Abstract
Despite the successes of combination antiretroviral therapy, HIV-associated neurocognitive disorders persist in many infected individuals. Earlier studies showed that neurocognitive impairment was associated with glutamate toxicity and synaptodendritic damage. We examined alterations in expression of four ephrin genes that are involved in synapse formation and recruitment of glutamate receptors to synapses, in the caudate and anterior cingulate in postmortem brain of cognitively characterized HIV-infected subjects, along with expression of neuronal and astroglial/macroglial markers. Postmortem tissues of HIV-infected and control subjects were obtained from the Manhattan HIV Brain Bank. HIV-infected subjects underwent neurocognitive assessment prior to death. Quantification of mRNA of genes of chemokine receptors and chemokines (CCR5, CXCR4, CCL2), astroglial/microglial markers (GFAP, CD163, CD68), the neuronal marker SNAP25, ephrin receptors EPHA4 and EPHB2, and ephrin ligands EFNB1 and EFNB2 was performed using SYBR Green RT-PCR. Proinflammatory chemokine and glial/macrophage mRNA levels in both regions were significantly greater in HIV+ than in HIV-subjects. Levels of EPHA4 and EFNB2 mRNA in the caudate, and EPHB2 mRNA in anterior cingulate were significantly lower in HIV+ subjects (p<0.002, p<0.02, p<0.05, respectively). These transcripts also showed correlations with immune status and cognitive function within the HIV-infected group. Decreased levels of EFNB2 mRNA in the caudate correlated with lower CD4 counts (P<0.05). Cognitive associations were limited to the cingulate, where decreased levels of EPHB2 mRNA were associated with better global cognitive status. Decreased cingulate expression of EPHB2 may represent a compensatory mechanism minimizing excitotoxic injury in the face of chronic inflammation.
Keywords: HIV dementia, human postmortem brain, ephrin receptors and ligands, chemokine receptors and ligands, neurocognitive impairment
Introduction
Despite the widespread availability and use of combination antiretroviral therapy (cART), HIV-associated neurocognitive disorders (HAND) persist in up to 45% of HIV-infected individuals (González-Scarano and Martín-García 2005; Ellis et al. 2007; Heaton et al. 2011). The cause of HAND remains poorly understood, but may result from a combination of factors, including poor CNS penetration of antiretroviral drugs, chronic inflammation with persistent microglia and macrophage activation, neurotoxicity/excitotoxicity, and loss of trophic factors. Morphologically, synaptodendritic degenerative changes in HIV have been correlated with the presence and severity of cognitive impairment. Specifically, it has been shown that the degree of neurocognitive impairment is associated with dendritic simplification and reduced synaptic density in the midfrontal cortex and putamen in AIDS patients at autopsy (Masliah et al. 1992, 1997; Everall et al. 1999; Moore et al. 2006). Synaptodendritic alterations in HIV may occur without major neuronal loss, and two underlying mechanisms - loss of trophic factors and elaboration of toxic factors – have been implicated (Ellis et al. 2007). For example, HIV-related decreases in brain-derived neurotrophic factor (BDNF) and basic fibroblast growth factors (FGF) have been described (reviewed in Mocchetti et al. 2008). With regard to toxicity, converging lines of evidence have implicated overstimulation of glutamate receptors in the generation of HAND (Erdmann et al. 2006; Kaul and Lipton 2006). No theories have unified these alternate mechanisms – loss of trophism and gain of toxicity – into one mechanistic paradigm.
Among brain neurotrophic factors, the ephrin receptor (EPH) tyrosine kinases and their ligands, ephrins (EFN) have been shown to be involved in synapse formation and neuronal plasticity (Klein 2001; Pasquale 2005, 2008). EPH receptors and ephrins have been implicated in modifying the properties of synapses, particularly density and morphology of dendritic spines at sites of neuron-neuron and neuron-astrocyte contacts. Ephrin receptors seem to play a role in immune processes, including differentiation of activated T-cells and proliferation of CD4+ T-lymphocytes in mice (Yu et al. 2003, Wu and Luo 2005). EPH receptors are divided into two classes, EPHA receptors (A1-A8, A10) and EPHB receptors (B1-B4, B6), based on their binding affinities for ephrin-A (A1-A5) or ephrin-B (B1-B3) ligands (Pasquale 2005; Murai and Pasquale 2011). Several EPH receptors are up-regulated by inflammatory cytokines, and may play multiple roles in inflammation (Ivanov and Romanovsky 2006). Importantly, some of the ephrins have been implicated in recruitment of glutamate receptors to synapses, and to clustering and stabilization of excitatory synapses (Hruska and Dalva 2012). Alterations in expression levels of ephrin ligands and receptors have been demonstrated in hippocampus in Alzheimer disease (AD) and animal models of AD. Simon et al reported reduction of EPHA4 and EPHB2 proteins in hippocampus of postmortem brain of subjects with AD (Simon et al. 2009). The ephrin system has not been well studied in human HIV-infected brain or HIV animal models. Recently, an expression microarray study detected down-regulation of ephrin receptors EPHA4 and EPHA7 in deep white matter of the anterior frontal lobe of HIV-infected patients (Borjabad et al. 2011).
Studies of different EPH receptors or ephrin knockout mice showed that pre- and post-synaptic EPH receptors and ephrins may mediate clustering of other synaptic molecules, such as the NMDA receptor, which is implicated in long-term potentiation (LTP), required for memory and learning, as well as HIV-related neurotoxicity (Grunwald et al. 2004, Kaul and Lipton 2006). Recent studies have revealed that EPH receptor tyrosine kinases and ephrins play an important role in contact-dependent neuron-glia communication at synapses (Murai and Pasquale 2011). In contrast to other receptor tyrosine kinases, EPH receptors are functional when binding membrane-bound, but not soluble, ephrin ligands. Therefore an exceptional property of the EPH proteins is that they signal bidirectionally; receptor/ligand binding leads to signaling events in both presynaptic and postsynaptic neurons.
Involvement of ephrin receptors and their ligands in HIV-associated neurocognitive disorders remains largely unknown. In the present study, we analyzed expression of four genes of the ephrin family, Eph A4 and Eph B2 receptors, and ephrin-B1 and ephrin-B2 ligands in the caudate and anterior cingulate of postmortem brain of cognitively characterized HIV-infected subjects, and of uninfected controls.
Subjects, materials and methods
Participants
Study subjects from whom postmortem brain samples were obtained are listed in Table 1. Brain tissues were obtained from the Manhattan HIV Brain Bank, member of the National NeuroAIDS Tissue Consortium (MHBB, The Mount Sinai Medical Center, New York, NY, U01MH083501). The MHBB operates under local IRB-approved ethical guidelines, and written informed consent was obtained from all subjects, or their primary next-of-kin gave consent, for collection and use of tissues for medical research and furthering medical knowledge. Specimens from subjects with protracted agonal state, as manifested by extensive anoxic-ischemic damage on histological evaluation, were excluded from this study. The caudate and anterior cingulate cortex samples were derived from 39 unrelated individuals of different ethnicities (11 Caucasians, 12 African Americans, 16 Hispanics, and one Asian). Twenty-four subjects were HIV seropositive and 15 were HIV seronegative. Mean ages (years ± SD) were 52 ± 10 in HIV seronegative and 45 ± 10 in HIV seropositive subjects, and corresponding postmortem intervals (hours, PMI) were 18.4 ± 6.2 and 9.3 ± 5.0, respectively. PDYN expression in the caudate and anterior cingulate in these brain samples has been reported earlier (Yuferov et al. 2009, 2011).
Table 1.
Study subjects information
| PID | Category | Race | Age | Sex | PMI (hrs) |
Plasma Viral load |
Brain Viral load |
Plasma CD4 |
Brain pH |
RNA RIN |
T cores: Global |
Motor | Abstraction and Executive Functioning |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mhbb516 | HIV neg | H | 58 | M | 13.5 | - | - | - | 6.36 | 7.9 | - | - | - |
| mhbb531 | HIV neg | C | 44 | M | 21.5 | - | - | - | 6.52 | 7 | - | - | - |
| mhbb553 | HIV neg | H | 46 | M | 16 | - | - | - | 6.58 | 8.7 | - | - | - |
| mhbb568 | HIV neg | H | 48 | F | 16 | - | - | - | 6.62 | 7 | - | - | - |
| mhbb577 | HIV neg | C | 65 | M | 16.5 | - | - | - | 6.89 | 7.7 | - | - | - |
| mhbb584 | HIV neg | C | 53 | M | 22.5 | - | - | - | 6.72 | 8.3 | - | - | - |
| mhbb588 | HIV neg | H | 21 | M | 18.5 | - | - | - | 6.57 | 7.7 | - | - | - |
| mhbb589 | HIV neg | H | 61 | M | 6.5 | - | - | - | 6.79 | 7 | - | - | - |
| mhbb594 | HIV neg | C | 57 | F | 24 | - | - | - | 6.84 | 7 | - | - | - |
| mhbb601 | HIV neg | H | 58 | F | 16.5 | - | - | - | 6.08 | 7.5 | - | - | - |
| mhbb604 | HIV neg | AA | 48 | M | 9 | - | - | - | 6.97 | 9.2 | - | - | - |
| mhbb606 | HIV neg | H | 50 | M | 16 | - | - | - | 6.78 | 7.7 | - | - | - |
| mhbb610 | HIV neg | AA | 54 | F | 27 | - | - | - | 6.48 | 6.9 | - | - | - |
| mhbb611 | HIV neg | AA | 59 | M | 28.5 | - | - | - | 6.03 | 6.3 | - | - | - |
| mhbb613 | HIV neg | A | 55 | F | 24 | - | - | - | 6.42 | 5.3 | - | - | - |
|
| |||||||||||||
| 10001 | HIV | AA | 64 | F | 4.5 | 359 | 960 | 72 | 6.97 | 8.7 | 42 | 37 | 43 |
| 10002 | HIV | AA | 58 | M | 15.5 | <50 | 1725 | 459 | 7.13 | 8.4 | 57 | 44 | 60 |
| 10003 | HIV | C | 45 | M | 8 | - | 5993 | - | 6.35 | 5 | 35 | 24 | 34 |
| 10011 | HIV | H | 44 | M | 8.5 | 162642 | <50 | 16 | 6.77 | 8 | 44 | 40 | 48 |
| 10013 | HIV | C | 33 | M | 17 | >750000 | - | 9 | 6.78 | 7 | 49 | 24 | 50 |
| 10015 | HIV | C | 33 | M | 20 | 176800 | 11276 | 66 | 6.79 | 6.9 | 35 | 18 | 28 |
| 10016 | HIV | AA | 58 | F | 17 | 576000 | - | 98 | 6.07 | 5.6 | 32 | 28 | 27 |
| 10023 | HIV | AA | 53 | M | 4.5 | 183 | <50 | 7 | 6.79 | 7.1 | 34 | 24 | 34 |
| 10025 | HIV | H | 46 | M | 7.5 | 73 | - | 93 | 6.53 | 7.9 | 28 | 22 | 36 |
| 10027 | HIV | H | 39 | M | 6 | 120714 | - | 109 | 6.67 | 6 | 27 | 39 | 36 |
| 10034 | HIV | H | 49 | M | 5 | 57712 | - | 77 | 6.74 | 8.2 | 28 | 18 | 38 |
| 10043 | HIV | AA | 34 | F | 17 | 2857 | - | 757 | 6.64 | 7.7 | 35 | 32 | 34 |
| 10045 | HIV | H | 31 | F | 9 | 744349 | 21019 | 4 | 6.77 | 5.4 | 27 | 14 | 35 |
| 10052 | HIV | AA | 39 | M | 5 | 174175 | 1611 | 79 | 7.06 | 8.5 | 40 | 26 | 32 |
| 10063 | HIV | H | 51 | M | 5 | 65 | 1983 | 136 | 6.82 | 8.4 | 24 | 26 | 36 |
| 10064 | HIV | C | 49 | M | 12 | - | - | 278 | 6.24 | 6.2 | 27 | 20 | 38 |
| 10065 | HIV | H | 46 | M | 7 | >750000 | 45741 | 18 | 6.61 | 7.4 | 41 | 38 | 49 |
| 10066 | HIV | AA | 54 | F | 7 | 469163 | - | 8 | 6.37 | 8.5 | 33 | 36 | 40 |
| 10074 | HIV | AA | 41 | M | 17.5 | >750000 | - | 3 | 6.53 | 7.8 | 31 | 28 | 34 |
| 10086 | HIV | AA | 48 | F | 7.5 | 22957 | - | 249 | 6.71 | 8.2 | 36 | 32 | 36 |
| 10094 | HIV | H | 41 | F | 6.5 | 45413 | 13323 | 1 | 7.38 | 9.5 | 26 | 30 | 23 |
| 10103 | HIV | H | 40 | M | 6 | >750000 | >750000 | 15 | 6.94 | 6.8 | 29 | 31 | 37 |
| 10133 | HIV | C | 48 | M | 7.5 | 173921 | 7401 | 3 | 6.78 | 7.4 | 47 | 54 | 46 |
| 20024 | HIV | C | 62 | M | 4 | <50 | <50 | 20 | 6.74 | 9.4 | 25 | 22 | 33 |
Abbreviatins: PID - subject I.D. at MHBB; Race: AA-African American; C-Caucasians; H- Hispanics; A-Asians; PMI- post-mortem interval; RIN – RNA
Integrity Number; T scores calculated using normative data as described in the literature (Woods et al., 2004); subjects with T score less than 40 are considered impaired
Patients with HIV were evaluated by a neuropsychological battery as previously described (Woods et al. 2004). The neuropsychological battery assessed the following domains known to be sensitive to HIV-related neurocognitive impairment: verbal fluency, working memory, executive functioning, learning, memory, information processing speed, and motor ability. Raw scores were converted into T-scores which adjusted for the following demographic factors, as available: age, gender, education, and ethnicity using normative data for each test. Individual test scores were summed and averaged to create domain T-scores. Domain T-scores were used in analyses, rather than individual tests, to provide a more balanced estimate of neurocognitive ability and to reduce the number of analyses. Consistent with previous studies, a T-score greater than one standard deviation below normative estimates was considered impaired. HIV-negative subjects were chosen on the basis of premortem normal neurological function and normal postmortem brain histology.
RNA preparation and cDNA synthesis
Brain tissues (50-60 mg) from the caudate and cingulate of each brain specimen were homogenized in RLT buffer (RNeasy Mini Kit, Qiagen, Valencia, CA) for isolation of total RNA according to the manufacturer’s protocol. RNA samples were treated with RNase-Free DNase (TURBO DNA-free, Ambion, Austin, Texas, USA). RNA preparations were analyzed using using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Mean RNA Integrity Number (RIN) values (± SD) were 7.4 ± 0.96 (range from 5.3 to 9.2) in HIV seronegative and 7.3 ± 1.5 (range from 5.0 to 9.5) in HIV seropositive subjects. Single strand cDNA was synthesized using approximately 1 μg of RNA and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems (ABI), Carlsbad, CA) in the presence of random primers and gene-specific antisense primer.
Quantitative real-time PCR
For quantification of the mRNA levels of 11 genes, CCL2/MCP-1, CCR5, CXCR4, the neuronal marker SNAP25, astroglial and microglial/macrophage markers GFAP, CD68 and CD163, ephrin receptors EPHA4 and EPHB2, and ephrins EFNB1 and EFNB2 in the caudate and anterior cingulate cortex, we used a quantitative real-time polymerase chain reaction (qRT-PCR). cDNA (2 μl) was amplified in a 20 μl solution that contained the Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix (Agilent Technologies) and 10 nM of primers with a PCR condition of 40 cycles of denaturation at 94°C for 5 sec, and annealing/extension at 60°C for 15 sec. Forward and reverse primers for amplification of cDNA of genes studied were ether custom designed or commercial (SABiosciences, Valencia, CA) (Supplemental Table S1). Levels of the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA/mRNA were used for normalization of levels of mRNA of the target genes. Experimental samples were amplified simultaneously with samples that contained serial dilutions of a target gene and GAPDH cDNAs from 101 to 106 copies/2 μl in sterile water, used to prepare standard curves. Copy number determination based on gene-specific cDNA PCR fragments concentration at OD260 was made using the following formula (Rose’Meyer et al. 2003):
where [C]=5×10–5 g/ml for DNA; Molecular Weight of the PCR product is number base pairs of cDNA fragment ×6.58×102. Final values for gene-specific expression are given as copies/2 μl of cDNA. qRT-PCR analysis was performed using SDS 2.2 software (ABI) on an ABI Prism® 7900 sequence detection system. The specificity of amplification was confirmed by agarose gel electrophoresis of PCR products, a melting curve profile, and, in some cases, by Sanger sequencing. Copy number of cDNA of chemokine receptors, neuronal and glial/macrophage markers and GAPDH was quantified by comparing threshold cycles (Ct) of an experimental sample to those in standard curves for specific genes and GAPDH cDNA as described (Rose’Meyer et al. 2003). cDNA copy number is expressed normalized to copies of GAPDH cDNA copy number.
Statistical analysis
Normalized values of copy numbers of mRNA of each gene studied were quantified as natural log of copy number of gene of interest/copy number of GAPDH cDNA in the caudate and in the anterior cingulate. For expression of each gene in each region, a t-test was used to determine the statistical significance of differences between HIV− and HIV+ subjects, and cognitively impaired and non-impaired HIV+ subjects. T-tests were also used to determine statistical significance in differences of ephrin gene expression between cognitively impaired (global T scores <40) and non-impaired HIV positive individuals. A comparison of expression of ephrin genes with inflammatory genes was made by a three-way analysis of variance (ANOVA), HIV Status X Gene Type X Brain Region. Relationship of expression of selected genes with neuronal and glial/macrophage mRNA levels was examined using Pearson correlation analysis. Correlation analysis was also used to examine whether there was a relationship between expression levels of ephrin genes and cognitive status (domain T scores), as well as expression of ephrin genes with immunological status (CD4+ counts and viral load). Since preliminary analyses showed no significant differences between genders or between HIV positive subjects on ART and those not on ART, these factors were not included in subsequent analyses.
Results
Methodological validation of the quantitative real-time PCR assay
In our previous study, using brain tissues from the very well characterized and well preserved brain specimens from the same subjects, we found no effect of the brain tissue pH or RNA quality (RIN) on expression of the PDYN gene in the caudate using the solution hybridization RNase protection assay (Yuferov et al. 2009). Quality of the quantitative real-time PCR assay depends in large part on efficiency of amplification of cDNA and the choice of a house-keeping gene used for data normalization. In this study, we provide detailed information on the quantitative RT-PCR experiments according to the Guidelines for Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE, Bustin et al. 2009). Efficiency of the primers in PCR, defined as the slope of the regression line derived from 10-fold dilutions of calibration standards for ephrin and GAPDH cDNA was 102%-112% (slope from −3.05 to −3.28, Supplement Table 2) which is generally regarded as acceptable (Nolan et al. 2006). We chose GAPDH mRNA levels for normalization of expression of genes of interest in this study since it has been validated as a normalizer in studies of Alzheimer’s and Parkinson’s diseases (Coulson et al. 2008; Quinn et al. 2012). Also, expression of several genes shown by microarray in deep white matter of the anterior frontal lobe in HIV− and HIV+ subjects from the same HIV Brain Bank was confirmed using RT-PCR with GAPDH as a normalizer (Borjabad et al. 2011).
Examination of GAPDH expression showed no significant difference between HIV− and HIV+ samples in the caudate (p=0.42) or anterior cingulate (p=0.67). Also, there was no significant correlation of GAPDH mRNA levels either with age (r2=0.05, p=0.19) or PMI (r2=0.08, p=0.10).
Gene expression and cognitive impairment
We have measured expression of 13 genes in two postmortem brain regions in 24 HIV-positive and 15 HIV-negative subjects. Results of t-test between HIV− and HIV+ subjects of each gene in each region are shown in Table 2A with mean (±SEM) of level of mRNA of each gene. Table 2B shows the results of t-test between cognitively impaired and non-impaired HIV+ individuals.
Table 2.
A. T-tests between HIV− and HIV+ individuals for each gene studied in each region
| Caudate |
Anterior Cingulate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | HIV− | HIV+ | HIV− | HIV+ | ||||||
| Mean±SEM | Mean±SEM | t | df | p value | Mean±SEM | Mean±SEM | t | df | p value | |
| CCR5 | 3.67 ± 0.15 | 4.50 ± 0.18 | 3.27 | 34 | <0.005 | 3.33 ± 0.34 | 4.78 ± 0.18 | 4.08 | 22 | <0.0005 |
| CXCR4 | 8.42 ± 0.20 | 8.77 ± 0.18 | 1.25 | 34 | N.S. | 6.62 ± 0.21 | 7.09 ± 0.34 | 1.09 | 23 | N.S. |
| CCL2 | 7.79 ± 0.23 | 9.88 ± 0.25 | 5.57 | 34 | <0.000005 | 6.69 ± 0.38 | 8.84 ± 0.25 | 4.89 | 21 | <0.0001 |
| SNAP25 | 7.89 ± 0.14 | 8.01 ± 0.12 | <1.0 | 34 | N.S. | 11.49 ± 0.21 | 11.62 ± 0.21 | <1.0 | 24 | N.S. |
| CD68 | 6.34 ± 0.14 | 6.99 ± 0.12 | 3.31 | 34 | <0.005 | 6.65 ± 0.24 | 7.56 ± 0.12 | 3.65 | 23 | <0.002 |
| CD163 | 6.85 ± 0.23 | 8.05 ± 0.21 | 3.71 | 34 | <0.001 | 6.14 ± 0.34 | 7.37 ± 0.28 | 2.79 | 22 | <0.02 |
| EPH A4 | 9.90 ± 0.08 | 9.43 ± 0.09 | 3.43 | 33 | <0.002 | 10.47 ± 0.10 | 10.37 ± 0.12 | <1.0 | 28 | N.S. |
| EPH B2 | 8.87 ± 0.11 | 9.02 ± 0.08 | 1.13 | 33 | N.S. | 9.30 ± 0.10 | 9.02 ± 0.08 | 2.19 | 26 | <0.05 |
| EFNB1 | 8.36 ± 0.13 | 8.1 ± 0.13 | 1.28 | 32 | N.S. | 8.16 ± 0.11 | 7.99 ± 0.12 | <1.0 | 25 | N.S. |
| EFNB2 | 10.22 ± 0.13 | 9.76 ± 0.11 | 2.59 | 33 | <0.02 | 10.19 ± 0.15 | 10.13 ± 0.14 | <1.0 | 28 | N.S. |
| B. T-tests between cognitively non-impaired and impaired HIV+ individuals for each gene studied in each region | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Caudate |
Anterior cingulate |
|||||||||
| Cognitive status of HIV+ individials |
Cognitive status of HIV+ individuals |
|||||||||
| Gene | Non-impaired Mean±SEM |
Impaired Mean±SEM |
t | df | p | Non-impaired Mean±SEM |
Impaired Mean±SEM |
t | df | p |
| CCR5 | 4.56 ± 0.23 | 4.48 ± 0.24 | <1.0 | 20 | NS | 4.72 ± 0.22 | 4.81 ± 0.26 | <1.0 | 12 | NS |
| CXCR4 | 8.56 ± 0.45 | 8.86 ± 0.17 | <1.0 | 21 | NS | 6.92 ± 0.87 | 7.18 ± 0.29 | <1.0 | 12 | NS |
| CCL2 | 9.92 ± 0.27 | 9.87 ± 0.35 | <1.0 | 21 | NS | 8.92 ± 0.23 | 8.79 ± 0.40 | <1.0 | 11 | NS |
| SNAP25 | 8.09 ± 0.14 | 7.98 ± 0.15 | <1.0 | 21 | NS | 11.48 ± 0.27 | 11.69 ± 0.29 | <1.0 | 13 | NS |
| GFAP | 11.63 ± 0.25 | 11.63 ± 0.16 | <1.0 | 21 | NS | 13.61 ± 0.35 | 13.00 ± 0.41 | <1.0 | 12 | NS |
| CD68 | 7.36 ± 0.21 | 6.82 ± 0.13 | 2.19 | 21 | <0.05 | 7.70 ± 0.22 | 7.49 ± 0.14 | <1.0 | 12 | NS |
| CD163 | 7.90 ± 0.50 | 8.11 ± 0.21 | <1.10 | 21 | NS | 7.61 ± 0.42 | 7.23 ± 0.38 | <1.0 | 12 | NS |
| EPHA4 | 9.63 ± 0.10 | 9.34 ± 0.12 | 1.52 | 20 | NS | 10.31 ± 0.19 | 10.39 ± 0.16 | <1.0 | 17 | NS |
| EPHB2 | 8.85 ± 0.18 | 9.09 ± 0.07 | −1.54 | 20 | NS | 8.79 ± 0.12 | 9.15 ± 0.09 | −2.45 | 15 | <0.05 |
| EFNB1 | 7.78 ± 0.28 | 8.26 ± 0.13 | −1.78 | 19 | NS | 7.68 ± 0.21 | 8.15 ± 0.13 | −1.98 | 15 | NS |
| EFNB2 | 9.97 ± 0.16 | 9.66 ± 0.15 | 1.29 | 20 | NS | 9.81 ± 0.25 | 10.27 ± 0.15 | −1.63 | 17 | NS |
Genes with significant expression differences between HIV− and HIV+ individuals are shown in bold
NS - no significant difference between HIV− and HIV+.
Down-regulation of mRNA levels of ephrin genes in HIV-infected subjects
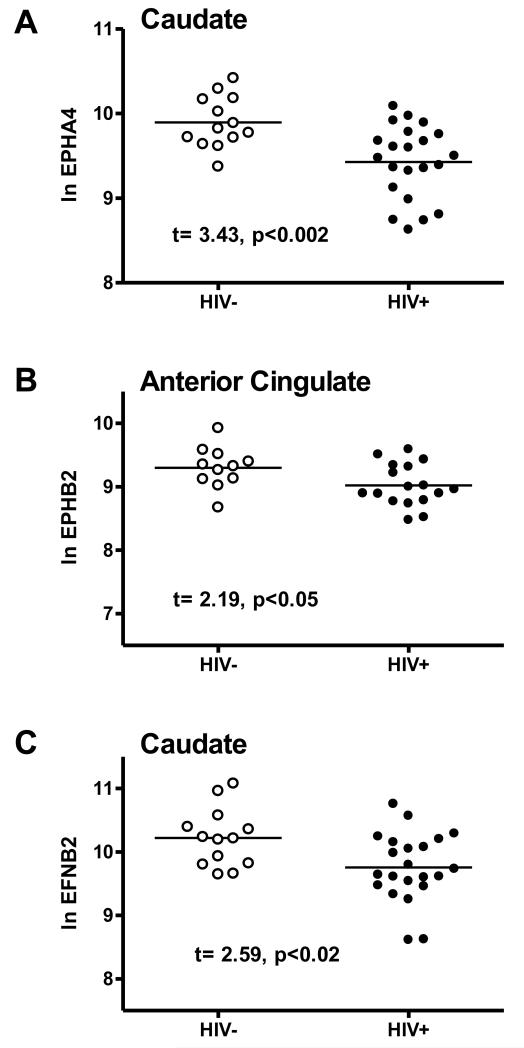
Levels of mRNA of the EPH A4 and EPH B2 ephrin receptors, and of the ephrin ligand EFNB2 in two brain regions of HIV− and HIV+ subjects are shown in Figure 1 and Table 2A. There were significantly lower mRNA levels of EPH A4 (p<0.002) and EFNB2 (p<0.02) in the caudate, and of EPH B2 (p<0.05) in anterior cingulate in HIV+ subjects.
Fig. 1.
Ephrin expression by HIV status. Significant differences in expression (Ln mRNA level/GAPDH mRNA) of ephrin genes between HIV-negative and HIV-infected individuals in each brain region are shown. A) ephrin receptor EPHA4 in the caudate. B) ephrin receptor EPHB2 in the anterior cingulate. C) ephrin ligand EFNB2 in the caudate. In each case there was significantly lower ephrin gene expression in HIV-infected individuals.
Up-regulation of mRNAs of proinflammatory genes in brain of HIV-infected subjects
Activation of proinflammatory genes is a hallmark of HIV infection reported in many studies, and inflammatory response within the CNS is considered to be the main mediator of neuronal alterations in brain. To characterize microglial and macrophage cell activation in HIV-infected postmortem brains, we analyzed mRNA levels of the chemokine receptors CCR5 and CXCR4, the chemokine CCL2/MCP-1 and microglial/macrophage markers, CD68 and CD163, as well the astroglial marker GFAP in two brain regions of HIV− and HIV+ subjects. T tests showed that there were significantly higher mRNA levels of CCR5, CCL2, GFAP, CD68 and CD163 in HIV+ subjects (Table 2A). In contrast, we did not observe alterations of the mRNA levels of the neuronal marker SNAP25 in either brain region in HIV+ subjects compared to HIV− subjects. Also, no difference was found in the levels of CXCR4 mRNA between HIV+ and control subjects in either brain region. Of note, in the caudate of control subjects, mRNA levels of CCR5 were more than four units (ln CCR5) lower than levels of CXCR4 (ln CXCR4) (Table 2B). A similar difference in expression of these receptors was found in the anterior cingulate. Taking into account the importance of these chemokine receptors in HIV infection in brain, these differences in their mRNA levels may reflect that CCR5 is expressed in a small subset of brain cells compared to the wider range of cell types expressing CXCR4.
Comparison of expression of ephrin genes with inflammatory genes
A three-way ANOVA, HIV Status X Gene Type X Brain Region, was conducted, including six inflammatory genes (CCR5, CXCR4, GFAP, CD68, CCL2, and CD163) and the four ephrin genes studied (EPHA4, EPHB2, EFNB1, EFNB2). There was a significant main effect of Gene Type, F(1,606) = 62.93, p<0.0001, a nonsignificant main effect of HIV status, F(1,606) = 3.27, p = 07, and no effect of brain region, F(1,606)<1.0. These results show that by far the strongest difference in expression level is that between ephrin and inflammatory genes. While the main effect of HIV status is modest, there was a strong interaction between HIV and Gene Type, F(1,606) = 7.94, p<0.01; that is, differences between ephrin and inflammatory genes depend upon HIV status. While inflammatory genes have been shown to be increased in HIV, what is new is that, as can be seen in Table 2A, three ephrin genes differed significantly by HIV status. Both EPHA4 and EFNB2 expression levels were lower in the caudate and EPHB2 expression was lower in the cingulate of brains from HIV+ subjects.
Relationship of expression of selected genes with neuronal and glial/macrophage mRNA levels
A number of studies have suggested that coordinated gene expression may indicate a functional relationship, although the coordinated expression does not necessarily indicate a causal relationship among transcript levels (e.g. Lee et al. 2004). Due to the known expression of chemokine receptors and ephrin genes in neuronal or glial/macrophage cells, we examined whether their mRNA levels are correlated with expression of the neuronal cell marker SNAP25, the astroglial marker GFAP, or microglial/macrophage markers, CD68 and CD163. Significant correlation between mRNA levels of the pairs of genes studied is shown in Table 3.
Table 3.
Correlation between mRNA levels in two brain region s of HIV− and HIV+ subjects
| Caudate | HIV− | HIV+ |
|---|---|---|
| EPHA4 and SNAP25 | NS | r=0.443, p<0.05 |
| CCL2 and SNAP25 | NS | inverse, r = −0.525, p<0.02 |
| CCR5 and GFAP | NS | r=0.440, p<0.05 |
| CCL2 and GFAP | NS | r=0.536, p<0.001 |
| CD68 and GFAP | NS | r=0.428, p<0.05 |
| CCR5 and CD68 | NS | r=0.647, p=0.001 |
| CXCR4 and CD68 | NS | r=0.500, p=0.08 |
| CCR5 and CD163 | NS | r=0.586, p<0.004 |
| EPHA4 and EFNB2 | NS | r=0.765, p<0.0001 |
| Anterior Cingulate | ||
|
| ||
| CXCR4 and SNAP25 | r=0.58, p=0.061 | r=0.595, p<0.05 |
| EPHB2 and SNAP25 | NS | r=0.565, p=0.056 |
| CD68 vs GFAP | NS | r=0.540 p<0.05 |
We found strikingly coordinated expression of the genes in both brain regions of HIV+ subjects but not in HIV− subjects. In the caudate, there was significant positive correlation between CCR5 and glial/macophage markers GFAP, CD68, and CD163. In the anterior cingulate, CXCR4 was significantly correlated with the neuronal marker SNAP25 in HIV+ subjects (r=0.595, p<0.05), but not significantly in HIV− subjects (r=0.580, p=0.061). In the caudate of HIV+ subjects, ephrin receptor EPHA4, was positively correlated with SNAP25 (r=0.443, p<0.05).
Relationship of expression of ephrin genes with cognitive and immunovirological status in HIV+ patients
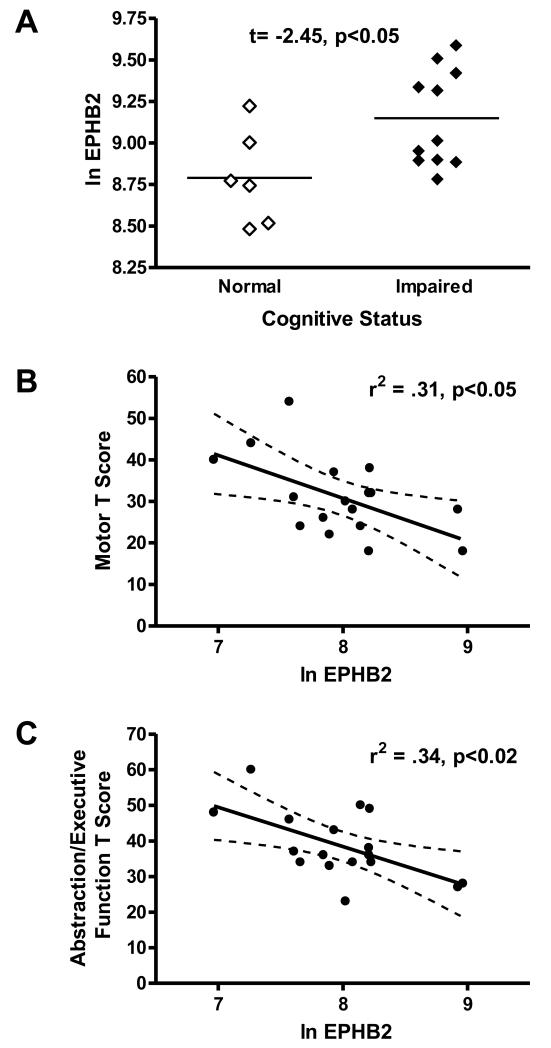
Cognitively impaired (global T score <40) HIV+ subjects were compared to cognitively normalHIV+ subjects in expression of the level of ephrin transcripts in each brain region. Of the transcripts found to differ between HIV− and HIV+ individuals, only cingulate levels of EPHB2 mRNA levels were associated with cognitive impairment (Table 2B). Unexpectedly, cognitively impaired HIV+ individuals demonstrated higher mean values of EPHB2 (9.15 ± 0.09) than cognitively intact (8.79 ± 0.12) (p<0.05) (Fig 2). A series of correlations was then examined between domain T scores and cingulate EPHB2. Motor T and abstraction/executive functioning T scores were significantly correlated with EPHB2 expression, and again, the direction was such that higher levels of EPHB2 were associated with worse neuropsychological test performance (for motor T, r2=0.301, p<0.05; for abstraction/executive function, r2=0.303, p<0.05). Finally, the impact of immunovirologic status (CD4 count and viral load) was assessed; in contrast to cognitive effects, associations with ephrins were seen only in the caudate. In the caudate, decreased CD4 count was associated with decreased levels of ephrin ligand EFNB2 (r2=0.196, p<0.05).
Fig.2.
EPHB2 expression in the anterior cingulate and cognitive function in HIV-infected subjects. A) EPHB2 expression (Ln mRNA level/GAPDH mRNA) was significantly higher in cognitively impaired (Global T score <40) than in cognitively normal individuals. Motor T (B) and abstraction/executive (C) functioning T scores were significantly correlated with EPHB2, showing that higher levels of EPHB2 were associated with worse neuropsychological test performance.
Discussion
Numerous studies suggest that HAND is primarily the result of neuronal loss/dysfunction from direct or indirect viral effects, inclusive of inflammation driven by chronic low-level infection, loss of trophic factors, and elaboration of excitotoxic molecules (e.g. Clifford 2008). Morphologically, HIV-associated cognitive impairment has been linked to alterations in the synaptodendritic network in HIV-infected brain (Mosliah et al. 1997; Ellis et al. 2007). Among other neurotrophins, ephrin receptors and associated ligands are known to be tightly involved in synaptic function, neuroadaptation, formation and maintenance of excitatory synapses, and response to brain injury (Pasquale 2005; Murai and Pasquale 2011).
Several studies implicate EPHA4 and EPHB2 receptors in the regulation of neuronal morphology. EphA4 knockout mice show irregularities in spine shape and densities (Murai et al. 2003). Animals lacking EphB2 display a 40% reduction in synapse-associated NMDARs (Gunwald et al. 2001). The receptors EphA4 and EphB2 have a distinct distribution in dendrites versus axon terminals in the adult mouse hippocampus and frontal cortex. EphA4 has been detected in axon terminals, dendritic spines, astrocyte processes, and axon shafts while EphB2 has been detected mainly in dendritic shafts and dendritic spines but not in axon terminals (Bouvier et al. 2008). Reduction in the EPHA4 and EPHB2 receptors, and the ligand EFNB2 in HIV-infected brain found in this study, are consistent with HIV-induced reduction of synaptic density, and dendritic morphology shown in previous studies (Masliah et al. 1997; Everall, et al. 1999; Murai et al. 2003).
Results of our study are also consistent, in part, with a recent report on alterations in the genome-wide transcriptome using Affymetrix U133 Plus 2.0 microarray in deep white matter of the anterior frontal lobe in 15 HIV-infected patients compared to six HIV-negative subjects (Borjabad et al. 2011). Brain tissues in this study were also provided by MHBB, and 11 subjects (four HIV-negative and seven HIV-positive subjects) are included in the present study. This microarray study showed significant elevation of proinflammatory genes (e.g. CD68, CD163, CCL2, CXCR4), and down-regulation of ephrin receptors EPHA4 and EPHA7, as well as down-regulation of presynaptic and postsynaptic markers MAP2 and synaptophysin in HIV-positive subjects with HAND. With cART therapy, however, the widespread inflammatory response and down-regulation of ephrins was not seen. In another cART era gene expression study, down-regulation of EPHA4, EPHA5, EFNA1 was seen in brains of cognitively impaired HIV+ individuals with florid inflammation due to HIV encephalitis, but not in those who were cognitively impaired without similar, substantive inflammatory increases (S Morgello, personal communication; Gelman et al, manuscript in preparation). The correlations between increased expression of inflammatory transcripts and decreased ephrin expression in our study, together with the prior gene expression analyses, suggest that down-regulation of ephrins may be a consequence of chronic immune activation and damage to the synaptodendritic structure of the brain. However, this does not necessarily imply that these changes are the primary factor in clinically-relevant cognitive impairments.
Indeed, in our study, when HIV patients were categorized by the presence or absence of impaired global T scores, an unexpected association of increased EPHB2 mRNA levels and impairment was seen in the anterior cingulate cortex. This was followed up with analysis of neuropsychological domains, and worse motor and abstraction/executive functioning showed significant correlations with greater EPHB2 expression. Thus, while neuroinflammation and HIV status were associated with cortical decreases in EPHB2, worsening cognitive status was paradoxically associated with increases.
Among the ephrin genes, EPHB2 is one of the best studied ephrin receptors. Recently, Cisse and colleagues provided evidence that AβPP-induced depletion of EphB2 and impairment in NMDA-dependent LTP can be reversed by an increase of EphB2 expression using a lentivirus in the dentate gyrus in the mouse model of Alzheimer’s disease (Cisse et al. 2011). The overexpression of EphB2 also reversed the AD-associated behavioral and cognitive deficits in this model. Another study showed that EphB2 in the amygdala regulates stress-induced plasticity and anxiety-like behavior in mice (Attwood et al. 2011). Restraint stress in mice activates the extracellular protease neuropsin which cleaves EphB2 in the amygdala, leading to disruption of EphB2-NMDA-receptor interaction resulting in increased expression of FKBP5, implicated in the development of anxiety, depression and post-traumatic stress disorder in humans (Binder et al. 2009).
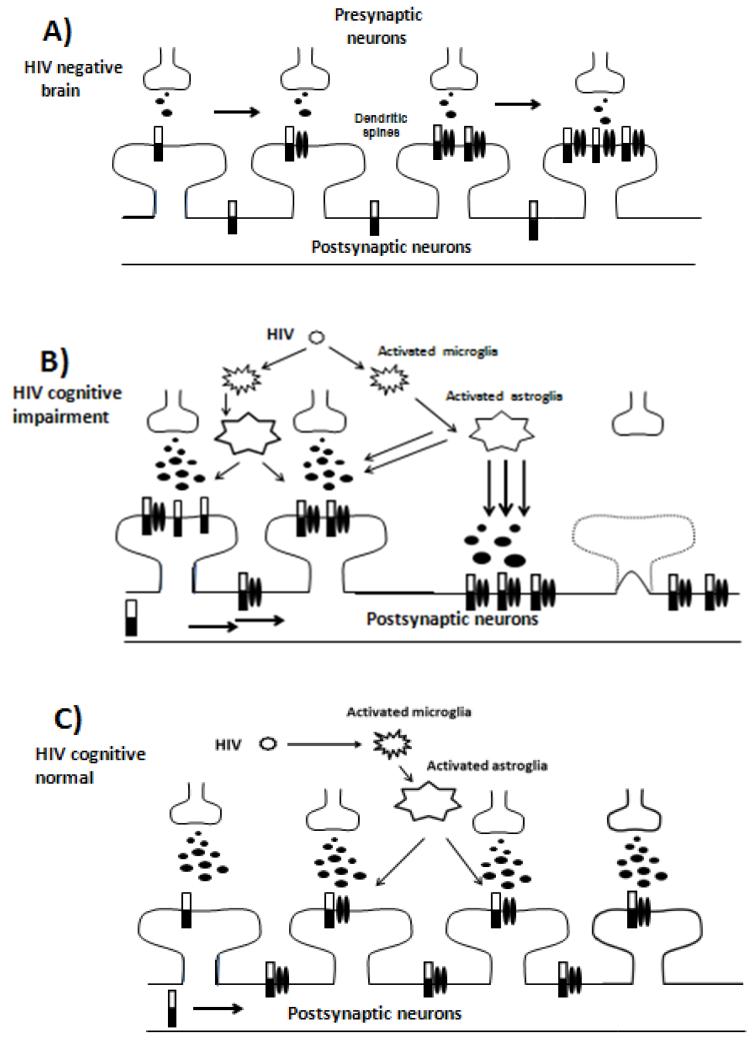
In the context of these experimental models, the increase of EPHB2 in the cognitively impaired phenotype of our patients is puzzling. The exact mechanism of the HIV-associated neurocognitive disorders is not known. In the absence of immunochemical and morphological evaluation of region-specific EPHB2 expression in brain of subjects used in this study, we can present only a hypothetical model (Fig 3) based on our own and other studies of HIV-infected brains, and in other neurogenerative disorders .Our finding on the reduction of EPHB2 expression in the anterior cingulate in HIV-infected brain in subjects with HAND compared to control brain is consistent with morphological studies of brains of HIV positive subjects that showed dendritic simplification and reduction in synaptic density and neuronal markers as the pathological features most closely associated with the clinical signs of HAND (e.g. Masliah et al, 1997; Everall et al, 1999). Although an exact mechanism of the HIV-induced reduction of dendritic branching and synaptic density in brain is not known, it is possible that HIV proteins (e.g. gp120, Tat) and cytokines and chemokines secreted by activated microglia and astrocytes in inflammation conditions lead to alterations in synapse and dendritic spine structure in HIV− infected subjects. Among many other proposed mechanisms of the HIV-induced neuroanatomical injury in brain, the glutamate neurotoxicity is now commonly accepted. Several studies demonstrated increased glutamate levels in CSF (Espey et al, 1999; Ferrarese et al, 2001), and lower neuronal glutamate levels in parietal cortex and frontal lobe in patients with HIV dementia (Sailasuta et al, 2009; Ernst et al, 2010). The latter was interpreted as inhibition of glutamate reaptake by astrocytes and of glutamine synthesis that leads to increased levels of extracellular glutamate. The elevated extracellular glutamate may lead to excessive activation of synaptic and extrasynaptic NMDA receptors, leading to deleterious glutamate and Ca++ overflow at synapses and extrasynaptic sites in neuronal spines and dendrites (Hardingham and Bading, 2010; Sheffler-Collins and Dalva, 2012). Ephrin receptors have been shown to participate in trafficking and clustering of NMDA receptors at specific sites on the neuronal membrane (Calo et al, 2006). A morphological study of ephrin A (including EPHA4) receptor and ligand expression in the temporal lobe of subjects with multiple sclerosis suggested that axonal ephrin/Eph expression was increased in samples immediately adjacent to active lesions compared with non-affected areas or adjacent to chronic lesions (Sobel, 2005). We hypothesize that higher level of EPHB2 found in the anterior cingulate in HIV-infected subjects in our study may also be related to the sites of neuronal injuries (e,g. dendritic and spine loss). It is likely that the total decrease of the EPHB2 in HIV-infected subjects compared to controls is related to HIV− mediated neuronal and dendritic loss regardless of a degree of neurocognitive deficit, but a degree of neurocognitive impairment depends on the level of synaptic or extrasynaptic EPHB2 expression in specific sites of brain lesions.
Fig.3.
Hypothetical model of HIV-induced increased in EPHB2 and redistribution of a pool of NMDA receptors in neuronal spines related to neurocognitive impairment.
A) EPHB2 receptor regulates excitatory synapse development and function at mammalian synapses by controlling dendritic morphology, synapse formation and excitatory neurotransmitter receptor content. In neurons, EPHB2 receptors mediate excitatory synaptogenesis and coordinate synaptic function by controlling synaptic glutamate receptor localization and function (Sheffler-Collins and Dalva, 2012).
B) High inflammatory response in HIV-infected brain in subjects with neurocognitive impairment causes an increase of EPHB2 receptor expression leading to alterations in balance of NMDA-R between synaptic and extrasynaptic areas in spines. In cultured neurons, the synaptic and extrasynaptic NMDA-R receptors have been shown to activate distinct transduction pathways and have different functions: synaptic NMDA-R provides normal fast excitatory neurotransmission and survival of neurons whereas an activation of the extrasynaptic NMDA-R induces expression of pro-apoptotic and cell death genes (Calo et al, 2006; Hardingham and Bading, 2010). Higher EPHB2 expression may lead to relatively higher extrasynaptic NMDA-R signaling and loss of synapses that contribute to the development of neurocognitive deficit in HIV seropositive subjects.
C) Relatively lower EPHB2 expression may be related to lower inflammatory response in HIV− infected subjects without neurocognitive impairment, and does not induce synaptic remodeling.
It is also possible that the increased level of transcript is a compensatory mechanism in individuals whose immune-mediated cortical damage has reached a threshold for dysfunction – that is, with a burden of synaptodendritic abnormality large enough to manifest in behavioral change, there is a compensatory up-regulation to attempt repair. Another possibility is that elevation in EPHB2 is a marker of vulnerability to inflammation-induced excitotoxic damage, and that individuals with higher EPHB2 expression are more prone to clinically significant damage due to recruitment or maintenance of excitatory synapses. There is a rich literature implicating excitatory pathways in the generation of HAND; if increased ephrin transcript levels result in increased excitatory receptor expression, clustering or stabilization (some of their known functions), this may promote an impaired phenotype in the face of chronic immune stimulation. Finally, it is possible that the increased level of cingulate EPHB2 is a function of some masked co-morbidity associated with the impaired phenotype, but not necessarily a factor in the genesis of HAND.
In contrast to the paradoxical association of increased EPHB2 expression with decreased cognitive function, analysis of the changes with regard to immunovirologic status revealed an association of decreasing caudate EFNB2 with decreasing CD4 counts in HIV positive individuals. This finding would be consistent with what is known about advancing HIV and the progressive atrophy of subcortical structures. Decreased caudate volume is commonly seen in individuals with advanced HIV, and it is plausible to hypothesize that with this atrophy, a loss of synaptic structures, dendritic complexity, and decrease in ephrin ligands would occur. The finding of decreased EPHA4 and EFNB2 in all HIV subjects is also consistent with this process.
It is of interest that cognitive associations were with EPHB2 in cortex, whereas immunologic associations (using CD4 count as proxy) were in the caudate. In the natural history of HIV disease, metabolic and structural changes in basal ganglia often precede cognitive change, as has been demonstrated by structural and functional neuroimaging. Recently, in a cART-era study of brain gene expression, it was demonstrated that in cognitively normal individuals dying with HIV, abnormal gene expression was essentially limited to the caudate, and was associated with abnormalities in immune function (Gelman et al, 2012). One might hypothesize that our findings in caudate reflect maladaptative changes to states of altered immunity in the context of HIV. In contrast, cortical changes might have more relevance to cognitive process, and hence show divergent patterns of association.
In summary, we found a decrease in EPHA4 and EFNB2 in the caudate and EPHB2 in the anterior cingulate in postmortem brain of HIV-infected subjects. Decreases in caudate EFNB2 were associated with worsening immune status, whereas increases in cingulate EPHB2 were associated with worse cognitive status. The complex changes in ephrin gene expression are worthy of future investigation, and may represent a target for future adjunctive therapy in the treatment of HAND.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH NIDA-P60-05130 (MJK), NIMH-U01-MH083501 (SM). We thank Allie Sperry for help in performance of RT-PCR assays, and the patients and staff of the Manhattan HIV Brain Bank. We acknowledge the help of Dr. Niikura, Keiichi, Adam J. Brownstein, and Molly Deutsch-Feldman in the art work of the diagram.
This work was supported by NIH NIDA-P60-05130 (MJK), NIMH-U01-MH083501 (SM), NSFC-10971210 (YY) (China Natural Science Foundation)
Footnotes
Conflict of interests: The authors declare that they have no conflict of interest.
Supplementary information is available online.
REFERENCES
- Attwood BK, Bourgognon JM, Patel S, Mucha M, Schiavon E, Skrzypiec AE, et al. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473:372–375. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Borjabad A, Morgello S, Chao W, Kim SY, Brooks AI, Murray J, et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 2011;7:e1002213. doi: 10.1371/journal.ppat.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier D, Corera AT, Tremblay ME, Riad M, Chagnon M, Murai KK, et al. Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem. 2008;106:682–695. doi: 10.1111/j.1471-4159.2008.05416.x. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci (Lond) 2005;109:365–379. doi: 10.1042/CS20050086. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Calò L, Cinque C, Patanè M, Schillaci D, Battaglia G, Melchiorri D, Nicoletti F, Bruno V. Interaction between ephrins/Eph receptors and excitatory amino acid receptors: possible relevance in the regulation of synaptic plasticity and in the pathophysiology of neuronal degeneration. J Neurochem. 2006;98:1–10. doi: 10.1111/j.1471-4159.2006.03844.x. [DOI] [PubMed] [Google Scholar]
- Cissé M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–98. [PubMed] [Google Scholar]
- Coulson DT, Brockbank S, Quinn JG, Murphy S, Ravid R, Irvine GB, Johnston JA. Identification of valid reference genes for the normalization of RT qPCR gene expression data in human brain tissue. BMC Mol Biol. 2008;9:46. doi: 10.1186/1471-2199-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Erdmann NB, Whitney NP, Zheng J. Potentiation of excitotoxicity in HIV-1 associated dementia and the significance of glutaminase. Clin Neurosci Res. 2006;6:315–328. doi: 10.1016/j.cnr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Ellis RJ, Heaton RK, Basile AS. Relevance of glutamate levels in the CSF of patients with HIV-1-associated dementia complex. Neurology. 1999;53:1144–1145. doi: 10.1212/wnl.53.5.1144. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, et al. HNRC Group. HIV Neurobehavioral Research Center Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA. Susan Morgello for the National NeuroAIDS Tissue Consortium.The National NeuroAIDS Tissue Consortium Brain Gene Array: Two Types of HIV-Associated Neurocognitive Impairment. PLoS One. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. doi: 10.1371. Epub 2012 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Adelmann G, Plueck A, Kullander K, Adams RH, et al. Hippocampal plasticity requires postsynaptic ephrin Bs. Nat Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. CHARTER Group; HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Dalva MB. Ephrin regulation of synapse formation, function and plasticity. Mol Cell Neurosci. 2012;50:35–44. doi: 10.1016/j.mcn.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life. 2006;58:389–394. doi: 10.1080/15216540600756004. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Klein R. Excitatory Eph receptors and adhesive ephrin ligands. Curr Opin Cell Biol. 2001;13:196–203. doi: 10.1016/s0955-0674(00)00197-6. [DOI] [PubMed] [Google Scholar]
- Lee HK, Hsu AK, Sajdak J, Qin J, Pavlidis P. Coexpression analysis of human genes across many microarray data sets. Genome Res. 2004;14:1085–1094. doi: 10.1101/gr.1910904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest. 1992;66:285–291. [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. HNRC Group. The HIV Neurobehavioral Research Center Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Masliah E. Chemokine receptors and neurotrophic factors: potential therapy against aids dementia? J Neurosci Res. 2008;86:243–255. doi: 10.1002/jnr.21492. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. HNRC Group Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia. 2011;59:1567–1578. doi: 10.1002/glia.21226. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signaling casts a wide net on cell behavior. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Quinn JG, Coulson DT, Brockbank S, Beyer N, Ravid R, Hellemans J, Irvine GB, Johnston JA. α-Synuclein mRNA and soluble α-synuclein protein levels in post-mortem brain from patients with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. Brain Res. 2012;1459:71–80. doi: 10.1016/j.brainres.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Rose’Meyer RB, Mellick AS, Garnham BG, Harrison GJ, Massa HM, Griffiths LR. The measurement of adenosine and estrogen receptor expression in rat brains following ovariectomy using quantitative PCR analysis. Brain Res Brain Res Protoc. 2003;11:9–18. doi: 10.1016/s1385-299x(02)00219-2. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed. 2009;22:326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- Sheffler-Collins SI, Dalva MB. EphBs: an integral link between synaptic function and synaptopathies. Trends Neurosci. 2012;35:293–304. doi: 10.1016/j.tins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, et al. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J Alzheimers Dis. 2009;17:773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Sobel RA. Ephrin A receptors and ligands in lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2005;15:35–45. doi: 10.1111/j.1750-3639.2005.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol. 2005;12:292–297. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol. 2003;171:106–114. doi: 10.4049/jimmunol.171.1.106. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, Shi R, Ott J, Kreek MJ. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–97. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Nielsen DA, Levran O, Randesi M, Hamon S, Ho A, Morgello S, Kreek MJ. Tissue-specific DNA methylation of the human prodynorphin gene in post-mortem brain tissues and PBMCs. Pharmacogenet Genomics. 2011;21:185–96. doi: 10.1097/FPC.0b013e32833eecbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.