Abstract
Background
Antimicrobial peptides (AMPs) are synthesized and secreted by immune and epithelial cells that are constantly exposed to environmental microbes. AMPs are essential for barrier defense, and deficiencies lead to increased susceptibility to infection. In addition to their ability to disrupt the integrity of bacterial, viral and fungal membranes, AMPs bind lipopolysaccharides, act as chemoattractants for immune cells and bind to cellular receptors and modulate the expression of cytokines and chemokines. These additional biological activities may explain the role of AMPs in inflammatory diseases and cancer. Modulating the endogenous expression of AMPs offers potential therapeutic treatments for infection and disease.
Methods
The present review examines the published data from both in vitro and in vivo studies reporting the effects of nutrients and by-products of microbial metabolism on the expression of antimicrobial peptide genes in order to highlight an emerging appreciation for the role of dietary compounds in modulating the innate immune response.
Results
Vitamins A and D, dietary histone deacetylases and by-products of intestinal microbial metabolism (butyrate and secondary bile acids) have been found to regulate the expression of AMPs in humans. Vitamin D deficiency correlates with increased susceptibility to infection, and supplementation studies indicate an improvement in defense against infection. Animal and human clinical studies with butyrate indicate that increasing expression of AMPs in the colon protects against infection.
Conclusion
These findings suggest that diet and/or consumption of nutritional supplements may be used to improve and/or modulate immune function. In addition, by-products of gut microbe metabolism could be important for communicating with intestinal epithelial and immune cells, thus affecting the expression of AMPs. This interaction may help establish a mucosal barrier to prevent invasion of the intestinal epithelium by either mutualistic or pathogenic microorganisms.
Keywords: Antimicrobial peptide, Innate immune, Vitamin, Dietary, Supplement, Infection
Introduction
Cathelicidins, defensins and other antimicrobial peptides (AMPs) are an evolutionarily conserved component of the innate immune system and play an important role in combating infection [1-4]. AMPs have two or more positively charged residues in acidic environments and can bind to the negatively charged membranes of both gram (−) and gram (+) bacteria, mycobacteria, fungi and enveloped viruses, which in turn kills the pathogens by disrupting their cell membranes [5, 6].
The human cathelicidin antimicrobial peptide (CAMP) gene encodes a preproprotein with an ~30 amino acid–long N-terminal signal sequence, a 94 amino acid cathelin domain and a 37 amino acid C-terminal cationic AMP domain (LL-37) [6]. The CAMP gene is primarily expressed in myeloid bone marrow cells, and the proprotein, hCAP18, is packaged in neutrophil specific granules [7]. CAMP is also expressed by epithelial cells in tissues exposed to environmental microbes, and the protein is secreted in semen, saliva and sweat providing barrier protection to mammals [8-10]. Mice and humans lacking CAMP are susceptible to bacterial infections in numerous tissues [4, 11, 12]. High circulating levels of hCAP18 are found in human plasma [13]. The hCAP18/LL37 peptide binds to and neutralizes LPS [14], thus preventing its interaction with the LPS-binding protein and subsequent activation of TLR-4 [15], NF-κB signaling and cytokine release from host cells [16]. LL-37 protects against LPS-induced sepsis in animals [17, 18] and kidney dialysis patients with low serum levels of hCAP18 are twice as likely to die from infectious disease or sepsis than patients with higher levels [19]. Therefore, circulating hCAP18 or LL-37 may protect against both bacterial infection and sepsis.
Like cathelicidin, defensins are expressed by immune cells and epithelial cells of tissues that are exposed to the environment [3]. Defensins have six highly conserved cysteines that form disulfide bonds [2]. Human defensins are classified into two families: α- and β-defensins [20]. All defensins are expressed as a biologically inactive preproprotein and activated by cleavage of a prosequence [20]. To date, six α-defensins have been identified with HNP-1–4 packaged in human neutrophil primary granules and HD-5 and HD-6 expressed by Paneth cells in the small intestine [3]; HD-5 is also expressed by epithelial cells of the female genitourinary tract [21]. The α-defensins have antimicrobial activity against a range of bacteria, viruses and fungi [20].
β-defensins are the most widely distributed of the antimicrobial peptides. They are typically 38–42 amino acids long [22] and expressed in monocytes, macrophages and dendritic cells (DCs) [23] as well as epithelial cells in the respiratory and urogenital tracts, skin and tonsil [20, 24]. Human β-defensin 1 (HBD-1) is constitutively expressed in many tissues while HBD-2, HBD-3 and HBD-4 expression is induced by inflammatory stimuli, such as bacterial infection, LPS, IL-1β, TNF-α or phorbol-myristate-acetate (PMA) [2, 3, 20, 22, 25]. β-defensins have shown activity against both gram (−) and gram (+) bacteria, fungi, viruses and parasites [26-28].
In this paper, we review the regulation of the CAMP gene and the defensin gene family by various nutritional compounds and/or microbial by-products of metabolism and discuss the potential importance of this regulation for human innate immune function.
Nutritional regulation of AMP expression
Vitamin D
Camp
There are two forms of vitamin D that humans can utilize: vitamin D2 (ergocalciferol) from fungi and vitamin D3 (cholecalciferol) from animal sources and synthesized in the skin [29]. Expression of the human CAMP gene is induced by 1,25(OH)2 D3 and its analogs in various cell lines and primary cells [30-32]. Vitamin D induction of CAMP is solely a human and non-human primate phenomenon [33, 34] due to a vitamin D response element (VDRE) located within a primate-specific retro-transposable element (Alu-SINE) found in the upstream promoter region of the CAMP gene [34].
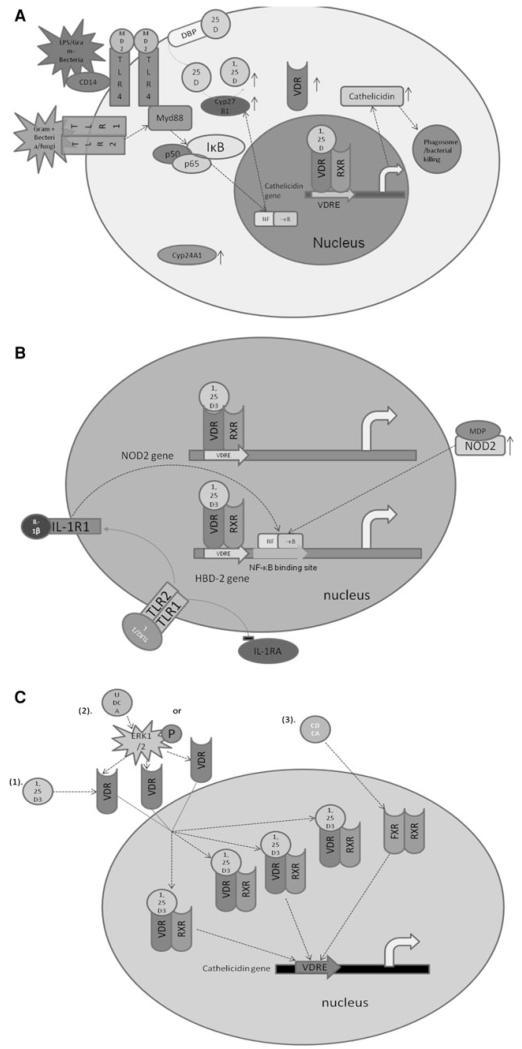
In subsequent studies, it was demonstrated in vitro that Toll-like receptor (TLR) activation induced CAMP expression via a vitamin D-dependent pathway in macrophages [35]. In the proposed model, sufficient levels of circulating 25(OH)D3, the precursor to 1,25(OH)2D3, are required for induction of CAMP gene expression and production of adequate LL-37 peptide levels to effectively combat infection (Fig. 1a) [35]. Cathelicidin expression is also increased in keratinocytes via a similar pathway after skin wounding [36].
Fig. 1.
Regulation of AMP gene expression through the VDR or FXR pathways. a Vitamin D-pathway-dependent TLR activation of CAMP gene expression. TLR-signaling activates NF-κB binding and induces VDR and CYP27B1 expression, the enzyme that catalyzes the conversion of 25(OH)D to 1,25(OH)2D. The expression of the vitamin D receptor (VDR) is increased. In the presence of locally high levels of 1,25(OH)2D, ligand-bound VDR:RXR heterodimers translocate into the nucleus and bind to the VDRE in the promoter of the human CAMP gene inducing its expression. b Vitamin D-mediated regulation of the human DEFB4 gene. (1) Direct induction: TLR stimulation activates the vitamin D pathway as described in a. Also it up-regulates the expression of IL-1β and IL-1R1 and down-regulates the expression of IL-1R antagonist (IL-1RA). IL-1R1 activates the NF-κB transcription factor that binds to the promoter proximal NF-κB binding site in the DEFB4 gene and induces HBD-2 expression together with the VDR:RXR heterodimer that binds to the VDRE in the promoter of DEFB4 gene. Indirect induction: In the presence of 1,25(OH)2D3, the VDR:RXR heterodimer binds to the VDRE in the NOD2 gene promoter and induces the expression of the NOD2 protein. Activation of NOD2 by its agonist muramyl dipeptide stimulates the NF-κB transcription factor that binds to the promoter proximal NF-κB binding site in the DEFB4 gene to induce its expression. c Regulation of human CAMP gene expression by vitamin D and bile salts in biliary cells. (1) 1,25(OH)2D3 activates the VDR:RXR heterodimer that then binds to the VDRE in the CAMP gene promoter; (2) UDCA activates the ERK1/2 signaling pathway that, in turn, induces VDR protein expression and induction of CAMP gene expression in the presence of 1,25(OH)2D3; and (3) CDCA binds to the FXR:RXR heterodimer that binds to the VDRE in the CAMP gene promoter
Interestingly, the vitamin D-mediated induction of CAMP potentially boosts antimicrobial activity against pathogens through direct killing by LL-37 and enhancing phagosome maturation [37]. In THP-1 and human primary monocytes, 1,25(OH)2D3 triggers the formation of autophagosomes and autophagolysosomes via a hCAP18/LL-37-mediated pathway [37].
Defensins
The HBD-2 gene (DEFB4) was induced by 1,25(OH)2D3 through a VDRE in the promoter, but the induction was not as robust as that observed for the CAMP gene [30]. Also, the induction of the HBD-2 gene in macrophages requires TLR activation and the convergence of the IL-1β and vitamin D pathways (Fig. 1b) [38]. In vitro studies demonstrated that activation of intracellular pattern recognition receptor nucleotide-binding oligomerization domain protein 2 (NOD2) by its ligand muramyl dipeptide (MDP), a lysosomal breakdown product of peptidoglycan from both gram-negative and gram-positive bacteria, induced the expression of the HBD-2 gene [39]. More recently, 1,25(OH)2D3 was shown to strongly induce the expression of NOD2/CARD15/IBD1 in primary human monocytic and epithelial cells [40]. In the absence of 1,25(OH)2D3, the activation of NOD2 by MDP activates NF-κB and there is a modest induction of the HBD-2 gene; however, pretreatment with 1,25(OH)2D3 followed by MDP leads to a robust, synergistic induction of the HBD-2 gene (Fig. 1b) [40]. Activation of the vitamin D pathway alone is not sufficient to induce robust expression of HBD-2, and additional signaling pathways are required [38, 40]. Treatment of normal human keratinocytes with 1,25(OH)2D3 increased HBD-3 mRNA levels in a dose-dependent manner [41]. Interestingly, treatment of lesional psoriatic plaques with the vitamin D analog calcipotriol increased IκB-α protein levels inhibiting the NF-κB signaling pathway and blocked IL-17A-induced expression of HBD-2 in the plaques [42]. Down-regulation of HBD-3 was also observed in the lesional psoriatic plaques after treatment with calcipotriol [42].
Primary and secondary bile acids
Bile acids play an important role in digestion and absorption of dietary fat and nutrients by the digestive system. They bind to and activate many nuclear receptors and thus modulate metabolism. In humans, the two major primary bile acids are cholic acid (CA) and chenodeoxycholic acid (CDCA) [43]. CDCA binds to farnesoid X receptor (FXR) [44] and up-regulates cathelicidin expression (Fig. 1c) [45]. This regulation may contribute to the sterility of the bile duct.
Lithocholic acid (LCA), a toxic secondary bile acid, is a by-product of CDCA metabolism by bacteria in the colon. It is a low-affinity ligand for FXR, pregnane X receptor (PXR) as well as the VDR [46-48]. LCA binds to the VDR and significantly increases both human CAMP transcript and protein levels in human primary keratinocytes (NHEK) in a time- and dose-dependent manner [49]. In the human colonic epithelial cell line HT-29, LCA and butyrate act synergistically to induce human CAMP gene expression [50]. The LCA derivatives LCA acetate and LCA propionate induced expression of human CAMP more efficiently than LCA itself and were less toxic in tissue culture cells and mice, suggesting they are more potent VDR agonists and might be safer potential therapeutic agents than LCA [51]. Unlike LCA, the secondary bile acid ursodeoxycholic acid (UDCA) is not a ligand for the VDR, but rather increases the levels of VDR in the nucleus that, in turn, increases CAMP expression (Fig. 1c) [45].
Butyrate
Camp
Butyrate is a short-chain fatty acid produced in the colon by fermentation of dietary fiber by anaerobic bacteria. It is found in many foods, such as butter and cheeses, and is a histone deacetylase inhibitor (HDACi) [52]. Shigella infections down-regulate LL37 and β-defensin-1 levels in the colon of adult patients [53], and rabbit CAP18 levels in colon surface epithelium are reduced during Shigella infection; however, CAP18 can be restored after oral administration of sodium butyrate [54].
Because of its unpleasant smell, butyrate is rarely used in clinical trials and instead 4-phenylbutyrate (PBA), an odorless and palatable derivative of butyrate, is used clinically. In several human cell lines PBA up-regulates CAMP gene expression more potently than butyrate [55]. It was recently shown that PBA also counteracts the down-regulation of cathelicidin in both the colon and lung by Shigella in rabbits [56]. Furthermore, butyrate and its derivatives up-regulate transcription of cathelicidin in human colon epithelial cells [57] and synergistically induces human CAMP mRNA levels with 1,25(OH)2D3 in lung epithelial and myeloid cells [32, 55]. A chemical analog of PBA, α-methylhydrocinnamate (ST7), can also dramatically up-regulate human CAMP mRNA transcription [55]. These studies support a potential role for sodium butyrate and its derivatives in the treatment of human infections.
Defensins
There are few publications reporting the regulation of defensins by butyrate; however, it has been shown to induce HBD-2 mRNA expression in colonocytes [58], and pretreatment of gingival epithelial cells with sodium butyrate significantly induced HBD-2 expression in response to bacterial challenge [59].
Sulforaphane
Defensins
Sulforaphane (SFN) is a dietary HDACi found in cruciferous vegetables (e.g., broccoli and broccoli sprouts) that reactivates epigenetically silenced genes [60]. Recently, it was demonstrated that SFN induces HBD-2 mRNA and protein expression in the Caco-2 human colon cancer cell line in a time- and dose-dependent manner [58]. Inhibition of VDR by an antagonist significantly blocked the SFN-induced HBD-2 mRNA expression in these cells, and SFN treatment increased the expression of VDR in both Caco-2 and HT-29 cell lines, indicating that induction of HBD-2 by SFN was mediated by the vitamin D pathway [58]. Whether these observations were due to epigenetic changes was not examined. The MAPK/ERK and NF-κB signaling pathways were also involved [58]. It has not been determined whether cathelicidin expression is regulated by SFN.
Retinoic acid
Retinoic acid (RA) is a metabolite of vitamin A, which is important in several aspects of immune function [61]. The retinoic acid receptor (RAR) forms a heterodimer with the retinoid X receptor (RXR) and interacts with specific retinoic acid response elements (RAREs) in the promoters of target genes [62]. All-trans RA was shown to induce both porcine cathelicidin, PR-39 expression [63] and hCAP18 promoter activity [64]. In contrast, induction of HBD-2, -3 and -4 by Ca2+, TNF-α, IL-1β, INF-γ, PMA or Pseudomonas aeruginosa in human keratinocytes is inhibited by RA [65]. The promoter regions of all the inducible human β-defensin genes have several possible AP-1 binding sites [65], and RA may suppress the expression of these genes by antagonizing AP-1 (c-Jun/c-Fos)-mediated gene expression pathways [66]. Therefore, RA may impair the innate immune response in human skin, thus increasing susceptibility to infections during RA therapeutic treatment [65]. On the other hand, α-defensin HNP-1 was induced by both all-trans RA and 9-cis-RA in a dose-dependent manner, suggesting that retinoic acid may be important for myeloid cell expression of HNP-1 [67].
Human health
Increasing endogenous cathelicidin and defensin expression may be particularly useful in the treatment of infections. Clinical studies have shown that asthma patients have reduced hCAP18 levels [68]. High levels of circulating hCAP18 in hemodialysis patients at the beginning of their treatment was indicative of a significant decrease in 1-year mortality and there was a modest correlation with 1,25(OH)2D3 levels, but not with 25(OH)D levels [19]. In sepsis patients, lower 25(OH)D, vitamin D binding protein (DBP) and cathelicidin levels were associated with severe illness, and a positive correlation between 25(OH)D and cathelicidin levels was seen in all subjects [69]. Additional studies are needed to substantiate these latest findings and determine whether supplementation of vitamin D-deficient individuals with vitamin D or therapy with active analogs of 1,25(OH)2D3 would boost plasma levels of cathelicidin and thus increase the protection against infection and sepsis.
Supplementation may increase cathelicidin and defensin expression in tissues and immune cells, thus enhancing barrier function. Atopic dermatitis patients suffer from frequent skin infections; therefore, induction of CAMP expression in the skin may increase protection from infection [70]. Patients supplemented with 4,000 IU/d of oral vitamin D for 21 days showed increased cathelicidin expression in skin lesions and a mild increase in unaffected skin, but a decrease in skin infection was not determined [70]. Ex vivo infection of urinary bladder biopsies from post-menopausal women after vitamin D supplementation resulted in an increased induction of the CAMP gene and protein expression when compared to biopsies taken prior to supplementation [71]. The studies to date would argue that it is important for individuals to have sufficient serum levels of 25(OH)D to allow for the production of adequate levels of cathelicidin during infection.
Epidemiological studies link vitamin D deficiency and increased rates of respiratory infections and there is interest in using vitamin D supplementation to reduce influenza infections and treat tuberculosis [72]. In a small randomized trial of school-age children, the vitamin D-supplemented group showed a nearly twofold reduction in influenza A rates than in the placebo group [73]. In another study with participants from different racial groups, it was shown that the maintenance of serum 25-hydroxyvitamin D levels at 38 ng/ml or higher was correlated with a twofold reduction in the incidence of acute viral respiratory tract infections [74]. In a double-blind randomized control trial of tuberculosis patients starting treatment, vitamin D supplementation did not significantly affect the time that patient sputum cultures converted from positive to negative for Mycobacterium tuberculosis growth in the study population as a whole; however, a significantly shortened time of conversion was observed in a sub-group of participants with the tt genotype of the TaqI vitamin D receptor polymorphism [75]. The latter study demonstrates the importance that genetic differences among individuals may play in the outcomes of trials involving supplementation.
In contrast to the positive findings above, a number of negative studies with vitamin D have been reported. A randomized controlled trial of vitamin D3 supplementation showed that there was no obvious difference in the incidence and duration of severity of upper respiratory tract infections (URIs) between vitamin D (2,000 IU/day) and placebo groups although 25(OH)D level increased significantly after 12 weeks in the vitamin D group compared to the placebo group [76]. Another study found that there was no difference in serum 25(OH)D levels between groups of patients aged 1-25 months admitted to hospital with uncomplicated acute lower respiratory tract infection (ALRI) and healthy, similarly aged patients without a history of hospitalization for ALRI. This study suggested that vitamin D status was not a risk factor in hospitalization for ALRI [77]. In a randomized, double-blind, placebo-controlled trial in TB clinics, the intervention and placebo groups were given 100,000 IU of cholecalciferol or vegetable oil at inclusion and again at 5 and 8 months after the start of treatment, but no significant difference was observed between the groups on mortality in patients with TB [78].
Very recently, a randomized, double-blind, placebo-controlled clinical trial to determine the efficacy of sodium butyrate as an adjunct therapy with antibiotics in the treatment of shigellosis in patients was performed. Reduced rectal luminal content of inflammatory cells and pro-inflammatory cytokines, increased expression of LL-37 in the rectal epithelia and improved rectal histopathology as compared to the placebo group were evident [79]. Nevertheless, the efficacy of sodium butyrate treatment in clinical recovery was not observed as in studies with rabbits [54, 79]. Important differences between the animal and human studies included oral delivery in rabbits versus delivery by enema in the humans and the lack of antibiotics in rabbits [79]. It remains to be determined whether oral delivery of butyrate compounds would be more efficacious as the treatment would not be eliminated as quickly as it is with an enema due to repeated bouts of diarrhea [79].
Conclusions
Accumulating evidence demonstrates that nutrients and microbial by-products derived from the metabolism of dietary factors may play a critical role in modulating the innate immune response via regulation of AMP gene expression. The by-products of gut microbe metabolism are potentially important for increasing AMP expression by epithelial cells of the luminal lining of the digestive tract, thus establishing a mucosal barrier preventing contact of microbes and pathogens with the intestinal epithelium [12, 50]. Increasing the consumption of dietary fiber, thus increasing short-chain fatty acid production, and/or food containing dietary HDAC inhibitors could increase the expression of AMPs in the digestive tract. This in turn would increase the barrier protection in the gut lumen and reduce the rate or severity of intestinal infections. This is nicely demonstrated in the studies using a rabbit model of shigellosis [54, 56] and has shown some promise in a human trial [79].
The potential for VDR and FXR to bind to the VDRE in the human CAMP gene offers an abundance of new compounds that can be synthesized or obtained from the diet to produce potent ligands for these steroid hormone receptors and used to enhance AMP expression. For example, the therapeutic use of active vitamin D is hampered by the toxic side effects of hypercalcemia [12], and although synthetic analogs reduce these side effects, they still exist. On the other hand, analogs of LCA have been shown to lack this side effect, but activate VDR-target genes like CAMP [51].
The VDR may act as a receptor for additional nutritional ligands including curcumin and polyunsaturated fats including α-linolenic acid, docosahexaenoic acid, eicosapentaenoic acid and arachidonic acid [80]; however, we recently demonstrated that in tissue culture, only curcumin modestly induced CAMP gene expression through a yet uncharacterized VDR-independent mechanism (Guo et al. in press). Identification of additional nutritional compounds and their synthetic analogs would provide further options for increasing AMP gene expression for therapeutic uses.
AMPs are critical to the barrier defense provided by the innate immune system, and deficits in AMP production can increase susceptibility to infections. As discussed, numerous nutritional compounds and microbial by-products from metabolism of dietary factors have been shown to regulate the expression of AMPs. The possibility of improving the innate immune response or boosting barrier defenses toward infection by increasing the consumption of food items rich in these nutrients is an exciting prospect, but well-designed clinical and animal model studies need to be performed to demonstrate that compounds obtained from the diet can improve immune function by increasing AMP levels.
Acknowledgments
We thank members of the laboratory for critically reading this manuscript. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [5R01AI065604 to A.F.G.].
References
- 1.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 2.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. doi:10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 4.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. doi:10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 5.Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv. 2003;21:465–499. doi: 10.1016/s0734-9750(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 7.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 8.Andersson E, Sorensen OE, Frohm B, Borregaard N, Egesten A, Malm J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum Reprod. 2002;17:2529–2534. doi: 10.1093/humrep/17.10.2529. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845–850. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. doi:10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 11.Agerberth B, Gudmundsson GH. Host antimicrobial defence peptides in human disease. Curr Top Microbiol Immunol. 2006;306:67–90. doi: 10.1007/3-540-29916-5_3. [DOI] [PubMed] [Google Scholar]
- 12.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. doi:10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 14.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol. 2001;167:3329–3338. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 16.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock RE. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 17.Kirikae T, Hirata M, Yamasu H, Kirikae F, Tamura H, Kayama F, Nakatsuka K, Yokochi T, Nakano M. Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against murine endotoxemia. Infect Immun. 1998;66:1861–1868. doi: 10.1128/iai.66.5.1861-1868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 19.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–424. doi: 10.1086/596314. doi:10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond G, Bevins CL. beta-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 21.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 23.Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–525. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. doi:10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Recent advances in the research and development of human defensins. Peptides. 2006;27:931–940. doi: 10.1016/j.peptides.2005.08.018. doi:10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaalouk TK, Bajaj-Elliott M, George JT, McDonald V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect Immun. 2004;72:2772–2779. doi: 10.1128/IAI.72.5.2772-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics. 2010;125:1237–1247. doi: 10.1542/peds.2009-3289. doi:10.1542/peds.2009-3289. [DOI] [PubMed] [Google Scholar]
- 29.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. doi:10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 31.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. doi:10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 32.Gombart AF, O’Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)2D3 in various tissues. J Steroid Biochem Mol Biol. 2007;103:552–557. doi: 10.1016/j.jsbmb.2006.12.095. doi:10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 33.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. doi:10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 34.Gombart A, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. doi:10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 36.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. doi:10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. doi:10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. doi:10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. doi:10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 40.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. doi:10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yang L, Hirakawa S, Hashimoto K. PPAR-gamma mediates innate immunity by regulating the 1alpha, 25-dihydroxyvitamin D3 induced hBD-3 and cathelicidin in human keratinocytes. J Dermatol Sci. 2010;60:179–186. doi: 10.1016/j.jdermsci.2010.09.008. doi:10.1016/j.jdermsci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Buchau A, Steinmeyer A, Zugel U, Ruzicka T, Schauber J. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS One. 2009;4:e6340. doi: 10.1371/journal.pone.0006340. doi:10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. doi:10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 45.D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C, Chignard N. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–1443. doi: 10.1053/j.gastro.2008.12.040. doi:10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. doi:10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 47.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11:160–166. doi: 10.1007/s11892-011-0187-x. doi:10.1007/s11892-011-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Ruzicka T, Schauber J. VDR and MEK-ERK dependent induction of the antimicrobial peptide cathelicidin in keratinocytes by lithocholic acid. Mol Immunol. 2009;46:3183–3187. doi: 10.1016/j.molimm.2009.08.010. doi:10.1016/j.molimm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Termen S, Tollin M, Rodriguez E, Sveinsdottir SH, Johannesson B, Cederlund A, Sjovall J, Agerberth B, Gudmundsson GH. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Mol Immunol. 2008;45:3947–3955. doi: 10.1016/j.molimm.2008.06.020. doi:10.1016/j.molimm.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, Shimizu M, Iwasaki K, Yamada S, Makishima M. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res. 2008;49:763–772. doi: 10.1194/jlr.M700293-JLR200. doi:10.1194/jlr.M700293-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Karagiannis TC, El-Osta A. The paradox of histone deacetylase inhibitor-mediated modulation of cellular responses to radiation. Cell Cycle. 2006;5:288–295. doi: 10.4161/cc.5.3.2421. [DOI] [PubMed] [Google Scholar]
- 53.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–185. doi: 10.1038/84627. doi:10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 54.Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. doi:10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53:5127–5133. doi: 10.1128/AAC.00818-09. doi:10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, Bergman P, Gudmundsson GH, Agerberth B, Raqib R. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One. 2011;6:e20637. doi: 10.1371/journal.pone.0020637. doi:10.1371/journal.pone.0020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. doi:10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwab M, Reynders V, Loitsch S, Steinhilber D, Schroder O, Stein J. The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology. 2008;125:241–251. doi: 10.1111/j.1365-2567.2008.02834.x. doi:10.1111/j.1365-2567.2008.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4:409–419. doi: 10.1038/mi.2010.83. doi:10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nian H, Delage B, Ho E, Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 2009;50:213–221. doi: 10.1002/em.20454. doi:10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. doi:10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamashev D, Vitoux D, De The H. PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation. J Exp Med. 2004;199:1163–1174. doi: 10.1084/jem.20032226. doi:10.1084/jem.20032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H, Zhang G, Minton JE, Ross CR, Blecha F. Regulation of cathelicidin gene expression: induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar typhimurium infection. Infect Immun. 2000;68:5552–5558. doi: 10.1128/iai.68.10.5552-5558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5′UTR negative regulatory element. Mol Immunol. 2008;45:204–217. doi: 10.1016/j.molimm.2007.04.023. doi:10.1016/j.molimm.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–529. doi: 10.1111/j.0022-202X.2004.23234.x. doi:10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 66.Schule R, Rangarajan P, Yang N, Kliewer S, Ransone LJ, Bolado J, Verma IM, Evans RM. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci USA. 1991;88:6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang N, Su Q, Boeckh-Herwig S, Yaneva M, Tempst P. Delayed-late activation of a myeloid defensin minimal promoter by retinoids and inflammatory mediators. Leuk Res. 2004;28:879–889. doi: 10.1016/j.leukres.2003.12.005. doi:10.1016/j.leukres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128:2316–2326. doi: 10.1378/chest.128.4.2316. doi:10.1378/chest.128.4.2316. [DOI] [PubMed] [Google Scholar]
- 69.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. doi:10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, Kanada K, Yamasaki K, Alexandrescu D, Gallo RL. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. doi:10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hertting O, Holm A, Luthje P, Brauner H, Dyrdak R, Jonasson AF, Wiklund P, Chromek M, Brauner A. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS One. 2010;5:e15580. doi: 10.1371/journal.pone.0015580. doi:10.1371/journal.pone.0015580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 73.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. doi:10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 74.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. doi:10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. doi:10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–1404. doi: 10.1017/S0950268809002404. doi:10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 77.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr. 2009;63:297–299. doi: 10.1038/sj.ejcn.1602946. doi:10.1038/sj.ejcn.1602946. [DOI] [PubMed] [Google Scholar]
- 78.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. doi:10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 79.Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman AS, Rekha RS, Andersson J, Gudmundsson GH, Cravioto A, Agerberth B. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo controlled clinical trial. BMC Infect Dis. 2012;12:111. doi: 10.1186/1471-2334-12-111. doi:10.1186/1471-2334-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, Hsieh JC, Kaczmarska M, Haussler CA, Haussler MR. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl 2):V2–V10. doi: 10.1359/jbmr.07s216. doi:10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]