Abstract
Acupuncture is a form of Eastern medicine that has been practiced for centuries. Despite its long history and worldwide application, the biological mechanisms of acupuncture in relieving pain have been poorly defined. Recent studies in mice, however, demonstrate that acupuncture triggers increases in interstitial adenosine, which reduces the severity of chronic pain through adenosine A1 receptors, suggesting that adenosine-mediated antinociception contributes to the clinical benefits of acupuncture. We asked here whether acupuncture in human subjects is also linked to a local increase in interstitial adenosine concentration. We collected microdialysis samples of interstitial fluid before, during, and after delivering 30 minutes of conventional acupuncture in the Zusanli point in human subjects. The interstitial adenosine concentration increased significantly during acupuncture and remained elevated for 30 minutes after the acupuncture. Acupuncture-mediated adenosine release was not observed if acupuncture was not delivered in the Zusanli point or if the acupuncture needle was inserted, but not rotated. This study strengthens the role of adenosine in acupuncture-mediated antinociception by directly providing such evidence in humans.
Keywords: Purinergic signaling, adenosine, antinociception, acupuncture, human
Chronic pain is a heterogeneous group of medical conditions that have significant impact on an individual’s physical, emotional, psychological, and financial distress. Chronic pain affects more than 100 million Americans and costs the nation up to $635 billion each year.13 The medical costs and loss of productivity also pose a significant financial burden to the society.20,38 Unfortunately, commonly used analgesics including nonsteroidal anti-inflammatory drugs and opioids often pose serious side effects, contraindications, physical dependence, and tolerance.23,25 Alternative treatments are thus critically needed.
Acupuncture is one of the most common nonpharma-cological pain-relieving alternative treatments and is being practiced worldwide.35 Despite its long history and worldwide practice, the mechanism of acupuncture-mediated analgesia has not been fully elucidated. One proposed mechanism involves the release of opioid peptides in the central nervous system in response to the long-lasting activation of ascending tracts during the intermittent stimulation.10,12,39 However, acupuncture is conventionally applied in close proximity to the locus of pain and the analgesic effects of acupuncture are often restricted to the ipsilateral side, thus pointing to a peripheral and local action of acupuncture.21 Consistent with this observation, using mouse models of chronic neuropathic or inflammatory pain, we found in a recent study that the analgesic effects of acupuncture were mediated by a local release of adenosine and activation of adenosine A1 receptors.8 Adenosine is a purine nucleoside that previously has been shown to have analgesic effects.28,30,36,41 Given the potential clinical and mechanistic impact of these animal studies to human acupuncture, we sought to further characterize this pathway in human subjects.
In the present study, human volunteers received conventional acupuncture in the Zusanli point (ST36), medial and lateral to the midline of the knee as described below and in our previous study.8 Our results indicate that acupuncture triggers adenosine release in humans comparable to that previously observed in mice. Interestingly, acupuncture needle placement in a nonacupuncture point in equal distance to the microdialysis probe did not trigger significant increases in local adenosine concentration. Similarly, insertion of the acupuncture needle without rotations also did not lead to significant increases in adenosine concentration. Thus, this study provides further credence to the critical role of peripheral adenosine in acupuncture-induced nociception.
Methods
Acupuncture and Microdialysis
All procedures were approved by the National Basic Research Program of China (973 Program, 2007CB51 03, Beijing Tiantan Hospital, Beijing, China). The 15 subjects enrolled in the study were healthy male volunteers, 24 to 30 years of age with weight ranging from 65 to 75 kg. None were taking medications during the period of this study, and those with a history of impaired renal and/or hepatic function were excluded. All volunteers were given a detailed description of the study and their written consent was obtained. During the procedure, the subjects were sitting in a comfortable chair with the right leg extended on a small table and were asked to keep their leg relaxed and motionless. Lidocaine (.4%) was administered subcutaneously in the skin at 5 cm caudal to the Zusanli acupoint prior to insertion of sterile human microdialysis probes (CMA 60; CMA Microdialysis, Stockholm, Sweden).1 Microdialysis probes (30-mm dialysis membrane length and .6-mm outer diameter, 20 kDa cut-off) were inserted so that the tip of the probe was positioned at .5 cm to the Zusanli acupoint aiming upward toward the peroneal nerve as shown in Fig 1. Saline was perfused at a rate of 1 μL per minute. After 150 minutes of equilibration period, the dialysates were collected every 30 minutes for an additional 120 minutes. One dialysate was collected before and 2 dialysates were collected after the 30-minute session of acupuncture, which was performed at the Zusanli point. In practice, the acupuncturist uses the patient’s fingers to adjust for differences in body size. Using this method, the Zusanli point is localized 4 fingers below the patella and 1 midfinger lateral to the midline.33 The acupuncture needle (.35-mm diameter, 75-mm total length; Hwato, Suzhou Medical Appliance Factory, Suzhou City, Jiangsu Province, China) was inserted vertically and rotated moderately to attain the propagated “Qi” reaction,18 alternating between 5 minutes of continuous, bidirectional rotations (2–3 rotations per direction) and 5 minutes of rest for 30 minutes, after which the acupuncture needle was removed. Samples were collected on ice every 30 minutes and immediately frozen at −80°C until high-performance liquid chromatography (HPLC) analysis. Additionally, 5 subjects received 1 more microdialysis probe at the exact location in the left leg in addition to the right leg. Dialysates were separately collected simultaneously, and the right leg received acupuncture as described above. Samples collected from the left leg were treated as control to the acupuncture effect. Alternatively, 5 subjects also received acupuncture but the needle was left untouched without rotations for the 30-minute acupuncture session. Another 5 subjects received acupuncture as described above, with rotations, but the needle was inserted at 2 cm lateral to the Zusanli acupoint with the needle tip aiming within .5 cm to the probe. In order to assess the effect of muscle movement, subjects were asked to gently move the tibialis anterior muscle during the 30-minute sample collection by ankle flexion-extension 15 times per minute without moving knee position.
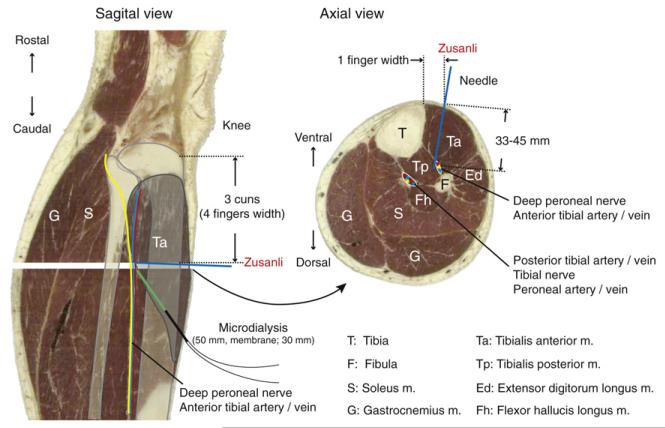
Figure 1.
Anatomical localization of the Zusanli acupoint. The Zusanli point is located close to the deep peroneal nerve as indicated in sagittal and axial cross-sections of a human leg (images used with permission from The Visible Human Project, NIH). Microdialysis probe was inserted distally and pushed upward pointing towards the deep peroneal nerve. The tip of the microdialysis probe is aimed at Zusanli point. Position of the deep peroneal nerve (yellow) and anterior tibial artery (red)/vein (blue) is indicated.
HPLC Analysis of Purines
The HPLC analyses were carried out using CoulArray 5600A HPLC System (ESA Inc, Sunnyvale, CA) and a model 526 UV detector (ESA Inc) as previously described.8 The relative recovery of the probe was calculated using an in vitro procedure in which the probe was dialysed against known concentrations (1–3 μM) of purines dissolved in saline (for CMA 60 probes) or in Ringer’s solution (for MD-2212 probes, 2-mm dialysis membrane length and .22-mm outer diameter, 38 kDa cut-off; Bioanalytical Systems, West Lafayette, IN) and was perfused at 1 μL/min for 30 minutes. The concentration of each purine in dialysates was divided by its known concentration to obtain the probe relative recovery rate.
Statistics
Data were expressed as mean ± standard deviation (SD), or mean ± standard error of the mean (SEM) where we made comparisons among the different groups. Outliers of the data were rejected based on Grubb’s test (α = .05), and normality of the data was examined with Shapiro-Wilk test (α = .05). Statistical significance was evaluated with Student t-test, paired t-test, analysis of variance (ANOVA), or repeated measures ANOVA with the Tukey-Kramer test.
Results
Prior to the experiment, the Zusanli acupoint was localized in each subject according to traditional description methods (see Methods section).37 The microdialysis probe was inserted at 5 cm caudal in the angle toward the Zusanli point so that the probe tip was positioned within .5 cm from the acupuncture needle tip when the needle was inserted at Zusanli (Fig 1). Collection of steady-state baseline dialysis sample was started 150 minutes after the implantation of the probe. The acupuncture needle was rotated every 5 minutes for 30 minutes and then removed. As illustrated in the flow chart of experimental design (Fig 2A, left panel), after a period of 150 minutes equilibration, dialysates were collected for 30 minutes and measured for the baseline levels of purines using HPLC. Adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monoposphate (AMP), and adenosine concentrations in the extracellular space are shown in the representative samples taken from 1 subject (Fig 2A, right panel). Two subjects were excluded from the study due to unusually high purine concentrations, indicative of muscle movement (see the latter part of the section). The remaining 13 subjects exhibited a baseline adenosine level ranging from 171.4 to 633.4 nM (Fig 2B). During the 30-minute session of acupuncture, a transient increase of adenosine was observed in all but 1 subject (Fig 2B). Out of the 12 responding subjects in the subsequent 2 fractions of dialysates following the completion of acupuncture (referred hereafter as Recovery 1 and 2 samples), 10 subjects showed gradual decrease of the concentrations of adenosine over time. In average of the 13 subjects, acupuncture caused significant transient increase of adenosine from 317.6 ± 132.6 nM (mean ± SD) baseline to 529.7 ± 200.3 nM (mean ± SD) during acupuncture (p < .01, repeated measures ANOVA with Tukey-Kramer; Fig 2C). In the subsequent samples the level of adenosine in Recovery 1 and 2 showed gradual decrease (470.4 ± 193.7 nM and 349.9 ± 145.4 nM, respectively) toward the baseline value. AMP concentration changes failed to show statistical significance (p > .05, Tukey-Kramer), from a baseline of 313.3 ± 135.2 nM to 462.3 ± 237.8 nM during acupuncture, then to (403.1 ± 229.5 nM) in Recovery 1 samples, and (381.7 ± 246.8 nM) in Recovery 2 samples (Fig 2C). Likewise, ADP and ATP exhibited less consistent changes in response to acupuncture. Concentrations in dialysates were 307.8 ± 211.3 nM ADP and 225.5 ± 156.7 nM ATP in baseline, then 350.3 ± 227.0 nM ADP and 248.1 ± 186.0 nM ATP during acupuncture. The concentrations remained high for the recovery phases, with 386.6 ± 266.6 nM ADP and 204.1 ± 126.1 ATP in the Recovery 1 period, and 330.8 ± 291.2 nM ADP and 241.7 ± 212.0 nM ATP in the Recovery 2 period.
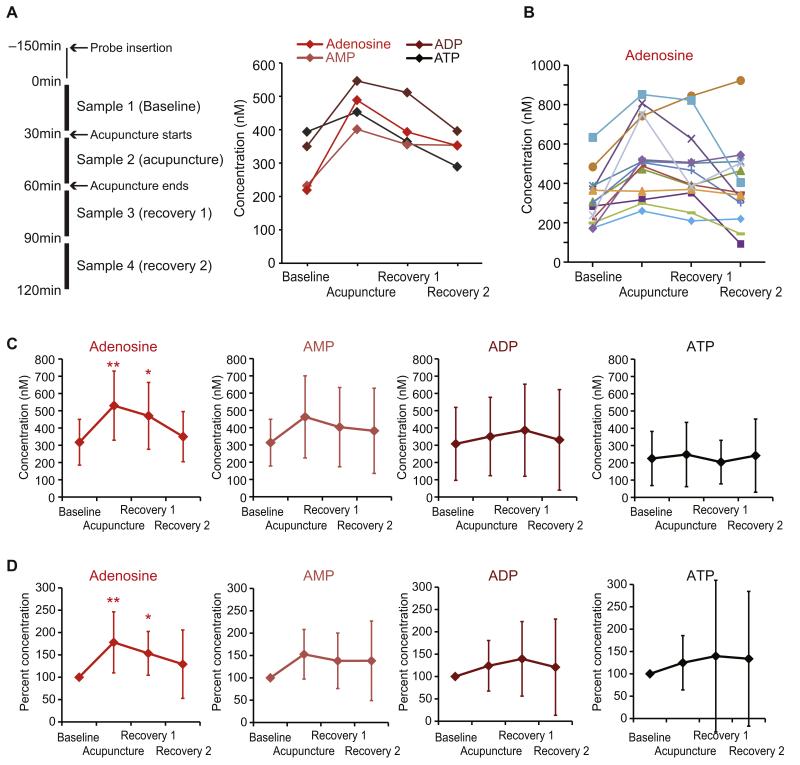
Figure 2.
Effects of acupuncture on interstitial concentrations of adenosine, AMP, ADP, and ATP. (A). Left: Experimental design. Microdialysis samples (30 μL) were collected every 30 minutes during the experiment. Right: Measurements of adenosine, AMP, ADP, and ATP in samples from a single representative subject. Purine concentrations are plotted as a function of time, before (Baseline), during (Acupuncture), and after (Recovery 1 and Recovery 2) acupuncture. (B) Interstitial concentration of adenosine for each of 13 subjects. Lines with different colors represent different subjects. (C) Summary of changes of all subjects in the interstitial concentration of adenosine, AMP, ADP, and ATP before, during, and after acupuncture (mean ± SD; **p < .01; *p < .05). (D) Changes in concentrations of purines observed in (C) were normalized by their baseline values for each subject (baseline was set to 100%; mean ± SD; **p < .01; *p < .05).
Because the baseline concentration values were highly variable, the changes in the concentrations of purines above were normalized to their respective baseline values and summarized in Fig 2D. Adenosine exhibited a relative increase during acupuncture, averaging 178.1 ± 19.0% (mean ± SEM, p < .01, repeated measures ANOVA with Tukey-Kramer). By comparison, the changes in other purines were statistically insignificant (p > .05, repeated measures ANOVA). During acupuncture, AMP was 152.7 ± 15.4%, ADP 124.0 ± 15.7%, and ATP 124.8 ± 18.3% relative to baseline. After acupuncture, adenosine remained significantly high, at 153.4 ± 13.6% at Recovery 1 relative to baseline (mean ± SEM, p < .05, repeated measures ANOVA with Tukey-Kramer). In the Recovery 2 period, adenosine was reduced to 129.2 ± 22.1% of baseline, whereas the changes of the rest of the purines were highly variable and thus not statistically significant overall (Fig 2D).
Next we attempted to examine if the changes of interstitial purines were unique to the tissue at the acupuncture position, with needle stimulation properly applied, and only when inserted at the acupoints. First, using 2 microdialysis probes inserted in both legs, we measured the purine concentrations at the same location of the leg that did not receive acupuncture while acupuncture was given to the other leg (Fig 3A). As reported in Fig 2, legs that received acupuncture showed increases of the adenosine (Fig 3B). The untreated opposite leg at the time of acupuncture at the other leg showed no changes in the concentration of all purines (Fig 3B). Normalized concentration change clearly showed that the opposite leg was not affected by the acupuncture, with 104.4 ± 19.2% for adenosine, 101.0 ± 24.3% for AMP, 95.7 ± 22.1% for ADP, and 98.4 ± 20.6% for ATP (n = 5, p > .05, t-test compared to respective baseline; Fig 3C). The adenosine concentration of the acupuncture side was significantly higher than the no-acupuncture side (p < .05, t-test compared right leg with left), indicating that the effect of acupuncture on tissue purines was local.
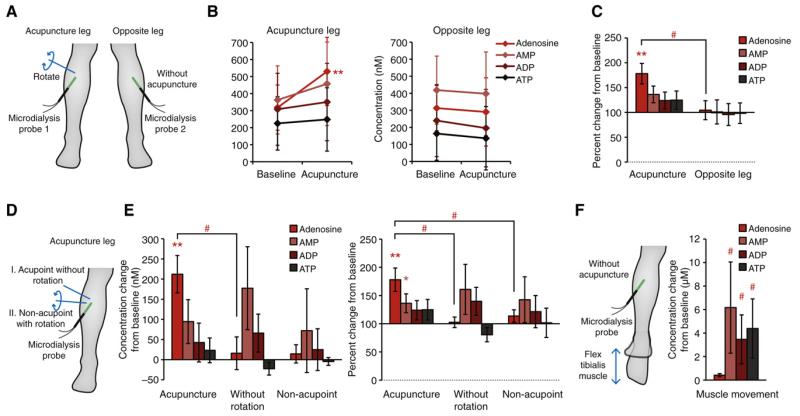
Figure 3.
Comparisons of acupuncture procedures on concentrations of adenosine, AMP, ADP, and ATP. (A) Experimental design. One microdialysis probe was inserted in a leg where acupuncture was applied, and a second microdialysis probe was inserted in the opposite leg to simultaneously collect samples while the other leg received acupuncture. (B). Increase in the interstitial concentration of adenosine, AMP, ADP, and ATP before and after acupuncture simultaneously collected from acupuncture-receiving legs and untreated opposite legs (n = 5–11; mean ± SD; *p < .05 compared to baseline). (C) Changes in concentrations of purines from acupuncture-receiving legs and untreated opposite legs, normalized by their respective baseline values (baseline was set to 100%; n = 5–11; mean ± SEM; *p < .05 compared to baseline; #p < .05 compared to acupuncture side). (D) Experimental design. Each subject received either acupuncture needle insertion but the needle was left untouched without intermittent rotations during 30 minutes, or acupuncture needle insertion with rotations but the needle was inserted outside the Zusanli acupoint. (E) Comparison of effects of acupuncture with acupuncture needle insertion without rotating needle, or acupuncture needle insertion at non-acupoint. Changes in concentration from their respective baseline values (right panel, baseline set to 0 nM) and changes in concentration normalized by their respective baseline values (left panel, baseline set to 100%. n = 5; mean ± SEM; *p < .05 compared to baseline; #p < .05 compared to mechanical acupuncture). (F) Subjects gently moved their ankle during sample collection to flex the tibialis muscle. Muscular movement while the microdialysis probe was implanted caused large increases of purines in the tissue (baseline set to 0 μM. n = 5; mean ± SEM; #p < .05 compared to mechanical acupuncture).
Next, we examined whether the acupuncture effect was due to the mere needle insertion, or a mixture of needle insertion and subsequent rotations of the needle. The subjects received acupuncture needle insertion the same way; then the needle was left untouched for the 30-minute period (Fig 3D). The result was the drastic reduction of adenosine level compared to acupuncture with rotations (n = 5, p < .05, t-test; Fig 3E). In fact, unlike the result for acupuncture with rotations, adenosine failed to show an increase from the baseline (n = 5, p > .05, t-test; Fig 3E).
We then also delivered acupuncture (with needle rotations) to a position outside established acupoints to test if acupuncture only releases adenosine in the acupoints (Fig 3D). Unexpectedly, the analysis showed that the relative change of adenosine was significantly reduced when acupuncture needle was inserted 2 cm lateral to the acupoint (n = 5, p < .05, t-test; Fig 3E). In addition, adenosine showed little overall changes in response to acupuncture in both concentration shift from baseline value and normalized values from baseline (n = 5, p > .05, t-test; Fig 3E). While acupuncture resulted in a large increase of tissue adenosine level, both acupuncture without rotations and acupuncture at non-acupoint had little impact on adenosine level.
Lastly, we tested the effects of movements of the tibialis muscle where microdialysis was inserted. In our acupuncture experiment we have excluded data from 2 subjects due to the high baseline purine levels. We suspected that the high purine concentrations likely resulted from movement of the legs during sample collection. To test this, we asked the subjects to stretch and contract their tibialis muscle by gently moving their ankle during the sample collection (Fig 3F). This movement resulted in large increase of AMP (6.2 ± 3.9 μM), ADP (3.5 ± 2.1 μM), and ATP (4.4 ± 2.5 μM) compared to acupuncture procedure, where we failed to see the increases of these 3 purines (n = 5; p < .05, t-tests; Fig 3F). Interestingly, adenosine exhibited a smaller amplitude of increase compared to other purines. The concentrations of nucleotides were highly variable and generally higher, suggesting that the muscle movement primarily results in release of ATP, ADP, and AMP, but less so of adenosine.
To compare the effect of acupuncture on the extracellular purine concentration in humans and mice,6 we next estimated the interstitial concentration of purines. The microdialysis probes used in mice were much shorter than the probes used in human volunteers (Fig 4A). The relative recovery of purines at a 1 μL/min flow rate was high for microdialysis probe used for human sampling, ranging from 68 to 92% (n = 4; Fig 4A), and much lower in mice, ranging from 6 to 16% (Fig 4A). Based on the relative recovery for the 2 microdialysis probes, we next estimated the actual concentrations of extracellular purines: Baseline steady-state extracellular adenosine concentration was estimated to be 366 ± 42 nM in humans, which was comparable to previous reports, which were in the range of 150 to 700 nM.5,11,26,27 However, these values were much higher than the estimated extracellular adenosine concentration of 66 ± 42 nM in mice (Fig 4B). During acupuncture, the adenosine in human subjects increased to 609 ± 64 nM (Fig 4B) but to 1,586 ± 507 nM in mice (Fig 4B). Interestingly, the extracellular purine concentrations normalized much more rapidly in mice than in humans. At 0 to 30 minutes after acupuncture, adenosine level in mice was reduced to 18.5% of concentration during acupuncture, and it nearly returned to baseline level (76 ± 33 nM) in the samples collected at 30 to 60 minutes after acupuncture (Fig 4B). In contrast, normalization of the extracellular concentration of adenosine after acupuncture was much slower in humans, in the range of 542 ± 62 nM at 0 to 30 minutes, and 403 ± 48 nM at 30 to 60 minutes after acupuncture in human subjects (Fig 4B).
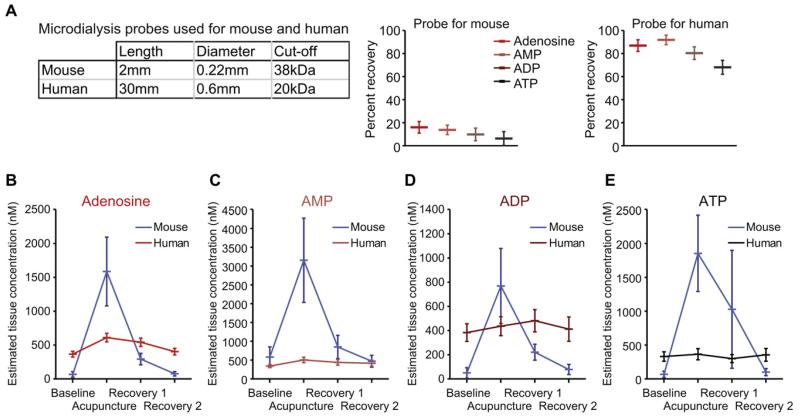
Figure 4.
Comparison of measurements of interstitial purine concentrations during acupuncture in humans and mice based on microdialysis. (A). Comparison of microdialysis probes used for human and mouse samplings. Upper table summarizes the physical characteristics of the probes, and lower histograms show the measured relative recoveries of adenosine, AMP, ADP, and ATP by the microdialysis probes. (B) Estimated interstitial concentrations of adenosine in humans (red) and mice (blue). Estimated tissue interstitial concentration of (C) AMP, (D) ADP, and (E) ATP in humans and mice.
Overall, this analysis shows that all purines, except for AMP, appeared to be present in higher concentrations at rest in humans than in mice. However, mice exhibited higher amplitudes of increases in purine during acupuncture and a greater capacity for rapid normalization following acupuncture (Figs 4C–E). Nevertheless, humans and mice displayed a similar pattern of purine release in response to acupuncture.
Discussion
This study used microdialysis to sample interstitial fluid in human subjects who received conventional acupuncture treatment. The microdialysis probe was placed along the peroneal nerve and samples of the interstitial fluid were collected before, during, and after acupuncture in the Zusanli point. Subsequent HPLC analysis of purines in the interstitial fluid samples showed that acupuncture induced a significant increase in adenosine concentration and that adenosine remained elevated for at least 30 minutes after the acupuncture needle was removed. The concentration of other purines including AMP, ADP, and ATP were also increased, albeit only AMP exhibited a significant elevation, suggesting that those purines may be quickly degraded to adenosine by extracellular nucleotidase.4,8 The pattern of acupuncture-induced changes in interstitial purine concentrations is in principle similar to earlier findings in mice. However, given that acupuncture needles are disproportionately large compared to the small body size of mice, it was important to obtain direct evidence in human volunteers who received conventional acupuncture. The observation that adenosine increases in human subjects during acupuncture strengthens the hypothesis that purinergic signaling plays a role in the analgesic actions of acupuncture by providing direct evidence in a clinically relevant setting.
Several lines of work indicate that acupuncture can produce analgesia by at least 2 distinct mechanisms acting centrally (endorphins) and peripherally.24 Based on mouse models of chronic neuropathic (nerve ligation) and inflammatory pain (Freund’s complete adjuvant injected into the plantar surface), our studies have shown that the interstitial concentration of adenosine increased in response to mechanical acupuncture and that acupuncture failed to reduce pain in transgenic mice lacking adenosine A1 receptors.8 Furthermore, local injection of an A1 receptor agonist in the Zusanli point potently reduced ipsilateral, but not contralateral, pain. Most importantly, systemic administration of a deaminase inhibitor, deoxycoformycin, prolonged the increase in adenosine concentration and the duration of acupuncture-induced analgesia almost 3-fold.8 When combined, these observations suggest that adenosine released during acupuncture binds to A1 receptors on afferent nerves that transmit pain information to spinal cord and temporarily reduces transmission of painful input. A1 receptors’ activation in dorsal root ganglions,29 afferent nerves,8,41 and nerve terminals17,28 have previously been linked to antinociception. This study expands prior work by demonstrating that conventional acupuncture in human subjects also is linked to significant increases in extracellular adenosine concentrations. The mouse studies demonstrated a significant increase in the extracellular concentrations of all purines analyzed, including ATP, ADP, AMP, and adenosine, suggesting that direct cellular injury triggers release of ATP, which subsequently is hydrolyzed in the extracellular space to adenosine.8 The present data collected from human subjects demonstrated a trend toward a general increase in the extracellular concentration of purine, albeit only adenosine reached significant levels. Technical differences related to the dimensions of the microdialysis probes (Fig 4A) may explain the difference in humans and mice. Additional studies are needed to firmly establish whether acupuncture in human subjects also triggers ATP release. Nevertheless, our data are compatible with the conclusion that the mechanism of acupuncture-induced purine release is shared by humans and mice.
The studies in neither humans nor mice established the source or mechanism of adenosine release. It is, however, of importance to note that remodeling of tissue fibroblast has been noted in response to mechanical acupuncture.15,16 Fibroblasts express high levels of connexin 43, which mediates purine release in multiple cell types.14,34
Differences in experimental designs may contribute to the difference observed in the resting baseline concentration of adenosine and the response to acupuncture in humans and mice. All experiments in mice were performed during anesthesia, while the human subjects were awake and alert. The local anesthetic did not likely play a major role, since its effect had worn off by the time sample collections begun. Also, local anesthetic was only applied where the microdialysis probe was inserted or at least 5 cm from the Zusanli point. The major difference between sample collections in the 2 species was the dimensions of the microdialysis probes. The probe used in human subjects was much larger than in mice, resulting in a recovery rate of ~80% in human volunteers versus 10% in mice. One consequence of the longer probe used in humans is that interstitial fluid collection includes tissue farther away from acupuncture point and thus effectively dampening the amplitude of acupuncture-induced purine release. The peak amplitudes of purine detected during acupuncture most likely underestimates the actual changes that occur in the acupuncture point. Another potential consequence of the larger microdialysis probe used in human subjects is that the tissue damage is more severe and the resting concentration therefore artificially high. Nevertheless, our measurements were within the range of other reports.5,11,26,27 It was not possible to use the same microdialysis probes as in mice, since we used the only commercial available microdialysis probes approved for use in human subjects.
Adenosine A1 receptors are Gi/o-coupled receptors, and similar to other Gi/o-coupled receptors, they are capable of initiating several intracellular signaling pathways, including inhibition of adenylate cyclase as well as with stimulation of phospholipase C.7,9 The inhibition of adenylate cyclase in response to adenosine A1 receptor activation is of particular interest, since chronic pain is linked to a persistent increase of cyclic AMP.19,31,32 In addition, acupuncture-induced release of adenosine may also suppress the inflammatory responses typically linked to chronic pain.3,8,40 Thus, it is possible that adenosine released during acupuncture can act via multiple mechanisms, including activation of A1 and A2a receptors to counteract intracellular signaling pathways involved in the development chronic pain.28 Consistent with the global changes in gene expression associated with chronic pain, acupuncture sessions typically are given repeatedly over several weeks to obtain maximal clinical benefits. Of note, the slow clearance of adenosine in humans compared to mice (Fig 4B) suggests that the antinociceptive effect of acupuncture lasts longer in humans than in rodents.2,6,8,22
Obviously more extensive studies are required to elucidate peripheral mechanisms of acupuncture-mediated analgesia. The relatively small sample size from healthy individuals used in this study might prevent us from identifying the true behavior of purines other than adenosine in response to acupuncture when patients need analgesic treatments. Nevertheless, the data reported here provide direct evidence for adenosine release in human subjects receiving traditional acupuncture in the Zusanli point. Our observation provides additional data important for defining the significance of purinergic signaling in acupuncture-mediated nociception.
Perspective.
This article presents further evidence of the role of adenosine in acupuncture-mediated antinociception by demonstrating that local adenosine concentrations increase in the acupoint in human subjects receiving traditional acu-puncture.
Acknowledgments
The authors would like to thank all the volunteers who participated in the study, Haomin Sun for technical assistance, and Ru-Rong Ji for comments on the manuscript.
The present study was funded by NIH/NINDS NS075177.
Footnotes
The authors declare no conflicts of interest.
Takahiro Takano, Xiaolin Chen, and Fang Luo contributed equally to the work.
References
- 1.Bangsbo J. Vasoactive substances in the interstitium of contracting skeletal muscle examined by microdialysis. Proc Nutr Soc. 1999;58:925–933. doi: 10.1017/s0029665199001238. [DOI] [PubMed] [Google Scholar]
- 2.Bing Z, Villanueva L, Le Bars D. Acupuncture-evoked responses of subnucleus reticularis dorsalis neurons in the rat medulla. Neuroscience. 1991;44:693–703. doi: 10.1016/0306-4522(91)90088-6. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 5.Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman B, Biaggioni I. Intravascular source of adenosine during forearm ischemia in humans: Implications for reactive hyperemia. Hypertension. 1999;33:1453–1457. doi: 10.1161/01.hyp.33.6.1453. [DOI] [PubMed] [Google Scholar]
- 6.Fox EJ, Melzack R. Transcutaneous electrical stimulation and acupuncture: Comparison of treatment for low-back pain. Pain. 1976;2:141–148. [PubMed] [Google Scholar]
- 7.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 10.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Han JS, Wan Y. Characteristics of electroacupuncture-induced analgesia in mice: Variation with strain, frequency, intensity and opioid involvement. Brain Res. 2002;945:20–25. doi: 10.1016/s0006-8993(02)02503-9. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine Committee on Advancing Pain Research Care and Education, in Relieving Pain in America . A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press (US); Washington, DC: 2011. [PubMed] [Google Scholar]
- 14.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neuroscience. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langevin HM, Bouffard NA, Badger GJ, Churchill DL, Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–774. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- 16.Langevin HM, Bouffard NA, Churchill DL, Badger GJ. Connective tissue fibroblast response to acupuncture: Dose-dependent effect of bidirectional needle rotation. J Altern Complem Med. 2007;13:355–360. doi: 10.1089/acm.2007.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima FO, Souza GR, Verri WA, Jr, Parada CA, Ferreira SH, Cunha FQ, Cunha TM. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: Involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain. 2010;151:506–515. doi: 10.1016/j.pain.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Limehouse JB, Taylor-Limehouse PA. Eastern concepts of acupuncture. In: Schoen A, editor. Veterinary Acupuncture: Ancient Art to Modern Medicine. Maryland Heights; MO, Mosby: 2000. pp. 79–93. [Google Scholar]
- 19.Liou JT, Liu FC, Hsin ST, Yang CY, Lui PW. Inhibition of the cyclic adenosine monophosphate pathway attenuates neuropathic pain and reduces phosphorylation of cyclic adenosine monophosphate response element-binding in the spinal cord after partial sciatic nerve ligation in rats. Anesth analg. 2007;105:1830–1837. doi: 10.1213/01.ane.0000287652.42309.5c. [DOI] [PubMed] [Google Scholar]
- 20.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 21.Melzack R, Stillwell DM, Fox EJ. Trigger points and acupuncture points for pain: Correlations and implications. Pain. 1977;3:3–23. doi: 10.1016/0304-3959(77)90032-X. [DOI] [PubMed] [Google Scholar]
- 22.Molsberger A, Hille E. The analgesic effect of acupuncture in chronic tennis elbow pain. Br J Rheumatol. 1994;33:1162–1165. doi: 10.1093/rheumatology/33.12.1162. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 24.Napadow V, Ahn A, Longhurst J, Lao L, Stener-Victorin E, Harris R, Langevin HM. The status and future of acupuncture mechanism research. J Altern Complem Med. 2008;14:861–869. doi: 10.1089/acm.2008.SAR-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010:CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyberg M, Mortensen SP, Thaning P, Saltin B, Hellsten Y. Interstitial and plasma adenosine stimulate nitric oxide and prostacyclin formation in human skeletal muscle. Hypertension. 2010;56:1102–1108. doi: 10.1161/HYPERTENSIONAHA.110.161521. [DOI] [PubMed] [Google Scholar]
- 27.Riksen NP, van Ginneken EE, van den Broek PH, Smits P, Rongen GA. In vivo evidence against a role for adenosine in the exercise pressor reflex in humans. J Appl Physiol. 2005;99:522–527. doi: 10.1152/japplphysiol.00108.2005. [DOI] [PubMed] [Google Scholar]
- 28.Sawynok J. Adenosine Receptors. In: Cairns RE, editor. Peripheral Receptor Targets for Analgesia and Novel Approaches to Pain Management. John Wiley and Sons; Hoboken, NJ: 2009. pp. 137–152. [Google Scholar]
- 29.Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003;121:907–916. doi: 10.1016/s0306-4522(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 30.Sjolund KF, Segerdahl M, Sollevi A. Adenosine reduces secondary hyperalgesia in two human models of cutaneous inflammatory pain. Anesth Analg. 1999;88:605–610. doi: 10.1097/00000539-199903000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 32.Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- 33.The World Federation of Acupuncture-Moxibustion Societies [Accessed 2011];Location of Points (GB 12346-90) Available at: http://www.wfas.org.cn/standard/dingwei/Index.html.
- 34.Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O. Expression of C×26, C×32 and C×43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer. 1992;51:522–529. doi: 10.1002/ijc.2910510404. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . Acupuncture: Review and analysis of reports on controlled clinical trials. World Health Organization; Geneva, CH: 2003. [Google Scholar]
- 36.Wu WP, Hao JX, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Lou X, Jiang S. Cross-section anatomy of Zusanli. J Wen Zhou Med Coll. 2000;30:224. [Google Scholar]
- 38.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Zijlstra FJ, van den Berg-de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. doi: 10.1080/0962935031000114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]