Abstract
Taeniasis is prevalent in all regions of Thailand, except the South. Infections were more frequently found in males than females of any age from 7-83 years. Taenia saginata is the most common species throughout the country. Taenia asiatica was reported only in the province of Kanchanaburi in the Central region. Co-infections, with Taenia solium and T. asiatica or T. solium and T. saginata, were found. Hybridization between T. asiatica and T. saginata is evidence that co-infection is never found between these 2 species. Finding more than 1 worm in a single patient was not entirely rare. Genetic variation was found without correlation to its geographic distribution in T. saginata, whereas no variation was found in T. asiatica.
Keywords: Taenia saginata, Taenia asiatica, Taenia solium, distribution, age, sex, co-infection, worm burden, intraspecies variation
DISTRIBUTION AND PREVALENCE
Taeniasis is still prevalent and thus a public health problem in Thailand. Investigation and survey information specific to taeniasis is not available. Previous prevalence reports have compiled taeniasis results with all other helminthiases and were obtained using microscopic stool examination techniques. Surveys on the nationwide prevalence of taeniasis have shown chronologic changes from 1957 from 2.53% to 0.63% in 2001 [1,2]. Its prevalence is always highest in the North and Northeast regions of Thailand as compared to Central and especially the South which has almost no reported infections. Recent studies done in communities in the North, Northeast, and Central regions, especially those along the Thai border, used fecal examinations to detect Taenia and other helminth eggs. They found a relatively high prevalence of infections. Among border communities in the North, 2.3% (11/475) of the population in the Tungchang District of Nan were found to be positive for Taenia egg. A higher prevalence of 3.7% (11/296) was found in Ubon Ratchathani of the Northeast, along the eastern border of Thailand (Anantaphruti et al., paper in preparation). In the Central region, where previously there has always been a low prevalence of taeniasis recorded, a prevalence of 3.3% (6/183) was found in a remote population of Kanchanaburi. This community is along the western border of Thailand, where more than 50% of the populations are immigrants from neighboring countries [3].
AGE AND SEX-PREVALENCE
In an investigation in the North and Northeast of Thailand, 68 Taenia infection cases were studied. Almost double the number of infections was found in males (44) than in females (24). The age ranged from the lowest at 12-years old to the maximum at 83-years old. In the North, there was a total of 44 T. saginata infection cases collected, 29 males and 15 females. The patient age range was 12-63 years (males 12-61 years, females 12-63 years). In the Northeast region, T. saginata infections were also found more in males (14) than in females (8). The patient age range was 14-83 years (males 14-83 years, females 19-77 years). This study only found 1 male of unknown age and an 18-year-old female with T. solium infections in Nan area (Anantaphruti et al., data in preparation). Similar results of T. solium infection cases in Kanchanaburi of the Central region, double the number of cases were reported in males (8) than in females (4) with a patient age range of 7-55 years [4,5]. However, in the same province, the opposite situation occurred with T. asiatica infection, which had double the number of cases in females (8) than in males (3) and an age range of 28-76 years (Table 1). It is possible that women are more likely to eat raw or partially cooked liver than men, which would lead to infections.
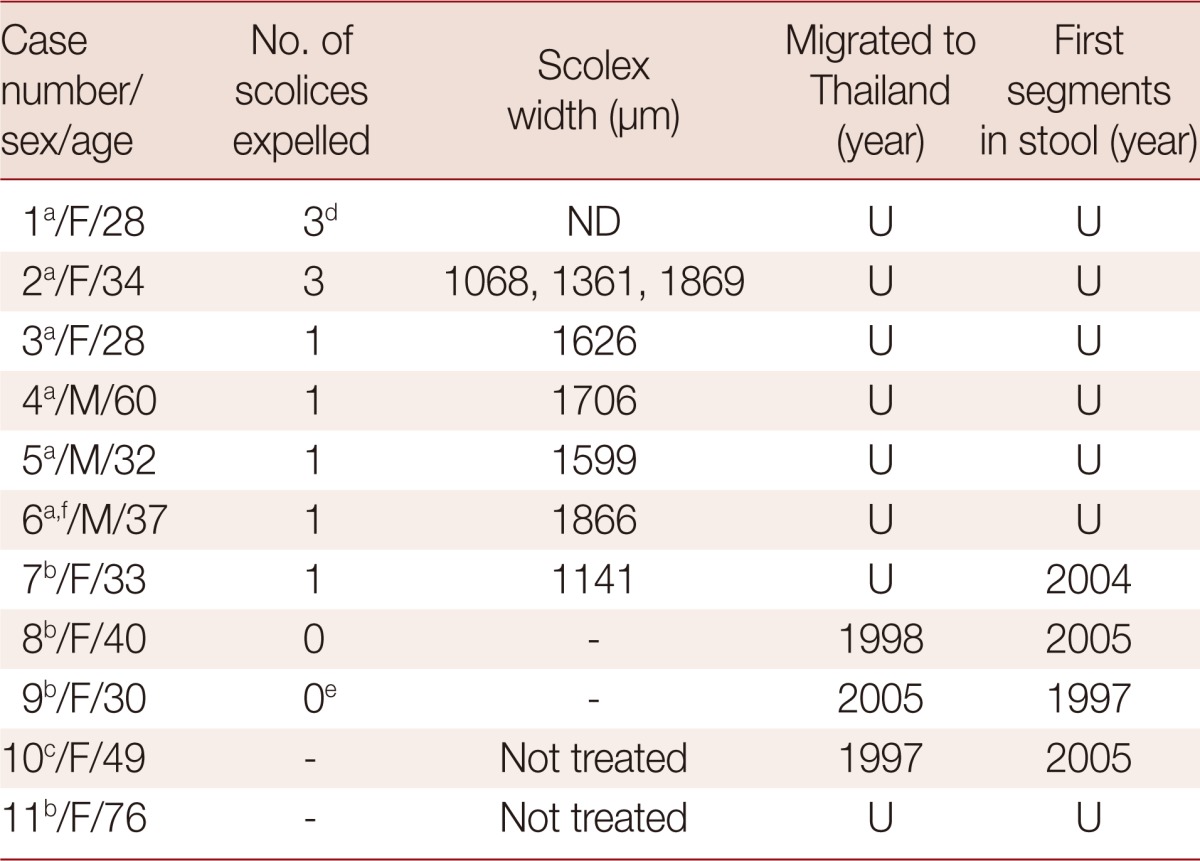
Table 1.
Taenia asiatica taeniasis cases from 2003-2007 from the province of Kanchanaburi, in west-central Thailand
SPECIES OF TAENIA
T. saginata is the most common species that infects humans throughout Thailand. The studies identified the species of worms based mainly on an examination of the morphology of either a scolex or proglottids that were expelled after drug administration. Charoenlarp et al. [6] used a Thai medicinal herb, Puag-Haad, for the treatment of taeniasis patients admitted in the hospital. Twenty-four of these patients passed worms with scolices and they were all identified as T. saginata. In a study before that one, the same author, Charoenlarp et al. [7] showed that 2 of 26 scolices were T. solium. In the Northeast region, a popular local food, larb, or raw or partially-cooked beef, is a common source of Taenia infection. In the upper-northeast province of Udon Thani, 23 volunteers tested positive for Taenia eggs discharged segments or strobila without scolex after niclosamide administration. The segments from all of those cases were T. saginata [8].
Using molecular identification techniques on Taenia species has been reported by a few investigators. PCR-based identification, using a 0.29 kb band, was done in 29 samples from taeniasis patients from several districts of Chiang Mai, in the North region of Thailand [9]. Twenty-seven of them were T. saginata cases [9]. In addition, 10 Taenia isolates from an unspecified area in Chiang Mai were all molecularly characterized as T. saginata [10]. Recently, molecular sequencing data of the cox1 gene was used to identify Taenia species in 2 regions of Thailand. In 2 provinces of the Northeast region, T. saginata is the only identified species of Taenia causing human taeniasis (22 cases). In the North region, partially-cooked pork is relatively common for local people to eat. However, like the above reports, T. saginata infections are more prevalent than those caused by other species. Forty-four of 46 (95.7%) niclosamide therapy cases in communities in Nan were T. saginata (Anantaphruti et al., paper in preparation).
Taeniasis caused by T. solium seems rather rare in Thailand, but cysticercosis, which is caused by the metacestode of T. solium is reported more often. In the Central region, an unexpectedly high infection rate of T. solium and a lower rate of T. saginata was found. Anantaphruti et al. [4] reported Taenia infection in a remote community along the Thai-Myanmar border in Kanchanaburi. After administering the medication, 24 taeniasis cases expelled proglottids, 16 of those with scolices. Based on morphologic characteristics of the scolex, 31% (5/16) of the cases were confirmed to be T. solium. Consequently, the expelled proglottids/scolex from these patients were also identified as T. solium based on molecular analysis with either the multiplex PCR, DNA sequencing or BESS T-base analysis (base excision sequence scanning thymine-base). A total of 11 cases (46%) of T. solium infection were found from 24 taeniasis patients.
The first reported evidence of T. asiatica in Thailand was in Kanchanaburi [4] and it was found exclusively in Kanchanaburi [5]. A total of 11 cases were found to be infected with more than 13 worms of this species. Among the 8 scolices collected, the average width was 1.5 mm and they ranged from 1.1 to 1.9 mm (Table 1). Three female cases had an infection with more than 1 worm. In 1 case, 28-year-old, discharged 3 scolices with a small number of segments. Two scolices had hooklets on their rostellum, indicating that they were T. solium, whereas the other was unarmed. The unarmed scolex was molecularly identified as T. asiatica. (case no. 1; Table 1). In another case, a 34-year-old person expelled a total of 5 m of strobilae of worms with 3 scolices. Each chain strobila was isolated separately and analyzed molecularly as T. asiatica (case no. 2; Table 1). In a third case, a 30-year-old female expelled a huge mass of strobila. Unfortunately, no scolex was found and no separation of strobilated chain was done, therefore no actual number of worms was stated (case no. 9; Table 1). The rest of the cases discharged 1 worm each with or without a scolex. The infected patients were all from the Karen hill tribe or of Karen origin. A number of them were immigrants who recently moved to Kanchanaburi from Myanmar. Particularly, case number 9 discharged segments in stool before migration to Thailand, which may indicate the existence of T. asiatica in Myanmar. Sources of infection may possibly be wild boars living in forests along the border of the 2 countries. The life cycle of T. asiatica is perpetuated as metacestode infection in wild boars can result from ingestion of human excreta containing T. asiatica, left from hunters. In addition, T. asiatica was reported sympatrically with T. saginata and T. solium in Kanchanaburi [4].
DUAL INFECTION
Mixed infections with multiple Taenia spp. were rare. A female patient was found with dual infections of 1 T. asiatica and 2 T. solium specimens. Each acetocarmine-stained hooklet-armed and unarmed scolex (as shown in Fig. 1) was identified by BESS T-base analysis [4]. Co-infections with T. saginata and T. solium were also reported. A female, aged 40 years, who resided in Nakhon Ratchasima Province in northeastern Thailand, had a history of discharging Taenia proglottids. After treatment with a local Thai herbal extract, 1 scolex of T. solium and segments of T. saginata were observed from this patient [12].
Fig. 1.
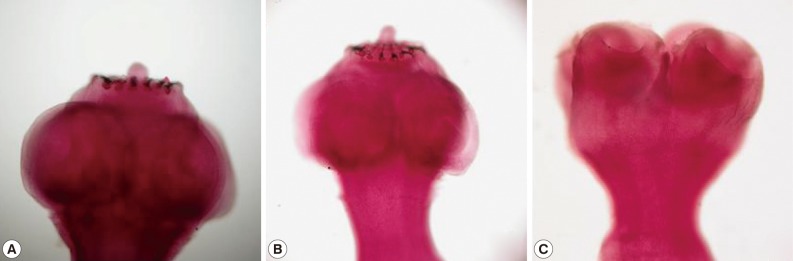
Scolices from dual infection of Taenia solium (A, B) and Taenia asiatica (C) in case no. 1 (Table 1).
WORM BURDEN
In general, with all species that cause human taeniasis, only 1 worm was found in each patient. However, more than 1 worm has been reported and is not entirely rare. The reported worm burden, assessed by counting of scolex expulsion, is summarized in Table 2.
Table 2.
Worm burden of Taenia species based on scolex expulsion
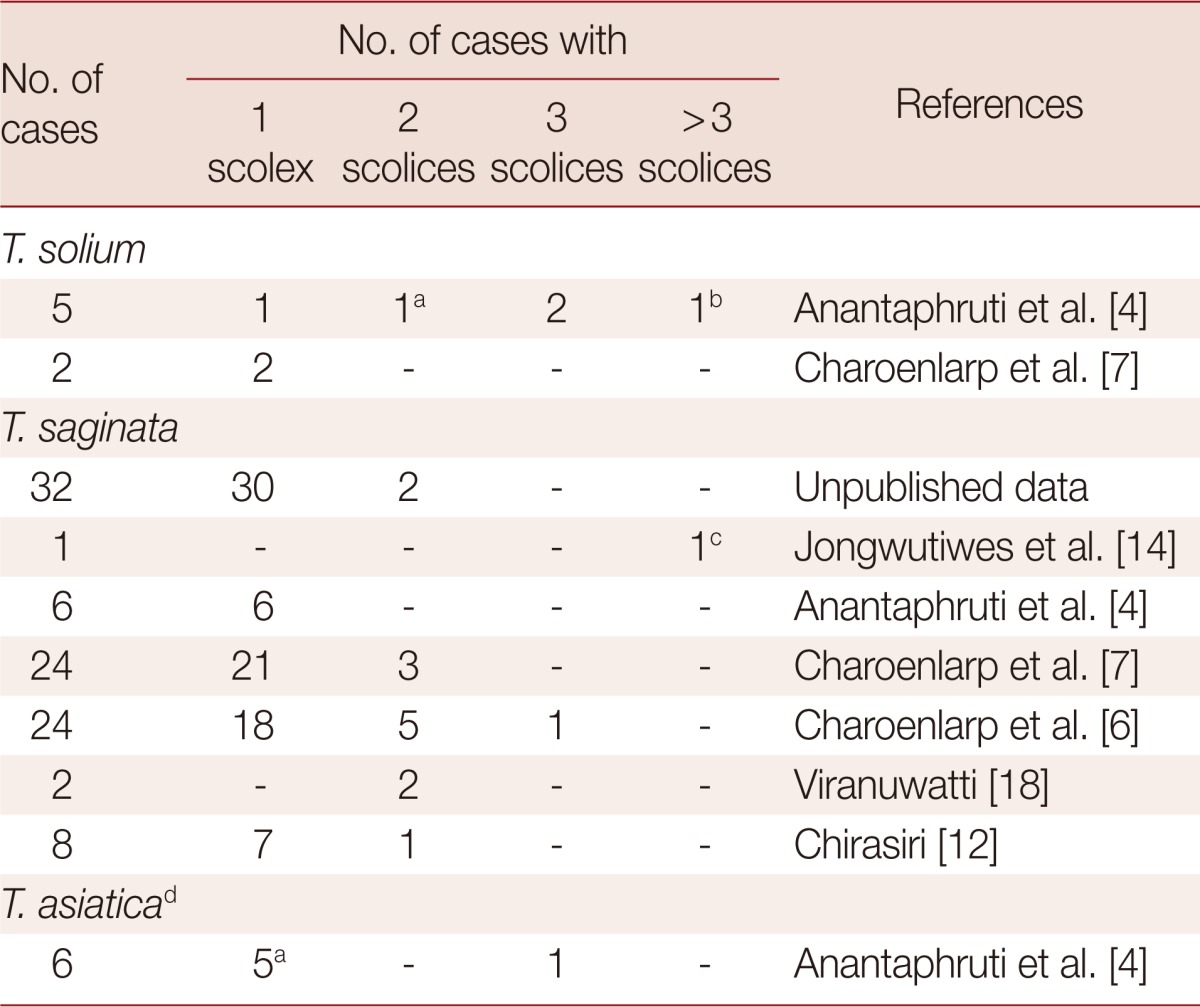
aA case of dual infection with 2 T. solium and 1 T. asiatica.
b6 scolices.
c5 scolices.
dMolecular identification.
T. solium
Anantaphruti et al. [4] reported 5 cases of T. solium infection in Kanchanaburi Province who discharged scolex/scolices after niclosamide therapy. A 29-year-old male had 6 scolices while another 2 cases, a 43-year-old male and a 34-year-old female, expelled 3 scolices each. Two scolices were found in a female co-infected with T. asiatica, and thus, this case had total 3 worms (case no. 1 Table 1). Two cases reported by Charoenlarp et al. [7] harbored 1 scolex each.
T. saginata
A trial testing the treatment of taeniasis with local Thai herbs reported the expulsion of T. saginata worms. Based on the proglottids evacuated, Chaneyayothin [13] reported 5 worms with a total length of 23 m discharged from a patient after treatment. The scolex portion of the worms was not mentioned, thus the actual worm numbers were not confirmed. Five T. saginata worms were confirmed in a female suffering from acute abdominal pain during an abdominal laparotomy. The tapeworms were 26-56 cm long and each worm scolex bore no hooklets. Molecular identification showed that they were all T. (saginata) saginata [14]. Based on scolex expulsion, Charoenlarp et al. [6] found 3 worms with scolices discharged in 1 patient. Another 5 patients discharged 2 scolices each. In an investigation of taeniasis in the North and Northeast Thailand, 2 worms, which were counted by scolex expulsion, were found in 2 of 32 patients discharging the scolex. In addition, 2 long chain strobilae, each of which included immature, mature, and gravid proglottids, indicated that 2 worms were found in 7 out of 66 patients (Anantaphruti et al., paper in preparation).
T. asiatica
Three worms assessed by scolices, with a total strobilate length of 5 m, were found in a female in Kanchanaburi Province. Other cases discharged only 1 scolex each [4]. It was observed that only a few or less segments of T. solium were discharged from infected cases, whereas long strobilae were more frequently observed in T. saginata. Worms in long chain strobilae, 9 to 17 feets, either with or without scolex, were discharged from almost half of the T. saginata patients (Anantaphruti et al., paper in preparation).
LIFE SPAN
A 42-year-old female residing in Nakhon Ratchasima had a history of continually discharging cestode proglottids since her age of 7. After herbal administration, a scolex of T. saginata was observed [12]. Therefore, a life span of 35 years for T. saginata could be calculated based on this case study. The actual life span must be longer than 35 years since the worm was treated before natural termination. In another case, a Japanese man became infected with T. saginata in Ethiopia (Addis Ababa) after eating raw beef. Proglottids were regularly observed in his stool from October 1969 and terminated in June 1997. Thus, the life span for T. saginata in this case was 27.8 years (Hara T., personal communication).
TAENIA SPECIES HYBRIDIZATION
Okamoto et al. [15] reported the evidence of hybridization between T. asiatica and T. saginata in 2 of 15 samples from Kanchanaburi. Phylogenetic analyses were carried out for the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene and 2 nuclear loci, for elongation factor-1α (ef1) and ezrin-radixin-moesin (ERM)-like protein (elp). Genetic identification was performed by analyzing complete nucleotide sequences of the cox1 gene (1,620 bp) and partial sequences of alleles at 2 nuclear loci, the ef1 (1,095-1,096 bp) and the elp genes (1,162-1,164 bp). The presence of a few heterozygous individuals suggested outcrossing in Taenia species. Two individual worms, 1 from a 30-year-old female and another from a 35-year-old male, showed the mitochondrial genomes of T. saginata and some alleles at 2 nuclear loci typical of T. asiatica, which suggested a hybrid origin. These findings are evidence of occasional hybridization between the 2 species. However, co-infection between T. asiatica and T. saginata in the same patient has never been reported.
ABNORMAL WORMS
Abnormal worms are sometimes observed, due to abnormal growth and development. In Taenia species, abnormalities were observed only in T. saginata and T. asiatica.
T. saginata
The normal character on the head part of Taenia species bears 4 suckers on the scolex. In Nan area (Northern region), 22 Taenia cases discharged tapeworm strobilae after treatment, and 17 scolices were recovered. Among these, 3 were morphologically abnormal, with 6 suckers on the scolex. The partial cox1 sequences (800 bp) of these abnormal tapeworms confirmed that they were T. saginata [16]. Abnormal strobilae were seen in a 32-year-old female with acute abdominal pain admitted to Chulalongkorn Hospital. During an abdominal laparotomy, jejunal perforation was observed and 5 Taenia tapeworms were recovered inside the jejunal lumen near the perforation. Two worms had terminal strobilate branching. A 45-cm-long worm showed posterior bifurcation of 1 and 2 cm. Another 30-cm worm possessed 0.9 cm protruded branch at 9 cm from the posterior end. Sequences of 28S rRNA and cox1 genes showed that they were T. (saginata) saginata [14].
T. asiatica
Abnormality was observed in a mature segment of a worm collected from a 37-year-old male. Two sets of reproductive organs with 2 genital pores were observed in 2 mature segments, while several of the other segments were normal (unpublished data, case no. 6; Table 1) (Fig. 2).
Fig. 2.
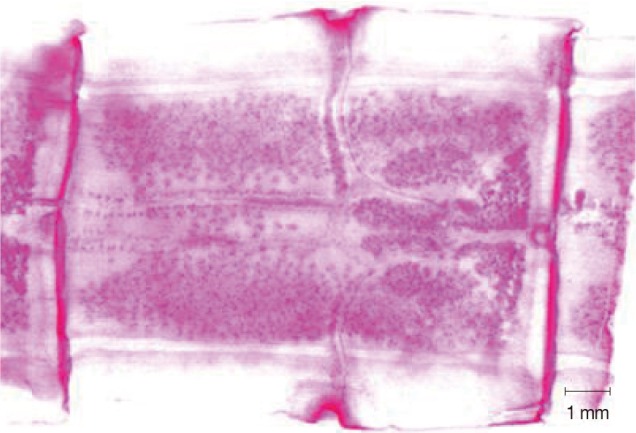
A mature segment from a T. asiatica worm showing 2 sets of reproductive organs and 2 genital pores in case no. 6 (Table 1).
ABNORMAL SITE OF INFECTION
The terminal gravid proglottids are normally separated from the strobilae and expell out from the host body in the feces. Often, a single free proglottid migrates out of the host anus, causing itching in the perianal area during the segment movement. In some instances, the detached proglottids in the intestine cause extra-intestinal infections. Poshakrisna [17] reported a chronic appendicitis case with a perforated tract between the ileum and bladder. The patient had a history of evacuating Taenia segments in their feces for 18 months before the onset of appendicitis. Later, a segment was discharged in the urine. In addition, at the Pathology Section of Siriraj Hospital, Bangkok, a T. saginata segment was found in the appendixes of 2 patients.
INTRASPECIFIC GENETIC VARIATION
T. saginata
T. saginata genetic variation was found without any correlation to its geographic distribution. Anantaphruti et al. (paper in preparation) studied T. saginata samples from provinces in the North and Northeast regions of Thailand. Genomic DNA sequencing of the cox1 gene DNA on 73 isolates from those study sites revealed differentiation into 14 haplotypes with a diversity equal to 0.6 and a maximum 11 nucleotide variation sites. A clustering diagram indicated no significant genetic differentiation among the isolated populations. The haplotype network showed a star-like pattern with a major haplotype of 38 isolates, indicating it to be an ancestor of T. saginata in Thailand. Over 50% of this major haplotype discharged scolex/scolices after niclosamide administration. The authors suggest further study in susceptibility to drug treatment of the major ancestor haplotype.
T. asiatica
T. asiatica has been reported exclusively in a district along the Thai-Myanmar border in Kanchanaburi, which is in the Central region of Thailand. Variation analysis was done in 12 T. asiatica isolates collected from 10 patients (cases no. 2-11; Table 1). Mitochondrial sequences of 924 bp cox1 gene of T. asiatica showed no genetic variation among the samples from this area. When compared with the cox1 sequences of 18 T. asiatica from several countries (4 from Thailand, 4 from China, 2 from Korea, 3 from Japan, 3 from Indonesia, 1 from the Philippines, and 1 from Taiwan), the haplotype network showed very low genetic variation (0.1% genetic differentiation) among T. asiatica of Thailand and that of other countries. The results indicate that T. asiatica has low genetic variation. This species may be at risk of becoming an endangered species. [11].
ACKNOWLEDGMENTS
The author sincerely thanks the "Symposium on current perspectives of Taenia asiatica researches" held at Osong, Korea in 2011, who invited the author to present this review.
References
- 1.Vajrasthira S, Harinasuta C. Study on helminthic infections in Thailand. J Med Assoc Thai. 1957;40:309–340. (In Thai) [PubMed] [Google Scholar]
- 2.Anonymous. The Evaluation on the Helminthiasis Control Program in Thailand by the end of the 8th health development plan in the year 2001. Report. Helminthiasis Control Program, Division of General Communicable Diseases, Department of Communicable Diseases Control, Ministry of Public Health. 2001. (in Thai) [Google Scholar]
- 3.Anantaphruti MT, Nuamtanong S, Watthanakulpanich D, Maipanich W, Pubampen S, Sanguankiat S, Kusolsuk T, Muennoo C, Waikagul J. Responses to albendazole treatment for hookworm infection in Ethnic Thai and Immigrant in West-central Thailand. J Health Sci. 2007;53:443–449. [Google Scholar]
- 4.Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, Maipanich W, Pubampen S, Sanguankiat S, Muennoo C, Nakaya K, Sato MO, Sako Y, Okamoto M, Ito A. Sympatric occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand. Emerg Infect Dis. 2007;13:1413–1416. doi: 10.3201/eid1309.061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anantaphruti MT, Okamoto M, Yoonuan T, Saguankiat S, Kusolsuk T, Sato M, Sato MO, Sako Y, Waikagul J, Ito A. Molecular and serological survey on taeniasis and cysticercosis in Kanchanaburi Province, Thailand. Parasitol Int. 2010;59:326–330. doi: 10.1016/j.parint.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Charoenlarp P, Radomyos P, Bunnag D. The optimum dose of Puag-Haad in the treatment of taeniasis. J Med Assoc Thai. 1989;72:71–73. [PubMed] [Google Scholar]
- 7.Charoenlarp P, Radomyos P, Harinasuta T. Treatment of taeniasis with Puag-Haad: A crude extract of Artocarpus lakoocha wood. Southeast Asian J Trop Med Public Health. 1981;12:568–570. [PubMed] [Google Scholar]
- 8.Chularerk P, Rasameeprabha K, Papasarathorn T, Chularerk U. Some aspects of epidemiology and mass treatment of taeniasis in Ban Tard, Udorn Thani. J Med Assoc Thai. 1967;50:666–671. [Google Scholar]
- 9.Morakote N, Wijit A, Uparanukraw P. Further search for Taenia saginata asiatica in Chiang Mai, Thailand. Ann Trop Med Parasitol. 2000;94:521–524. doi: 10.1080/00034983.2000.11813572. [DOI] [PubMed] [Google Scholar]
- 10.Bowles J, McManus DP. Genetic characterization of the Asian Taenia, a newly described taeniid cestode of humans. Am J Trop Med Hyg. 1994;50:33–44. [PubMed] [Google Scholar]
- 11.Anantaphruti MT, Thaenkham U, Maipanich W, Watthanakulpanich D, Nuamtanong S, Yoonuan T, Sanguankiat S, Pubampen S, Phuphisut O. Genetic diversity of Taenia asiatica from Thailand and other geographical locations as revealed by cytochrome c oxidase subunit 1 sequences. Korean J Parasitol. 2013;51:55–59. doi: 10.3347/kjp.2013.51.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirasiri L. Treatment of intestinal parasites with 2:4:3':5' tetrahydroxystilbene (preliminary report) Vejasarn. 1963;12:439–453. (in Thai) [Google Scholar]
- 13.Chaneyayothin T. Treatment of taeniasis with crystal of Ma-Hard. News Lett Parasitol Trop Med Assoc Thail. 1971;2(4):3. (in Thai) [Google Scholar]
- 14.Jongwutiwes S, Putaporntip C, Chantachum N, Sampatanukul P. Jejunal perforation caused by morphologically abnormal Taenia saginata saginata infection. J Infect. 2004;49:324–328. doi: 10.1016/j.jinf.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto M, Nakao M, Blair D, Anantaphruti MT, Waikagul J, Ito A. Evidence of hybridization between Taenia saginata and Taenia asiatica. Parasitol Int. 2010;59:70–74. doi: 10.1016/j.parint.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Maipanich W, Sato M, Pubampen S, Sanguankiat S, Kusolsuk T, Thaenkham U, Waikagul J. Abnormal Taenia saginata tapeworms in Thailand. Southeast Asian J Trop Med Public Health. 2011;42:1065–1071. [PubMed] [Google Scholar]
- 17.Poshakrisna U. Vesical taeniasis. J Med Assoc Thai. 1961;44:143–150. (in Thai) [Google Scholar]
- 18.Viranuvatti V. Clinical trial of treating taeniasis by atabrine. J Med Assoc Thai. 1952;35(5):27–36. (in Thai) [Google Scholar]


