Abstract
Twelve 924 bp cytochrome c oxidase subunit 1 (cox1) mitochondrial DNA sequences from Taenia asiatica isolates from Thailand were aligned and compared with multiple sequence isolates from Thailand and 6 other countries from the GenBank database. The genetic divergence of T. asiatica was also compared with Taenia saginata database sequences from 6 different countries in Asia, including Thailand, and 3 countries from other continents. The results showed that there were minor genetic variations within T. asiatica species, while high intraspecies variation was found in T. saginata. There were only 2 haplotypes and 1 polymorphic site found in T. asiatica, but 8 haplotypes and 9 polymorphic sites in T. saginata. Haplotype diversity was very low, 0.067, in T. asiatica and high, 0.700, in T. saginata. The very low genetic diversity suggested that T. asiatica may be at a risk due to the loss of potential adaptive alleles, resulting in reduced viability and decreased responses to environmental changes, which may endanger the species.
Keywords: Taenia asiatica, Taenia saginata, cox1 gene, genetic diversity, Thailand, different geographical location
INTRODUCTION
Taenia asiatica is one of the 3 species of parasitic tapeworms (Taenia solium, Taenia saginata, and T. asiatica) reported as human pathogens [1,2]. This parasite is distributed in Asian countries where people eat inappropriately cooked viscera of pigs, especially the liver contaminated with metacestodes. T. asiatica was first described as a new species, based on morphologic characters of adult worms and metacestodes [3]. Studies on the morphology of adult worms showed that T. asiatica is very similar to T. saginata; therefore, it is very difficult to distinguish one from the other [4,5]. The 2 species can be discriminated by genetic data such as restriction fragment length polymorphism (RFLP) data and/or mitochondrial DNA sequence data [6-8].
T. asiatica and T. saginata diverged from a shared common ancestor around 0.16 million years ago [9]. After separation, these 2 lineages have evolved to have different intermediate hosts in their life cycle. T. asiatica infects the visceral organs of pigs while T. saginata infects the muscle of cattle [5,10,11]. Moreover, geographical localities between these parasites are different. T. asiatica is distributed in specific areas, e.g., in several countries of Asia, including Taiwan [11], Korea [3], China [12], Vietnam [13], Indonesia [14], and Thailand [15], but T. saginata can be found worldwide [16].
In Thailand, Thong Pha Phum District, northwest of Kanchanaburi, a west-central province, is the only reported site of T. asiatica. It is still unknown if differences in geographical locations and ecological niches affect intra- and inter-specific variations. In this study, we aimed to reveal genetic variations within the species of T. asiatica and of T. saginata using cytochrome c oxidase subunit 1 (cox1) as a DNA marker.
MATERIALS AND METHODS
Parasites and source of gene sequences
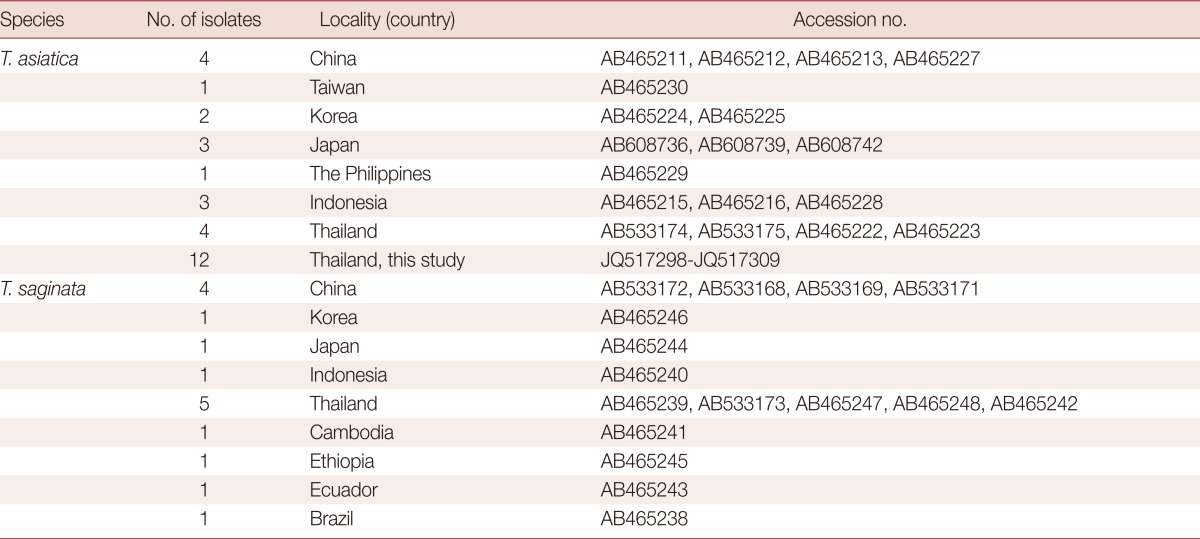
Taenia proglottids used in this study were previously collected from patients in Kanchanaburi Province during 2003-7. A rough gross morphology of either gravid segment or scolex was identified as T. saginata were collected. Worms were preserved in 70% ethanol and kept frozen (-70℃) until used. The scolex, with mature and gravid proglottids from some cases were fixed with 10% formalin, stained with acetic carmine and examined morphologically. Twelve molecularly identified T. asiatica isolate samples were used in this study [15,17,18]. The other sequences of T. asiatica and T. saginata used for analysis in this study were compiled from the GenBank database (Table 1).
Table 1.
The cox1 sequences of 30 T. asiatica isolates and 16 T. saginata isolates from different geographical localities
DNA preparation, PCR, and sequencing
Partial fragments of the strobilated proglottids from each isolate were separated and washed with distilled water, to remove any ethanol remaining from the fixation process. The genomic DNA of each worm was extracted using a Genomic DNA Mini Kit (Geneaid Biotech Ltd, Sijhih City, Taiwan), according to the manufacturer's instructions. DNA was re-suspended in 50 µl of elution buffer (provided in the kit). The 1,140 bp PCR amplicons were amplified using 2 oligonucleotide primers: cox1 (forward); 5'-CATGGAATAATAATGATTTTC-3' and cox1 (reverse); 5'-ACAGTACACACAATTTTAAC-3'. These primers were designed from the alignment of T. saginata and T. asiatica mitochondrial cox1 genes (AB533171 and AB533175, respectively). PCR amplicons were produced in 50 µl of reaction mixture containing 10 ng genomic DNA, 0.5 µM of each primer, 1X TopTaq™ Master Mix Kit (Containing TopTaq DNA Polymerase, PCR Buffer (with 1.5 mM MgCl2), and 200 µM each dNTP) (QIAGEN, Hilden, Germany). Amplification conditions were as follows: initial heating at 94℃ for 3 min, followed by 30 amplification cycles, consisting of denaturation at 95℃ for 30 sec, annealing at 53℃ for 30 sec, and elongation at 72℃ for 50 sec. PCR products were run on 1.2% agarose gel and visualized with a UV illuminator. The PCR amplicons were purified and sequenced by dideoxy-termination method and ABI3730XL sequencer and BigDye v3.1 (Applied Biosystems, Foster City, California, USA) at Macrogen Inc. (Geumcheon-gu, Seoul, Korea).
Clustering diagram and Genetic diversity analysis
The cox1 sequences were aligned by Clustal X version 2.0 [19], and haplotypes were distinguished. A neighbor-joining (NJ) phylogram was constructed under p-distance model by MEGA version 5.0 [20]. Bootstrap analyses were conducted using 1,000 replicates. The genetic diversity values including polymorphic sites (S), haplotype numbers (h), haplotype diversity (Hd), and nucleotide diversity (π) were calculated by using the DnaSP 5.0 program [21]. This program was also used to evaluate the genetic structure of the parasites under the population expansion effect via Tajima's D test.
RESULTS
We found significant differences in the amount of genetic variations within T. asiatica and within T. saginata (Table 2). The sequences of the 924 bp cox1 gene of T. asiatica from 12 Thai samples were added. The genetic variations among 30 samples of T. asiatica from 7 different countries (China, Taiwan, Korea, Japan, the Philippines, Indonesia, and Thailand) (Table 1) were almost identical (Table 2). Only 2 haplotypes (A and B) of the cox1 gene were found. The major genotype of T. asiatica was haplotype A, composed of 29 samples. Only 1 sample from China was haplotype B, with only 1 polymorphic site found at nucleotide position 115 (Tables 3, 4). On the other hand, for T. saginata there were 8 different genotypes (haplotype C to J) with 9 polymorphic sites (Tables 3, 4) in 16 samples from 9 countries in both Asia and other countries (Table 1). The major genotype is haplotype C which is composed of 8 isolates. The nucleotide variations were 1-3 positions in each haplotype. The genetic variation, therefore, in T. saginata is high. The segregation site S (polymorphic site) and haplotype number h of T. asiatica was 1 and 2, respectively, while those of T. saginata was 9 and 8, respectively (Table 2). Significant differences between T. asiatica and T. saginata were seen when comparing the haplotype diversity: Hd, which was 0.067 in T. asiatica and 0.700 in T. saginata. However, the value of nucleotide diversity: π of each 2 species was very low (Table 2). The genetic variations of T. asiatica and T. saginata were indicated by genetic divergence on the clustering diagram (Fig. 1).
Table 2.
Genetic diversity and neutrality values estimated from cox1 sequences of T. asiatica and T. saginata population
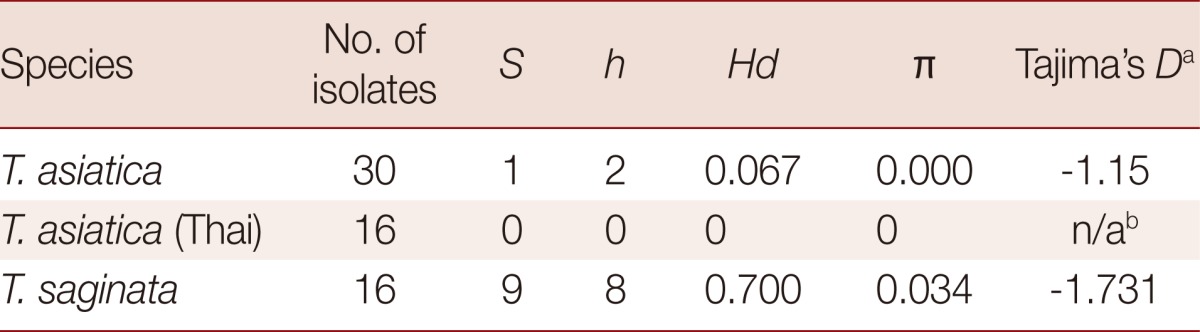
S: segregation (polymorphic) site, h: haplotype number, Hd: haplotype diversity, π: nucleotide diversity.
aP<0.05.
bNot analyzed.
Table 3.
Nucleotide variation sites of partial cox1 gene (924 bp length) between T. asiatica and T. saginata from 11 different countriesa
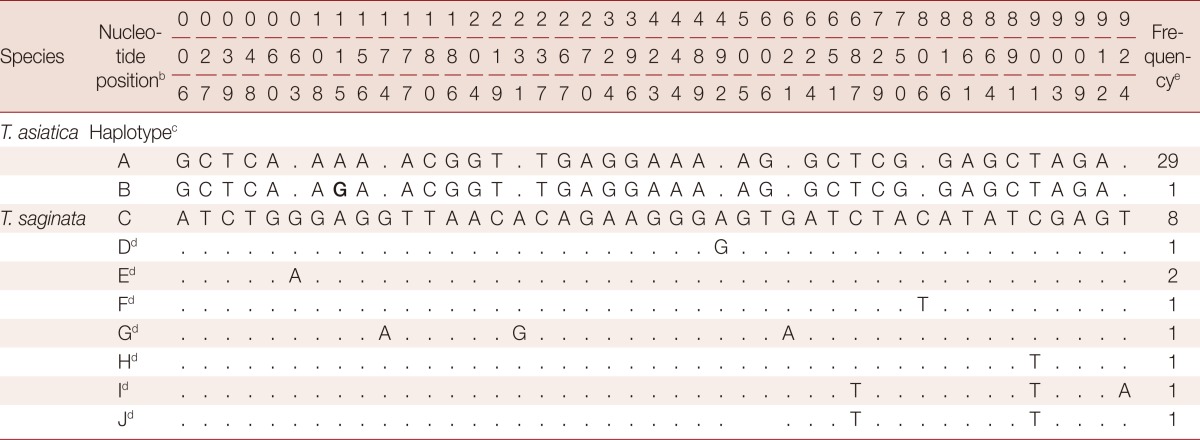
aEleven countries here involved in the genetic analysis include Thailand, Taiwan, Korea, China, Japan, Indonesia, the Philippines, Cambodia, Ecaudor, Brazil, and Ethiopia. The bold G font indicates nucleotide variation of T. asiatica.
bThe numbering of nucleotide positions 1-924 refers to position 400-1,324 of the complete mtDNA sequence (1,620 bp) of T. asiatica (GenBank Acc. No. AB465227).
cHaplotype A and B are genotypes of T. asiatica while haplotype C to J are genotypes of T. saginata.
dDots represent homology with haplotype C sequence of T. saginata.
eNo. of samples in each haplotype.
Table 4.
The frequency of worm numbers (46) in each haplotype of T. asiatica and T. saginata from 11 different geographical countries
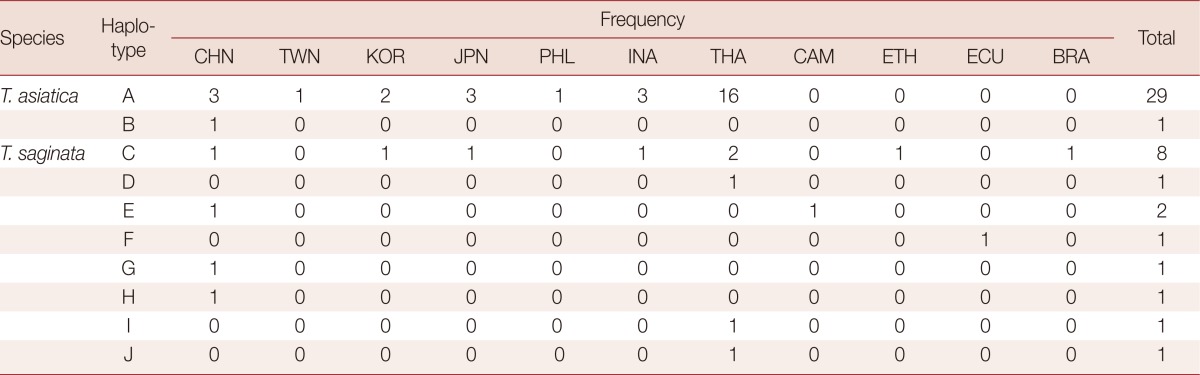
CHN, China; TWN, Taiwan; KOR, Korea; JPN, Japan; PHL, The Philippines; INA, Indonesia; THA, Thailand; CAM, Cambodia; ETH, Ethiopia; ECU, Ecuador; BRA, Brazil.
Fig. 1.
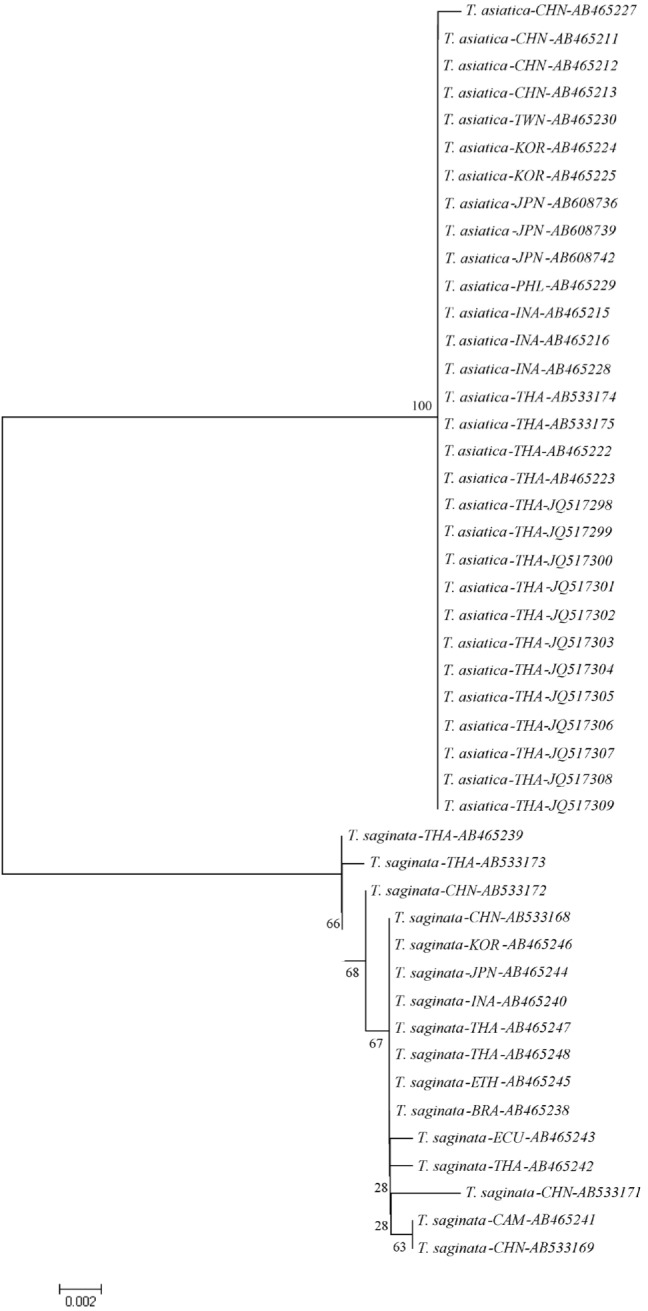
Phylogenetic tree of T. asiatica and T. saginata constructed by the neighbor-joining method from genetic distance estimated by the p-distance model. The numbers on each internal node is a bootstrap proportion. JQ517298-JQ517309 are samples in this study.
DISCUSSION
Although T. asiatica and T. saginata derived from a shared common ancestor a long time ago, their morphological characters are still similar [5]. DNA variation is a clear information to clarify their genetic divergence. The mitochondrial genes, cox1 and cytochrome b (cob), showed a distant relationship between T. asiatica and T. saginata [8]. The different intermediate hosts in their life cycle between these species might be one of the conditions causing genetic divergence. The intermediate host of T. asiatica is the pig while that of T. saginata is cattle [5]. The obvious difference between the farming of pigs and of cattle is population migration. Cattle frequently move from one locality to another to find food, while pigs are restricted to their pigsty.
The phylogenetic tree showed genetic variations of both parasites were not related to geographical locality. For T. asiatica, the low genetic variation might suggest that populations of the parasite might be small, i.e., prevalence of T. asiatica is low when compared to T. saginata in most countries; with very low recombination. For T. saginata, the results showed high genetic variations but no specific locality for each genotype. It might suggest that T. saginata populations migrated during cattle farming. The gene flow among T. saginata might be one effect influenced by population migration. The Tajima's D value was tested for gene flow of T. asiatica and T. saginata. The test revealed no gene flow in both species samples studied. It is possible that the sample size tested for T. saginata is small. The results suggested that more samples of T. saginata are needed to clarify its population structure. In this study, it is suggested that very low genetic diversity in T. asiatica (Table 2) might lead to a reduction of the parasite species, due to loss of potentially adaptive alleles for surviving in changing environments as similarly recognized with other endangered species [22].
ACKNOWLEDGMENTS
This study was supported by a research grant from the Faculty of Tropical Medicine, Mahidol University, academic year 2009. Sincere thanks are extended to the "Symposium on current perspectives of Taenia asiatica researches" held at Osong, Korea in 2011, who invited the authors of this paper to present their work.
References
- 1.Fan PC. Taiwan Taenia and taeniasis. Parasitol Today. 1988;4:86–88. doi: 10.1016/0169-4758(88)90204-9. [DOI] [PubMed] [Google Scholar]
- 2.Eom KS, Jeon HK, Rim HJ. Geographical distribution of Taenia asiatica and related species. Korean J Parasitol. 2009;47:S115–S124. doi: 10.3347/kjp.2009.47.S.S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eom KS, Rim HJ. Morphologic descriptions of Taenia asiatica sp. n. Korean J Parasitol. 1993;31:1–6. doi: 10.3347/kjp.1993.31.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Fan PC, Lin CY, Chen CC, Chung WC. Morphologic description of Taenia saginata asiatica (Cyclophyllidea: Taeniidae) from man in Asia. J Helminthol. 1995;69:299–303. doi: 10.1017/s0022149x00014863. [DOI] [PubMed] [Google Scholar]
- 5.Eom KS. What is Asian Taenia? Parasitol Int. 2006;55:S137–S141. doi: 10.1016/j.parint.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Zarlenga DS, McManus DP, Fan PC, Cross JH. Characterization and detection of a newly described Asian taeniid using cloned ribosomal DNA fragments and sequence amplification by the polymerase chain reaction. Exp Parasitol. 1991;72:174–183. doi: 10.1016/0014-4894(91)90135-j. [DOI] [PubMed] [Google Scholar]
- 7.Bowles J, McManus DP. Genetic characterization of the Asian Taenia, a newly described taeniid cestode of humans. Am J Trop Med Hyg. 1994;50:33–44. [PubMed] [Google Scholar]
- 8.Jeon HK, Eom KS. Taenia asiatica and Taenia saginata: Genetic divergence estimated from their mitochondrial genomes. Exp Parasitol. 2006;113:58–61. doi: 10.1016/j.exppara.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Hoberg EP, Alkire NL, Queiroz AD, Jones A. Out of Africa: origins of the Taenia tapeworms in humans. Proc Biol Sci. 2001;268:781–787. doi: 10.1098/rspb.2000.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eom KS, Rim HJ. Natural infection of Asian Taenia saginata metacestodes in the livers of Korean domestic pigs. Korean J Parasitol. 1992;30:15–20. doi: 10.3347/kjp.1992.30.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Eom KS, Rim HJ, Geerts S. Experimental infection of pigs and cattle with eggs of Asian Taenia saginata with special reference to its extrahepatic viscerotropism. Korean J Parasitol. 1992;30:269–275. doi: 10.3347/kjp.1992.30.4.269. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Tao H, Zhang B, Wang H, Wang Y, Li Z, Yang J, Yang B, Li Y, Pang Y, Zhang H, Li Y, Wu Y. First discovery of Taenia saginata asiatica infection in Yunnan province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1999;17:95–96. (in Chinese) [PubMed] [Google Scholar]
- 13.De NV, Hoa LT, Doanh NQ, Ngoc NB, Cong LD. Report on a new species of Taenia (Taenia asiatica) in Hanoi, Vietnam. J Malaria Parasit Dis Control. 2001;3:80–85. [Google Scholar]
- 14.Wandra T, Depary AA, Sutisna P, Margono SS, Suroso T, Okamoto M, Craig PS, Ito A. Taeniasis and cysticercosis in Bali and North Sumatra, Indonesia. Parasitol Int. 2006;55:S155–S160. doi: 10.1016/j.parint.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, Maipanich W, Pubampen S, Sanguankiat S, Muennoo C, Nakaya K, Sato MO, Sako Y, Okamoto M, Ito A. Sympatric occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand. Emerg Infect Dis. 2007;13:1413–1416. doi: 10.3201/eid1309.061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markell EK, Voge M, John DT. Medical Parasitology. 7th ed. Philadelphia, USA: WB Saunders Company; 1992. pp. 242–244. [Google Scholar]
- 17.Anantaphruti MT, Okamoto M, Yoonuan T, Saguankiat S, Kusolsuk T, Sato M, Sato MO, Sako Y, Waikagul J, Ito A. Molecular and serological survey on taeniasis and cysticercosis in Kanchanaburi Province, Thailand. Parasitol Int. 2010;59:326–330. doi: 10.1016/j.parint.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Anantaphruti MT. Current status of taeniasis in Thailand. Korean J Parasitol. 2013;51:37–42. doi: 10.3347/kjp.2013.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4885. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetic analysis using maximum likelihood, evolutionary distance, maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 22.Matocq MD, Villablanca FX. Low genetic diversity in an endangered species: recent or historic pattern? Biol Conserv. 2001;98:61–68. [Google Scholar]


