires for cognitive status rather than patient rated. Unlike patient self-report, informant approach has been found useful as a valid and sensitive screen of cognitive impairment in individuals with multiple sclerosis.40 A direct approach would be administrating in clinical settings of brief objective measures of cognitive performance. Currently, measures such as the Mini Mental State Exam41 and Montreal Cognitive Assessment42 administered by trained staff may have some usefulness. Development of brief cognitive self-assessments with monitoring by staff may prove useful for this population in a similar to recent attempts for dementia screening.43–45 A promising approach is computer-administered cognitive tests with a recent review by Tierney et al. of appropriateness for use in clinics.44
The study has limitations. The sample size is small and consisted mainly of women. The clinics used for participant recruitment had a disproportionate number of women patients. Although the sample is representative of the population at these clinics, it may not be representative of the more general population of patients with significantly different medical histories. We also do not know if the findings are generalizable to both sexes. Some of the participants had medical conditions in addition to the disorder for which they receive corticosteroids. Strengths of the study include the administration of an extensive objective cognitive test battery and mood assessment.
CONCLUSION
In a sample of patients with asthma and rheumatologic conditions, current use of prednisone was associated with greater likelihood of depressive symptoms, cognitive complaints, and cognitive impairment on neuropsychological tests. Cognitive complaints were more strongly associated with current depressive symptom severity than performance on clinician-administered neuropsychological tests.
Footnotes
Funded by the University of Texas Southwestern Medical Student Research Program, Science Teacher Access to Resources at Southwestern (STARS) program, Summer Research with National Institute on Drug Abuse (NIDA) program, National Alliance for Research on Schizophrenia and Depression (NARSAD), and NIH Grant K08 MH01725
ES Brown receives grant support from Sunovion. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Appenzeller S, Bertolo MB, Costallat LT. Cognitive impairment in rheumatoid arthritis. Meth Find Exp Clin Pharmacol 26:339, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Brown ES, Denniston D, Gabrielson B, et al. Randomized, double-blind, placebo-controlled trial of acetaminophen for preventing mood and memory effects of prednisone bursts. Allergy Asthma Proc 31:331–336, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Schraa JC, Dirks JF, Jones NF, Kinsman RA. Bender-Gestalt performance and recall in an asthmatic sample. J Asthma 18:7–9, 1981. [DOI] [PubMed] [Google Scholar]
- 4. Feldmann R, Weglage J, Roth J, et al. Systemic juvenile rheumatoid arthritis: Cognitive function and social adjustment. Ann Neurol 58:605–609, 2005. [DOI] [PubMed] [Google Scholar]
- 5. aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ 180:305–313, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown ES, Woolston JD, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry 55:538–545, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Keenan PA, Jacobson MW, Soleymani RM, et al. The effect on memory of chronic prednisone treatment in patients with systemic disease. Neurology 47:1396–1402, 1996. [DOI] [PubMed] [Google Scholar]
- 8. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology 21:25–31, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Bender BG, Lerner JA, Poland JE. Association between corticosteroids and psychologic change in hospitalized asthmatic children. Ann Allergy 66:414–419, 1991. [PubMed] [Google Scholar]
- 10. Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci 1179:41–55, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry 141:369–372, 1984. [DOI] [PubMed] [Google Scholar]
- 12. Wolkowitz OM, Rubinow D, Doran AR, et al. Prednisone effects on neurochemistry and behavior. Preliminary findings. Arch Gen Psychiatry 47:963–968, 1990. [DOI] [PubMed] [Google Scholar]
- 13. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 353:487–497, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uttl B. North American Adult Reading Test: Age norms, reliability, and validity. J Clin Exp Neuropsychol 24:1123, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Kaufman AS, Lichtenberger EO. Essentials of WAIS-III Assessment. Hoboken, NJ: John Wiley & Sons, Inc., 77–163, 1999. [Google Scholar]
- 17. Gordon NG. The trial making test in neuropsychological diagnosis. J Clin Psychol 28:167–169, 1972. [DOI] [PubMed] [Google Scholar]
- 18. Troyer AK, Leach L, Strauss E. Aging and response inhibition: Normative data for the Victoria Stroop Test. Aging Neuropsychol Cognition 13:20–35, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol 40:785–787, 1984. [DOI] [PubMed] [Google Scholar]
- 20. Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press, 447–459, 1998. [Google Scholar]
- 21. Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26:759–778, 2004. [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell WE, De Soto CB, De Soto JL, et al. The Neuropsychological Impairment Scale (NIS) Manual. Torrence, CA: Western Psychological Services, 13–35, 1984. [Google Scholar]
- 23. Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol 92:500–505, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Brown ES, Suppes T, Khan DA, Carmody TJ., 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol 22:55–61, 2002. [DOI] [PubMed] [Google Scholar]
- 25. McEwen BS. Allostasis, allostatic load, and the aging nervous system: Role of excitatory amino acids and excitotoxicity. Neurochem Res 25:1219–1231, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Magariños AM, McEwen BS, Fl[umlat]ugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16:3534–3540, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience 69:83–88, 1995. [DOI] [PubMed] [Google Scholar]
- 28. Brown ES, Wolfshohl J, Shad MU, et al. Attenuation of the effects of corticosteroids on declarative memory with lamotrigine. Neuropsychopharmacology 33:2376–2383, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown ES, Vazquez M, Nakamura A. Randomized, placebo-controlled, crossover trial of memantine for cognitive changes with corticosteroid therapy. Biol Psychiatry 64:727–729, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Moore LH, van Gorp WG, Hinkin CH, et al. Subjective complaints versus actual cognitive deficits in predominantly symptomatic HIV-1 seropositive individuals. J Neuropsychiatry Clin Neurosci 9:37–44, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Vance DE, Ross LA, Downs CA. Self-reported cognitive ability and global cognitive performance in adults with HIV. J Neurosci Nurs 40:6–13, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Brown ES, Khan DA, Mahadi S. Psychiatric diagnoses in inner city outpatients with moderate to severe asthma. Int J Psychiatry Med 30:319–327, 2000. [DOI] [PubMed] [Google Scholar]
- 33. Goodwin RD, Olfson M, Shea S, et al. Asthma and mental disorders in primary care. Gen Hosp Psychiatry 25:479–483, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: A systematic review of the literature with meta-analysis. Psychosom Med 64:52–60, 2002. [DOI] [PubMed] [Google Scholar]
- 35. Dickens C, Jackson J, Tomenson B, et al. Association of depression and rheumatoid arthritis. Psychosomatics 44:209–215, 2003. [DOI] [PubMed] [Google Scholar]
- 36. Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain 96:279–284, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res 63:20556, 2011. [DOI] [PubMed] [Google Scholar]
- 38. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370, 1983. [DOI] [PubMed] [Google Scholar]
- 40. O'Brien A, Gaudino-Goering E, Shawaryn M, et al. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol 22:933–948, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Dick JP, Guiloff RJ, Stewart A, et al. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry 47:496–499, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Hancock P, Larner AJ. Test your memory test: Diagnostic utility in a memory clinic population. Int J Geriatr Psychiatry 26:976–980, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Tierney MC, Lermer MA. Computerized cognitive assessment in primary care to identify patients with suspected cognitive impairment. J Alzheimers Dis 20:823–832, 2010. [DOI] [PubMed] [Google Scholar]
- 45. Chester JG, Grande LJ, Milberg WP, et al. Cognitive screening in community-dwelling elders: Performance on the clock-in-the-box. Am J Med 124:662–669, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


