Abstract
This study aimed to measure the levels of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6), and nitrite/nitrate (NOx) in serum of dogs experimentally infected with Rangelia vitalii. Twelve female mongrel dogs were divided into 2 groups; group A (uninfected controls) composed by healthy dogs (n=5) and group B consisting of dogs inoculated with R. vitalii (n=7). Animals were monitored by blood smear examinations, which showed intraerythrocytic forms of the parasite on day 5 post-infection (PI). Blood samples were collected through the jugular vein on days 0, 10, and 20 PI to determine the serum levels of IFN-γ, TNF-α, IL-1, IL-6, and NOx. Cytokines were assessed by ELISA quantitative sandwich technique, and NOx was measured by the modified Griess method. Cytokine levels (IFN-γ, TNF-α, IL-1, and IL-6) were increased (P<0.01) in serum of infected animals. Serum levels of NOx were also increased on days 10 PI (P<0.01) and 20 PI (P<0.05) in infected animals. Therefore, the infection with R. vitalii causes an increase in proinflammatory cytokines and nitric oxide content. These alterations may be associated with host immune protection against the parasite.
Keywords: Rangelia vitalii, IFN-γ, TNF-α, IL-1, IL-6, nitric oxide, immunity, rangeliosis
Canine rangeliosis is a tick-borne protozoan disease caused by Rangelia vitalii that primarily affects rural and periurban young dogs in southern Brazil [1,2]. Infection with R. vitalii causes severe anemia, jaundice, fever, splenomegaly, lymphadenopathy, hemorrhage along the gastrointestinal tract, and persistent bleeding through the tips of the pinnae, external surface of the ears, nose, and oral cavity [3-6]. The influence of this disease on immune system parameters of infected animals is unknown.
Cytokines represent the major molecules involved in the communication among T-cells, macrophages, and other immune cells in the course of an immune response to infectious agents [7]. IFN-γ is the cytokine responsible for the function of CD4+ Th1 cells, which is the activation of macrophages to kill intracellular microorganisms and synthesize other proinflammatory cytokines (TNF-α, IL-1, and IL-6) and nitric oxide (NO) [8].
NO is an important cytotoxic mediator on immune activated cells, capable to kill pathogenic agents [9]. This reactive nitrogen species is synthesized by the isoenzymes nitric oxide synthases (NOS), which include inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS) that catalyze the oxidation of L-arginine and molecular oxygen to citrulline and NO [10]. The iNOS is highly expressed in macrophages when activated by proinflammatory cytokines [11]. The half-life of NO is very short, and its indirect determination can be made by measurements of its oxidation products, nitrite (NO2-) and nitrate (NO3-), referred to as NOx [12].
Numerous studies on the immune response of Th1 cells, characterized by the synthesis of proinflammatory cytokines and iNOS, have been performed in different hemoparasites [13-15]. However, the mechanism of host immune responses has not been explained in R. vitalii infection. Therefore, the aim of this study was to measure the levels of IFN-γ, TNF-α, IL-1, IL-6, and NOx in serum of dogs experimentally infected with R. vitalii.
Twelve female mongrel dogs (6- to 12-months old) were used in this study as previously described [16]. The animals, previously determined by molecular tests to be free from Babesia spp., Hepatozoon spp., and Ehrlichia spp. infection [16], were inoculated with R. vitalii (n=7) or served as uninfected controls (n=5). The R. vitalii strain used in this study was obtained from a naturally infected dog [17]. A fresh blood sample of this animal was inoculated (2 mL through the jugular vein) in another dog (Dog 13: male, 5-months old) for maintenance of the isolate in the laboratory. Each animal of Group B was infected through intravenous inoculation with 2 ml of fresh blood collected from Dog 13, containing an average of 6 parasites per slide, which were found inside of erythrocytes and leukocytes of blood smears [16].
The presence and degree of parasitemia were estimated for each animal every 2 days throughout the experiment. Peripheral blood smears were collected from the tip of the ear of each dog. The smears were Romanovsky stained and then examined under a microscope (×1,000 magnification), as previously described [16]. Blood collection of the jugular vein (3 ml) was performed on days 0, 10, and 20 post-infection (PI). The blood was placed in tubes without anticoagulant, centrifuged for 10 min to obtain serum and stored under freezing (-20℃). The serum was used for determination of proinflammatory cytokines (IFN-γ, TNF-α, IL-1, and IL-6) and NOx.
The proinflammatory cytokines were quantified by ELISA, using the commercial Quantikine canine immunoassay kits (IFN-γ, TNF-α, IL-1, and IL-6), according to manufacturer's instructions (R&D Systems, Minneapolis, Minnesota, USA). Briefly, 96-well microplates were sensitized with the primary antibodies at room temperature (RT) for 30 min; the sample was added and incubated for 30 min at 37℃. After washing, the secondary antibody conjugated with peroxidase was added to each well, and a period of incubation followed. The presence and concentration of the cytokines were determined by the intensity of color measured by spectrometry by a micro-ELISA reader, Sunrine-Tecan (Tecan, Sunrise, Melbourne, Australia).
Nitric oxide levels in serum of dogs infected with R. vitalii were analyzed indirectly, by the quantification of nitrite/nitrate (NOx) according to the technique described in detail by Tatsch et al. [12]. Therefore, NOx was measured by the modified Griess method using the Cobas Mira automated analyzer (Roche Diagnostics, Basel, Switzerland). Results were expressed in µmol/L. The data were evaluated by the Student's t-test. Values with probability less than 5% were considered statistically different.
On day 5 PI, there were blood smears positive to R. vitalii. Parasitemia increased progressively until day 10 PI when a peak was observed. Then, the number of parasites was significantly reduced as described in detail by Da Silva et al. [16], and the parasite was found within erythrocytes, leukocytes, and the extracellular milieu. During the experimental period, clinical signs such as anorexia, apathy, weight loss, fever, anemia, and diarrhea were observed in dogs infected with R. vitalii [16]. The infected dogs showed no bleeding (a common finding in rangeliosis) despite severe thrombocytopenia, reduction of platelet activity, and alteration in the concentrations of nucleotides and nucleosides present in platelets, participants in the process of homeostasis [18,19]. The infection by R. vitalii caused in these dogs reduction in serum levels of iron, zinc, and copper [20], as well as oxidative stress with increased lipid peroxidation, protein oxidation and, consequently, increase of antioxidant status to reduce cellular injury [21].
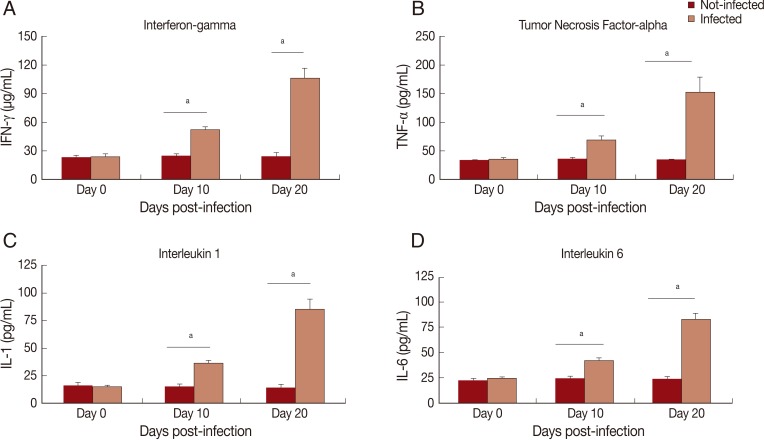
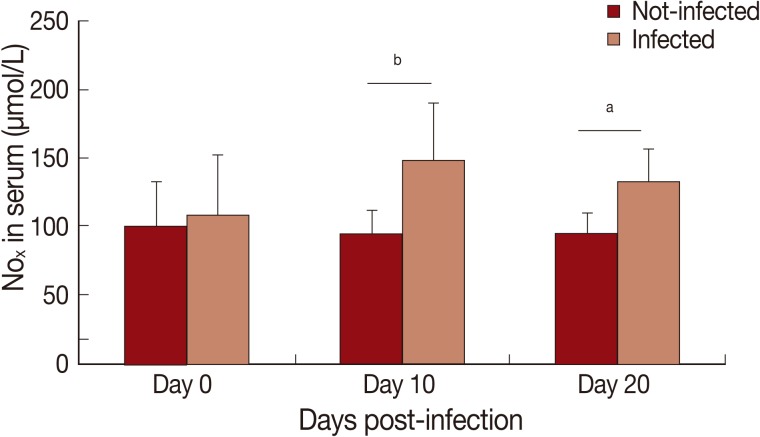
In this study, cytokine levels increased (P<0.01) in infected animals compared to the control group (Fig. 1). A progressive increase in serum levels of IFN-γ (Fig. 1A), TNF-α (Fig. 1B), IL-1 (Fig. 1C), and IL-6 (Fig. 1D) was observed. An increase of nitrite/nitrate (NOx) in serum of infected animals was observed on day 10 PI (P<0.01) and 20 PI (P<0.05) (Fig. 2).
Fig. 1.
Levels of proinflammatory cytokines in serum of Rangelia vitalii-infected dogs on days 0, 10, and 20 post-infection compared with uninfected controls. (A) IFN-γ, (B) TNF-α, (C) IL-1. (D) IL-6. aRepresents statistical difference between infected and control group (P<0.001).
Fig. 2.
Levels of nitrite/nitrate (NOx) in serum of Rangelia vitalii-infected dogs on days 0, 10, and 20 post-infection compared with uninfected controls (aP<0.05, bP<0.01).
This study is the first to demonstrate the increased serum levels of IFN-γ, TNF-α, IL-1, and IL-6 in dogs experimentally infected with R. vitalii. The increase of these proinflammatory cytokines can be attributed to activation of the immune response and parasitemia control, as related in other infections caused by hemoparasites, as babesiosis (22), ehrlichiosis [15], anaplasmosis [23], leishmaniasis [24], hepatozoonosis [14], and trypanosomosis [25].
On day 10 PI occurred the peak of parasitemia, when the levels of cytokines and NO increased significantly compared to the control group; thus this increase in the inflammatory mediators were able to reduce parasitemia of day 15 PI [16]. Serum levels of cytokines and NO increased during the course of infection, as detected on day 20 PI. Probably the high levels of inflammatory mediators on day 20 PI was the cause of low parasitemia observed during this period. According to the literature, these immunological parameters are responsible for controlling parasitemia in infections by other hemoparasites [14,15,22,24,25]. In trypanosomosis by T. cruzi, researchers concluded that NO may be directly related to parasitemia, severity of lesions, chronicity, and mortality of mice infected [26,27]. In vitro studies showed that NO has the ability to kill parasites like Plasmodium [28] and T. cruzi [26]. Therefore, this study suggests that both cytokines and NO are responsible for reduction and maintenance of low parasitemia in dogs infected with R. vitalii, as detailed below.
One early reaction of the host to infection with protozoan parasites is the secretion of an array of potent cytokines, including TNF, IL-1, and IL-6. These early responses contribute significantly to the outcome of infection by influencing its course directly and by regulating the specific immune responses against the parasite [29]. The overproduction of proinflammatory cytokines observed in this study contributes not only to control the infection, but also for the disease progress, similarly to which occurs in babesiosis [30]. The increased serum levels of proinflammatory cytokines (TNF-α, IL-1, and IL-6) could be associated with the onset of clinical signs in the acute phase of inflammatory responses (anorexia, apathy, weight loss, and fever) [8], as observed in this study.
This study found that the increase of proinflammatory cytokines coincides with the reduction of parasites in the bloodstream. A high expression of TNF-α was found in dogs experimentally infected with Ehrlichia canis [31]. Furthermore, TNF-α also played a role in controlling bacterial number because TNF-α receptor-knockout mice were highly susceptible in this infection [32]. Researchers reported an association between low parasite and increased IFN-γ and TNF-α expression in dogs naturally infected with Leishmania chagasi, indicating that these cytokines play a role in protection against infection [24]. Serum levels of TNF-α and IFN-γ increased in anaplasmosis, indicating a role of cellular immunity activation during the infection [33]. Enhanced levels of IL-1, IL-6, and TNF-α in bovine babesiosis are important for stimulating immunity against protozoan pathogens [22].
High levels of NO are mediated by upregulated expression of the iNOS gene in response to the activating signals, in particular to the secretion of proinflammatory cytokines by Thl cells [34]. Accumulating evidence indicates that parasitic diseases are commonly associated with elevated production of NO [35]. Production of proinflammatory cytokines predisposes to the increased synthesis of NO, which mediates host protection through either direct parasite killing or by limiting parasite growth [34]. The half-life of NO is very short. Because of this, the measurement of the circulatory stable end products of NO, i.e., nitrite/nitrate (NOx), are most often used to evaluate the NO production [12].
In this study, it was observed that an increased level of NOx which can be related to the induction of immune responses as demonstrated in Babesia bovis infection, in which increased NO production reflected a complex host-parasite interaction [36]. Furthermore, researchers have demonstrated that IFN-γ is responsible by the synthesis of NO by monocytes/macrophages, which show a high babesicidal activity [37]. In dogs infected by Hepatozoon canis, it was demonstrated that an increased production of NO enhances the host's defense against parasitic infections and helps to eliminate it [14]. In addition, protective responses were reported through high concentrations of nitrate in equids infected with Theileria equi and Babesia caballi [38]. Increased levels of NO and IL-6 were shown in cattle with anaplasmosis and may indicate the stimulation of the host immune system [23]. However, the determination of reactive nitrogen intermediates (RNI) is unlikely to be useful indicators of severity or outcome in dogs infected with Babesia canis [13].
We conclude that infection with R. vitalii causes an increase in proinflammatory cytokines and NO metabolites. This alteration may be associated with the host immune protection against the piroplasms, similar to what occurs in other hemoparasite infections.
ACKNOWLEDGMENT
This study was approved by the Ethics and Animal Welfare Committee of the Federal University of Santa Maria (UFSM), Brazil, protocol number 15/2010.
References
- 1.Loretti AP, Barros SS. Parasitism by Rangelia vitalii in dogs ("nambiuvú", "peste de sangue") - a critical review on the subject. Arq Inst Biol. 2004;71:101–131. [Google Scholar]
- 2.França RT, Silva AS, Paim FC, Costa MM, Soares JF, Mazzanti CM, Lopes STA. Rangelia vitalii in dogs in southern Brazil. Comp Clin Pathol. 2010;19:383–387. [Google Scholar]
- 3.Pestana BR. The Nambyuvú (Preliminar Note) Rev Soc Cient São Paulo. 1910;5:14–17. [Google Scholar]
- 4.Krauspenhar C, Fighera RA, Graça DL. Protozoan-associated hemolytic disease in dogs. Medvep - Rev Cien Med Vet Peq An Esti. 2003;1:273–281. [Google Scholar]
- 5.Loretti AP, Barros SS. Hemorrhagic disease in dogs infected with an unclassified intraendothelial piroplasm in southem Brazil. Vet Parasitol. 2005;134:193–213. doi: 10.1016/j.vetpar.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Fighera RA, Souza TM, Kommers GG, Irogoyen LF, Barros CSC. Pathogenesis, clinical, hematological, and pathological aspects of Rangelia vitalii infection in 35 dogs (1985-2009) Pesq Vet Bras. 2010;30:974–987. [Google Scholar]
- 7.Belardelli F. Role of interferons and other cytokines in the regulation of the immune response. APMIS. 1995;103:161–179. doi: 10.1111/j.1699-0463.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 8.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 7 ed. Philadelphia, USA: Saunders/Elsevier; 2012. p. 527. [Google Scholar]
- 9.Dusse LMS, Vieira LM, Carvalho MD. Review on nitric oxide. J Bras Patol Med Lab. 2003;39:343–349. [Google Scholar]
- 10.Flora Filho R, Zilberstein B. Nitric oxide: a simple messenger passing through the complexity. Metabolism, synthesis and functions. Rev Assoc Med Bras. 2000;46:265–271. doi: 10.1590/s0104-42302000000300012. [DOI] [PubMed] [Google Scholar]
- 11.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 12.Tatsch E, Bochi GV, Pereira RD, Kober H, Agertt VA, De Campo MMA, Gomes P, Duarte MMMF, Moresco RN. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–350. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson LS, Lobetti RG, Becker P, Reyers F, Vaughan-Scott T. Nitric oxide metabolites in naturally occurring canine babesiosis. Vet Parasitol. 2002;104:27–41. doi: 10.1016/s0304-4017(01)00606-9. [DOI] [PubMed] [Google Scholar]
- 14.Kiral F, Karagenc T, Pasa S, Yenisey C, Seyrek K. Dogs with Hepatozoon canis respond to the oxidative stress by increased production of glutathione and nitric oxide. Vet Parasitol. 2005;131:15–21. doi: 10.1016/j.vetpar.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Tajima T, Rikihisa Y. Cytokine responses in dogs infected with Ehrlichia canis Oklahoma strain. Ann NY Acad Sci. 2005;1063:429–432. doi: 10.1196/annals.1355.078. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva AS, França RT, Costa MM, Paim CB, Paim FC, Dornelles GL, Soares JF, Labruna MB, Mazzanti CM, Monteiro SG, Lopes STA. Experimental infection with Rangelia vitalii in dogs: acute phase, parasitemia, biological cycle, clinical-pathological aspects and treatment. Exp Parasitol. 2011;128:347–352. doi: 10.1016/j.exppara.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Soares JF, Girotto A, Brandão PE, França RT, Da Silva AS, Lopes STA, Labruna MB. Detection and molecular characterization of a canine piroplasm from Brazil. Vet Parasitol. 2011;180:203–208. doi: 10.1016/j.vetpar.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Paim CB, Paim FC, Da Silva AS, Franca RT, Costa MM, Leal CA, Soares JF, Labruna MB, Schetinger MR, Mazzanti A, Mazzanti CM, Monteiro SG, Lopes ST. Thrombocytopenia and platelet activity in dogs experimentally infected with Rangelia vitalii. Vet Parasitol. 2012;185:131–137. doi: 10.1016/j.vetpar.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Paim CB, Da Silva AS, Paim FC, Franca RT, Costa MM, Souza VC, Pimentel VC, Jaques JA, Mazzanti CM, Leal DB, Monteiro SG, Schetinger MR, Lopes ST. Activities of ectonucleotidases and adenosine deaminase in platelets of dogs experimentally infected with Rangelia vitalii. Exp Parasitol. 2012;131:252–257. doi: 10.1016/j.exppara.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva A, Franca R, Costa M, Paim C, Paim F, Santos C, Flores E, Eilers T, Mazzanti C, Monteiro S, Amaral C, Lopes S. Influence of Rangelia vitalii (Apicomplexa: Piroplasmorida) on copper, iron and zinc bloodstream levels in experimentally infected dogs. J Parasitol. 2012 doi: 10.1645/GE-2985.1. DOI: 10.1645/GE-2985.1. [DOI] [PubMed] [Google Scholar]
- 21.Franca RT, Da Silva AS, Costa MM, Paim FC, Paim CB, Thome GR, Wolkmer P, Pereira ME, Schetinger MR, Moresco RN, Mazzanti CM, Monteiro SG, Lopes ST. Relationship between oxidative stress and clinical-pathological aspects in dogs experimentally infected with Rangelia vitalii. Res Vet Sci. 2012 doi: 10.1016/j.rvsc.2012.02.001. DOI: 10.1016/j.rvsc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Shoda LK, Palmer GH, Florin-Christensen J, Florin-Christensen M, Godson DL, Brown WC. Babesia bovis-stimulated macrophages express interleukin-1β, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect Immun. 2000;68:5139–5145. doi: 10.1128/iai.68.9.5139-5145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ergonul S, Askar TK. The investigation of heat shock protein (HSP 27), malondialdehyde (MDA), nitric oxide (NO) and interleukin (IL-6, IL-10) levels in cattle with anaplasmosis. Vet Sci. 2009;15:575–579. [Google Scholar]
- 24.Alves CF, De Amorim IFG, Moura EP, Ribeiro RR, Alves CF, Michalick MS, Kalapothakis E, Bruna-Romero O, Tafuri WL, Teixeira MM, Melo MN. Expression of IFN-gamma, TNF-alpha, IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol. 2009;128:349–358. doi: 10.1016/j.vetimm.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Paim FC, Duarte M, Wolkmer P, Da Silva AS, Monteiro SG, Mazzantti CM, Lopes STA. Cytokines in rats experimentally infected with Trypanosoma evansi. Exp Parasitol. 2011;128:365–370. doi: 10.1016/j.exppara.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naviliat M, Gualco G, Cayota A, Radi R. Protein 3-nitrotyrosine formation during Trypanosoma cruzi infection in mice. Braz J Med Biol Res. 2005;38:1825–1834. doi: 10.1590/s0100-879x2005001200011. [DOI] [PubMed] [Google Scholar]
- 28.Boutlis CS, Weinberg JB, Baker J, Bockarie MJ, Mgone CS, Cheng Q, Anstey NM. Nitric oxide production and nitric oxide synthase activity in malaria-exposed Papua New Guinean children and adults show longitudinal stability and no association with parasitemia. Infect Immun. 2004;72:6932–6938. doi: 10.1128/IAI.72.12.6932-6938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titus RG, Sherry B, Cerami A. The involvement of TNF, IL-1 and IL-6 in the immune response to protozoan parasites. Immunol Today. 1991;12:A13–A16. doi: 10.1016/S0167-5699(05)80005-2. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed JS. The role of cytokines in immunity and immunopathogenesis of piroplasmoses. Parasitol Res. 2002;88:S48–S50. doi: 10.1007/s00436-001-0573-4. [DOI] [PubMed] [Google Scholar]
- 31.Faria JLM, Munhoz TD, João CF, Vargas-Hernández G, André MR, Pereira WAB, Machado RZ, Tinucci-Costa M. Ehrlichia canis (Jaboticabal strain) induces the expression of TNF-α in leukocytes and splenocytes of experimentally infected dogs. Rev Bras Parasitol Vet. 2011;20:71–74. doi: 10.1590/s1984-29612011000100015. [DOI] [PubMed] [Google Scholar]
- 32.Bitsaktsis C, Huntington J, Winslow G. Production of IFN-γ by CD4 T cells is essential for resolving Ehrlichia infection. J Immunol. 2004;172:6894–6901. doi: 10.4049/jimmunol.172.11.6894. [DOI] [PubMed] [Google Scholar]
- 33.Nazifi S, Razavi SM, Kaviani F, Rakhshandehroo E. Acute phase response in cattle infected with Anaplasma marginale. Vet Microbiol. 2011;155:267–271. doi: 10.1016/j.vetmic.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Wandurska-Nowak E. The role of nitric oxide (NO) in parasitic infections. Wiad Parazytol. 2004;50:665–678. [PubMed] [Google Scholar]
- 35.Nahrevanian H. Involvement of nitric oxide and its up/down stream molecules in the immunity against parasitic infections. Braz J Infect Dis. 2009;13:440–448. doi: 10.1590/s1413-86702009000600010. [DOI] [PubMed] [Google Scholar]
- 36.Stich RW, Shoda LKM, Dreewes M, Adler B, Jungi TW, Brown WC. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect Immun. 1998;66:4130–4136. doi: 10.1128/iai.66.9.4130-4136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown WC, Norimine J, Knowles DP, Goff WL. Immune control of Babesia bovis infection. Vet Parasitol. 2006;138:75–87. doi: 10.1016/j.vetpar.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Deger S, Deger Y, Bicek K, Ozdal N, Abdurrahman G. Status of lipid peroxidation, antioxidants, and oxidation products of nitric oxide in equine babesiosis: status of antioxidant and oxidant in equine babesiosis. J Eq Vet Sci. 2009;29:743–747. [Google Scholar]