Abstract
History and current status of human taeniasis in the Republic of Korea, due to Taenia solium, Taenia asiatica, and Taenia saginata, are briefly reviewed. Until the 1980s, human taeniasis had been quite common in various localities of Korea. A study from 1924 reported 12.0% egg prevalence in fecal examinations. Thereafter, the prevalence of Taenia spp. ranged from 3% to 14% depending on the time and locality. Jeju-do, where pigs were reared in a conventional way, was the highest endemic area of taeniasis. An analysis of internal transcribed spacer 2 and mitochondrial cytochrome c oxidase 1 genes of 68 taeniasis cases reported from 1935 to 2005 in Korea by a research group revealed the relative occurrence of the 3 Taenia spp. as follows: T. solium (4.4%), T. asiatica (75.0%), and T. saginata (20.6%). However, national surveys on intestinal helminths conducted every 5 years on randomly selected people revealed that the Taenia egg prevalence dropped from 1.9% in 1971 to 0.02% in 1997 and finally to 0.0% in 2004. With the exception of 3 egg-positive cases reported in 2008 and 2 worm-proven cases in 2011, no more cases have been officially recorded. Based on these surveys and also on other literature, it can be concluded that taeniasis has virtually disappeared from Korea, although a few sporadic cases may remain hidden. Human cysticercosis is also expected to disappear within a couple of decades in Korea.
Keywords: Taenia asiatica, Taenia solium, Taenia saginata, taeniasis, prevalence
INTRODUCTION
Human taeniases are distributed worldwide [1]. The diseases are zoonotic because they involve pigs or cattle as the intermediate host and humans as the definitive host [1]. They are caused by 3 species of Taenia (their scolices are shown in Fig. 1) - T. solium, T. asiatica, and T. saginata - with the first 2 being transmitted by pigs and the third by cattle [1]. T. saginata and T. solium were undoubtedly observed from ancient times, including the dates of Hippocrates and possibly Moses [2]. However, the 2 species were not specifically differentiated until 1782 [2]. The Greeks (Aristophanes and Aristotle) mentioned the larval stage of T. solium in the tongue of the swine [2]. The larval stage of T. saginata was first observed in cattle in 1675 and was experimentally confirmed in 1863 [2]. The existence of T. asiatica, which is transmitted by swine (liver and viscera) was first notified in Taiwan in the 1930s [1,3], and was subsequently also found in the Republic of Korea (hereafter Korea) and Indonesia from the 1960s [1]. T. asiatica was reported as a distinct species in 1993 [4].
Fig. 1.
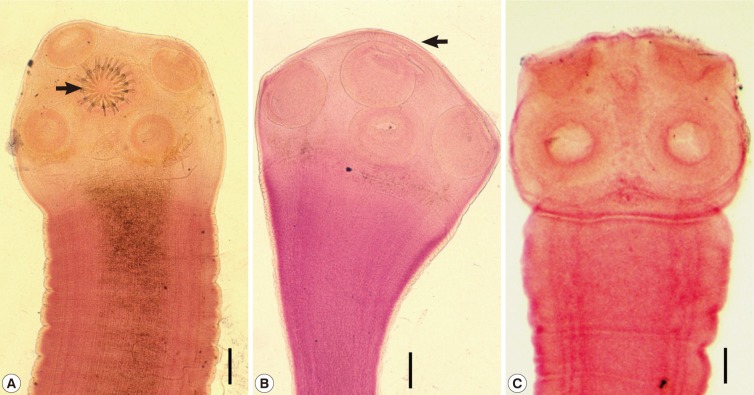
Scolices of human Taenia tapeworms recovered from Korean patients. (A) A scolex of Taenia solium having 4 suckers and 30 hooklets (arrow) on the rostellum. Scale bar=0.15 mm. (B) A scolex of T. asiatica having 4 suckers but no hooklets. It has more or less elevated rostellum (arrow). Scale bar=0.20 mm. (C) A scolex of T. saginata showing the same morphology as T. asiatica with the exception of the morphology of the rostellum, which is flat or invaginated. Scale bar=0.20 mm.
Human taeniasis was quite common in wide localities of Korea until the 1980s. However, recent national surveys showed that the Taenia egg prevalence became as low as 0.02% in 1997 and 0% in 2004. Thereafter, only 5 sporadic cases were detected during 2006-2011. We can presume that human taeniasis has almost disappeared from Korea. The present paper presents a brief review of the history of human taeniasis and discusses on their current status in Korea.
PREVALENCE OF TAENIASIS NATIONWIDE
Human Taenia tapeworms are mentioned in a medical book in Korea, "Dong-Eui-Bo-Gam", published in 1613. They were termed as "Chon-Baek-Chung", which means "small whitish worm", recognizing the morphology of spontaneously discharged gravid proglottids. However, it was not until 1914 that human taeniasis was first documented by the recovery of eggs in the feces of North Korean individuals [5]. The egg positive rate was 7.8% among 206 hospital patients (Suan Mine Hospital) examined (Table 1) [5]. Various studies conducted up to 1924 reported prevalences higher than 7.2% [6-8]. A report from a prison in Seoul stated that the prevalence of Taenia eggs was 12.0% among 1,280 prisoners in 1924 [8]. However, after 1927, the prevalence became lower than 5.0% and was maintained around 1-2% until the 1980s [9-19]. A notable figure was the 1.9% prevalence in 1971 among 24,887 nationwidely and randomly selected Korean people [17]. At that time, the province with the highest Taenia prevalence was Jeju-do (Island), which recorded the prevalence of 9.4% in rural areas and 5.9% in urban areas, followed by Jeollanam-do, with a prevalence of 4.7% in rural areas and 3.6% in urban areas [17].
Table 1.
Prevalence of taeniasis among Koreansa (1914-2008)
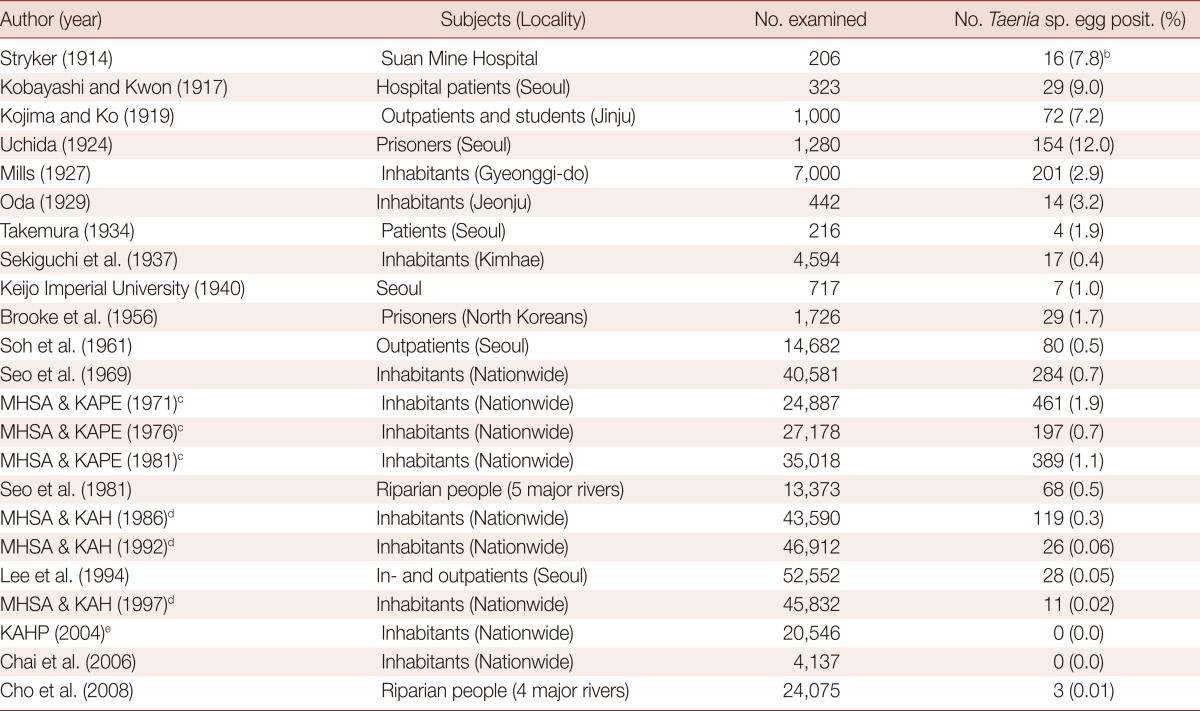
aPeople from nationwide or local areas, except Cheju-do (Island).
bTaenia saginata eggs.
cMinistry of Health and Social Affairs & Korea Association for Parasite Eradication.
dMinistry of Health and Social Affairs & Korea Association of Health.
eKorea Association of Health Promotion.
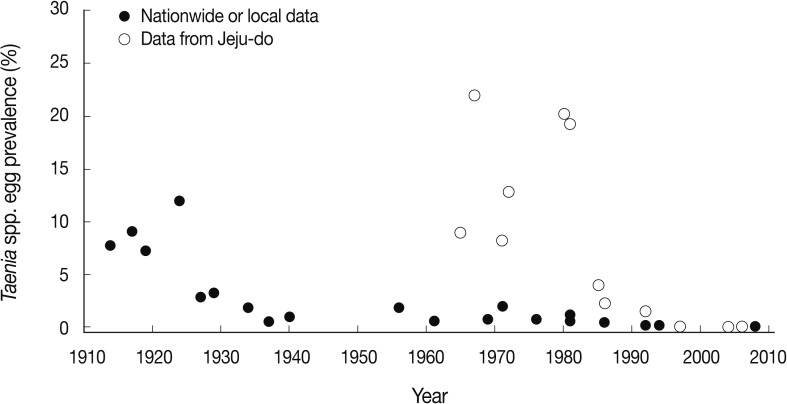
Ten years later, in 1981, the national prevalence had decreased slightly to 1.1% [19]. However, the prevalence in Jeju-do and Jeollanam-do remained high, at 19.2% and 3.0%, respectively [19]. From 1981-1986, the prevalence of taeniasis (national or local) became lower than 1% [20,21], and dropped even lower, to less than 0.1%, from 1992 [22-24]. The prevalence finally dropped to 0-0.01% during 2004-2008 (Fig. 2) [25-27]. Since 2008, only 2 cases have been detected, which were diagnosed as T. saginata by sequencing of the gene encoding mitochondrial cytochrome c oxidase 1 (cox1), in the Department of Parasitology, Chungbuk National University School of Medicine, Cheongju, Korea (Eom KS, personal communication). This temporal pattern is an evidence of the declined prevalence of human taeniasis and its present near-virtual absence in Korea.
Fig. 2.
Graphic presentation of the decreasing patterns of Taenia spp. egg positive rates in Korea. Data are from nationwide or local figures presented in Table 1 from 1914-2008 and from Jeju-do in Table 2 from 1965-2006. Note the remarkably higher egg positive rates in Jeju-do compared to the national figures or data from other local areas during 1965 and 1990.
The exact reasons for this decline of taeniasis in Korea are unclear. However, several factors could have been involved. One is the availability and widespread use of effective anthelmintics, for example, praziquantel and niclosamide. The second is the introduction and subsequent popularity of the flush toilet nationwide, including Jeju-do. The third is improvement of food for pigs (commercial fodders) and strengthening of the meat inspection system, especially for pigs and cattle. Still another factor includes the greatly improved socioeconomic status and health educational level of Koreans in recent generations.
PREVALENCE OF TAENIASIS IN JEJU-DO
A high prevalence (26%) of human taeniasis on Jeju-do was reported by Soh and coworkers in 1963 in an abstract to the 5th Annual Meeting of the Korean Society for Parasitology (KSP); however, the results were not published. In 1964, Kang et al. [28] reported in an abstract to the 6th Annual Meeting of KSP that the prevalence of Taenia spp. eggs (termed tapeworm eggs) among primary schoolchildren in Puk-Jeju-gun was 8.9% in 1963 and 4.3% in 1964 (Table 2). Kang et al. [29], in 1965, further detected 136 proglottid-discharging patients in Jeju-do and successfully treated them with bithionol. In 1967, very high prevalences of taeniasis (37.4% in rural areas and 16.4% in urban areas residents) were found in Aewol-myon, Puk-Jeju-gun, through recovery of eggs from feces [30].
Table 2.
Prevalence of taeniases among people in Cheju-do
aquestionnaire study.
In 1971, during a national fecal survey on randomly selected Koreans, the positive rate of Taenia spp. eggs among Jeju-do residents was a little lower, 8.2% (38 positives among 462 examined) [17]. Thereafter, the prevalence remained high, from 12.7% to 20.1%, until 1980 [19,31,32]. From 1985, however, the prevalence began to drop quickly in that year, and until 1992, Taenia egg prevalence rates were 1.5-6.9% in several areas of Jeju-do [21,22,33,34]. In a 1997 survey, the egg positive rate of Taenia spp. was 0%, where it remained through 2006 (Fig. 2) [24-26]. Since 2006, there have been no reports of fecal surveys on Jeju-do residents.
One of the reasons for such a high Taenia tapeworm prevalence until the 1970s in Jeju-do was undoubtedly the traditional way of raising pigs on the island (Fig. 3). Until then, toilets in Jeju-do were located in close contact with pigs. The pigs, usually housed immediately below the toilet, were allowed to eat the freshly defecated human feces, which would encourage active transmission of the life cycle of T. solium and T. asiatica. However, this type of traditional pig raising ceased during the 1980s, and today is exhibited only in several traditional villages used for sightseeing. The change in pig raising drove the decreased prevalence of taeniasis, and also cysticercosis, in Jeju-do.
Fig. 3.
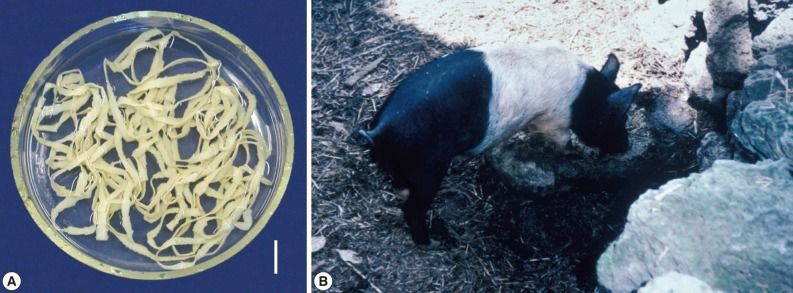
Taenia asiatica adult worm and life cycle in Korea. (A) An adult worm of T. asiatica recovered from a Korean patient. Scale bar=2 cm. (B) A figure showing a traditionally reared pig in Jeju-do. The toilet used by humans is located far right side of the figure (upper level of the rock).
STUDIES ON T. ASIATICA
The presence of T. asiatica, which is morphologically almost indistinguishable from T. saginata but biologically similar to T. solium, which adopts pigs as the intermediate host, was first reported in Taiwan in the 1930s [3]. The adult tapeworms collected from the mountain tribes (aborigines) of Taiwan morphologically resembled T. saginata rather than T. solium, despite the popular practices of eating pig liver and the unavailability of cattle around the oboriginal environments [35,36]. From 1982-1986, Fan and coworkers [35,37] raised the possibility that this Taenia tapeworm represented a new species, and beginning in 1987 [38,39] began to use the term Taiwan Taenia for this tapeworm. However, as the same kind of Taenia tapeworm was also discovered in Korea and Indonesia in 1988 [40], the name 'Asian Taenia' was more accurately adopted [41]. In 1995, a subspecies of T. saginata, i.e., T. saginata asiatica, was proposed by Fan and co-workers [42,43]. However, in 1993, this peculiar Taenia tapeworm had been proposed as a new species, T. asiatica, in Korea [4].
The existence of this non-solium non-saginata human Taenia tapeworm in Korea was suggested in the 1960s [29,30,44,45]. In particular, a study published in 1969 [44] reported the perflexing epidemiologic finding in Jeju-do that, whereas the majority of adult tapeworms recovered from residents appeared morphologically to be T. saginata, there were little or no Cysticercus bovis-infected cattle in Jeju-do. A similar discrepancy was repeatedly noted in different endemic areas of taeniasis, including Jeollabuk-do [45] and Jeollanam-do [46]. In 1983, a leprosarium in Sorok-do (Island), Jeollanam-do, was reported to show a peculiar finding in the occurrence of Taenia tapeworm infections [46]. Whereas 26 adult specimens recovered from the patients after chemotherapy were all T. saginata based on their morphology, no cattle were reared on this island, no beaf was imported, and only pigs were raised and consumed as pork [46].
In 1990, Min [47] strongly suggested the presence of a third species of Taenia in Korea. Subsequently, the new species description of T. asiatica was made in 1993 by Eom and Rim [4]. In the meantime, the cysticerci of T. asiatica (under the name Asian T. saginata) were reported in the liver of naturally infected pigs from Jeollabuk-do [48]. A human experimental infection was performed using the cysticerci from the aforementioned pig liver, and 2 adult tapeworms were recovered after 2 years, which were morphologically close to T. saginata, 1 being 330 cm in length [49]. Subsequently, pigs and cattle were experimentally infected with eggs from this experimentally infected human, and viscerotropism was found in the distribution of cysticerci in pigs and cattle after 30-165 days in the liver, omentum, and lung in pigs, and only in the liver in cattle [50].
RELATIVE OCCURRENCE OF THE THREE TAENIA SPECIES
Before T. asiatica was reported as a new species, the human Taenia tapeworms in Korea were diagnosed as either T. solium or T. saginata, or were undetermined (this does not represent T. asiatica) [1]. T. asiatica was included among T. saginata because of the almost indistinguishable morphology of the gravid proglottids between the 2 species. Eom and Rim [1] reviewed 7 previous reports on a total of 874 specimens of Taenia tapeworms collected from Koreans during 1965 and 1988, and concluded that the ratio of T. solium and T. saginata was about 1:5. This ratio was either higher, 2:3, in Jeollabuk-do [45], 3:4, in Seoul and Gyeongsangnam-do, and 1:3 or 1:4, in Jeju-do [51,52], or lower, 1:9, in Jeju-do [30]. No T. solium worms were recovered among 10 Taenia worm-recovered cases in Chungcheongbuk-do [51], 26 cases in Jeollanam-do [46], and 58 cases in Jeju-do [34]. Considerable numbers of these T. saginata cases must have been actually T. asiatica cases.
After 1993, no article which properly analyzed the ratio had been available until a study was done in 2005 which reported a ratio of 1 (T. solium): 17 (T. asiatica): 5 (T. saginata) based on the analysis of cox1 and internal transcribed spacer 2 (ITS2) genes from 68 specimens obtained from university museum collections from 1935-2005 [53]. These results obtained before and after 1993 may suggest that T. solium had decreased more quickly than T. asiatica and T. saginata in Korea. It also seems to be true that T. asiatica had been the dominating species over T. solium and T. saginata, and the next was T. saginata followed by T. solium over the past 50-100 years in Korea.
ARE TAENIASES HIDDEN OR GONE?
All 3 species of Taenia tapeworms seem to have now almost disappeared from Korea. However, it cannot be completely ruled out that some sporadic cases may remain hidden, for example, in remote or previously endemic areas. The 2 cases referred to the Department of Parasitology, Chungbuk National University School of Medicine in 2011 (Eom KS, personal communication) are such examples.
The detectability of Taenia cases may be different by different Taenia spp. For example, T. solium infection can be completely silent unlike T. saginata or T. asiatica. Whereas the terminal gravid proglottids of T. saginata or T. asiatica tend to actively move and crawl out of the intestine to the anus, thereby eliciting symptoms such as anal itching, those of T. solium pass out of the intestine quite passively [2]. For this reason, T. solium infection is detected on examination of stools only, and may be more easily missed and hidden than T. saginata or T. asiatica infection. To the author's own experience, a substantial number of Taenia egg-negative people in Lao Peoples' Democratic Republic actually expelled adult Taenia tapeworms (mostly T. saginata) after treatment with praziquantel and purging with magnesium salt which was done for recovery of trematode infections. Therefore, to confirm whether taeniasis has completely disappeared from Korea, attention should be paid to such false egg negative cases.
DECREASE OF HUMAN CYSTICERCOSIS
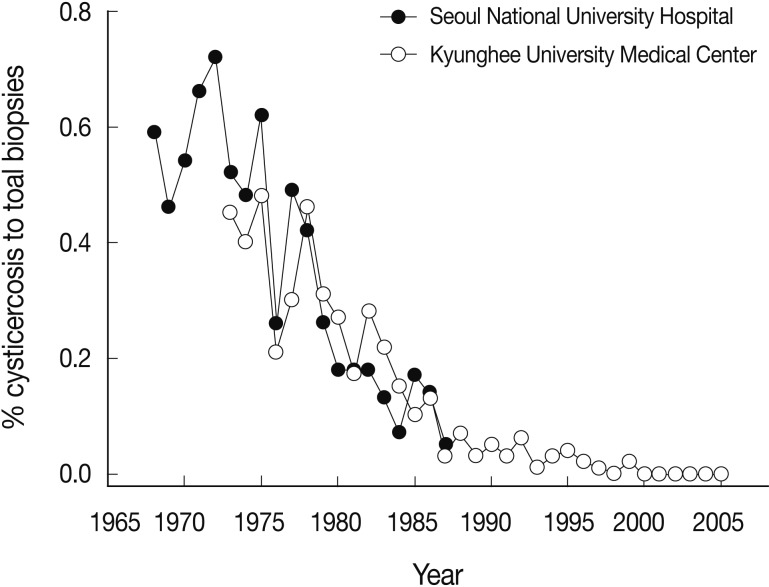
Human cysticercosis is caused by T. solium, but not by T. saginata or T. asiatica, though some possibility remains for T. asiatica [54,55]. In Korea, the incidence of human cysticercosis had been high before, but recently a decreasing tendency has been demonstrated. For example, during 1968 and 1987, 425 (0.24%) cysticercosis cases were diagnosed out of a total of 174,770 surgical biopsy specimens obtained in the Department of Pathology, Seoul National University Hospital [56]. Similarly, in 1972-1983, 136 (0.30%) cysticercosis cases were detected out of a total of 45,651 surgical biopsies examined in the Department of Pathology, Kyunghee University Medical Center in Seoul [57]. Another study during 1980-1989 found 112 (0.14%) cysticercosis cases out of a total of 80,947 biopsies in the Department of Pathology, Chonnam National University Hospital [58]. However, this ratio became much lower in a follow-up study (1984-2005) in Kyunghee University Medical Center; only 62 (0.029%) were found to be cysticercosis out of a total of 211,859 surgical biopsies [59], which is only 1/5-1/15 that of previous reports [56-58]. These trends of the decrease of cysticercosis among the toal biopsy cases, in particular, data derived from Seoul National University Hospital (1968-1987) [56] and Kyunghee University Medical Center (1972-2005) [57,59] are depicted in Fig. 4.
Fig. 4.
Decreasing patterns of cysticercosis in Korea based on data from Seoul National University Hospital (1968-1987) [56] and those from Kyunghee University Medical Center (1972-2005) [57,59] both of which are located in Seoul.
The prevalence of cysticercosis can also be estimated by serologic tests, in particular, ELISA. In 1993, Kong et al. [60] reported a seropositive rate for cysticercosis of 2.1% by ELISA among 750 individuals selected from general population selected from different areas of Korea, whereas 4.0% of 2,667 epileptic patients revealed positive reaction. In 2003, a study reported 26 (3.0%) cysticercus positive cases out of 865 serum samples referred from various localities [61]. A serologic reference center located in Seoul (Department of Laboratory Medicine, Chung-Ang University College of Medicine) reported an overall seropositive rate of 4.2% for cysticercosis out of a total of 74,448 serum or cerebrospinal fluid samples suspected as neurologic diseases, which were referred from 121 hospitals nationwide during 1993-2006 [62]. An interesting finding in the latter study was that the seropositive rate of cysticercosis apparently showed a decreasing trend, from 7.3-8.3% in 1993-1994 to 1.6-2.2% in 2005-2006 [62].
Cysticerci may live 5-10 years in the human body and thereafter begin to calcify (this may also take years) [63]. Most of the symptoms develop after cysticerci die and undergo calcification [63,64]. Therefore, symptomatic and also seropositive cysticercosis patients would continue to be found until 10-20 years after complete disappearance of human T. solium infection in a community. Therefore, it is presumed that human cysticercosis disappears within 10-20 years after disappearance of human T. solium infection.
PERSPECTIVES
In Korea, there was a high prevalence of soil-transmitted helminths (Ascaris lumbricoides, hookworms, and Trichuris trichiura) in the past, but the prevalence has now decreased markedly [65] causing no more serious problems due to these parasitic infections. Lymphatic filariasis, due to Brugia malayi, has also posed a problem in several endemic areas, including Jeju-do [66]. However, a declaration was made in 2009 that lymphatic filariasis has been eliminated from Korea [66]. Now, we expect the complete disappearance of taeniasis from Korea in the near future. One thing to consider is the relative lack of data from North Korea on the prevalence of taeniasis and cysticercosis. An available document on North Koreans reported that the seropositive rate of cysticercosis by ELISA was 9.3% (29/270 people) among people in Cheongjin area in 2004 [67], which was higher than the rates observed in 1993-1994 in South Korea [62]. We do not know the current situation of taeniasis and cysticercosis in North Korea. However, when free communication becomes possible between North Korea and South Korea, the risk for taeniasis transmission should again be taken into considerations.
References
- 1.Eom KS, Rim HJ. Epidemiological understanding of Taenia tapeworm infections with special reference to Taenia asiatica in Korea. Korean J Parasitol. 2001;39:267–283. doi: 10.3347/kjp.2001.39.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. 9th ed. Philadelphia, USA: Lea & Febiger; 1984. pp. 512–522. [Google Scholar]
- 3.Liu HY, Chao D, Fan PC. Prevalence and chemotherapy of taeniasis among the aborigines in Nan-ao District, I-lan County, Northeast Taiwan; Proceed Nat Sci Council Taiwan (Part A: Applied Sciences); 1981. pp. 188–195. [Google Scholar]
- 4.Eom KS, Rim JH. Morphologic descriptions of Taenia asiatica sp. n. Korean J Parasitol. 1993;31:1–6. doi: 10.3347/kjp.1993.31.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Stryker E de M. Extracts from report for 1913, Suan Mine Hospital, Korea. China Med J. 1914;28:277–280. [Google Scholar]
- 6.Kobayashi H, Kwon N. Studies on the intestinal parasites of Koreans: First report. J Chosen Med Ass. 1917;19(suppl):72–78. (in Japanese) [Google Scholar]
- 7.Kojima R, Ko T. Researches on intestinal parasites of Korea in South Gyeongsang-do, especially on the distribution of the liver fluke. J Chosen Med Ass. 1919;26:42–86. (in Japanese) [Google Scholar]
- 8.Uchida R. Results of fecal examination on intestinal helminths among Korean prisoners in the Seodaemoon Jail. J Chosen Med Ass. 1924;46:74–83. (in Japanese) [Google Scholar]
- 9.Mills RG. Parasites, chiefly metazoan, observed in 7,000 specimens of feces from Koreans, with an attempt to interpret the findings. Am J Hyg. 1927;7:222–263. [Google Scholar]
- 10.Oda M. On the distribution of human parasites in Jeonju area. J Chosen Med Ass. 1929;19:1044–1049. (in Japanese) [Google Scholar]
- 11.Takemura E. Microscopical examination for intestinal parasites of 500 out and in-patients in our Department. J Med Coll Keijo. 1934;4:115–121. (in Japanese) [Google Scholar]
- 12.Sekiguchi I, Nagada K, Hanesaka T. Report on intestinal helminthic infections in the inhabitants of Daejo-myon, Kimhae-gun, South Gyeongsang-do (2nd report) Masen No Ikai. 1937;196:1–15. (in Japanese) [Google Scholar]
- 13.Keijo Imperial University. Surveys on living, hygiene and health of a slum quarter in Keijo, Chosen. (1) Results of parasite examination. Mansen No Ikai. 1940;237:23–32. (in Japanese) [Google Scholar]
- 14.Brooke MM, Swartzwelder C, Payne FJ, Weinstein P, Frye WW. Intestinal parasits survey of Korean prisoner of war camp. U S Armed Forces Med J. 1956;7:708–714. [PubMed] [Google Scholar]
- 15.Soh CT, Lee KT, Shin EW, Kang TC. Incidence of parasites in Seoul area based on an examination of the Severance Hospital out-patients. Yonsei Med J. 1961;2:31–41. [Google Scholar]
- 16.Seo BS, Rim HJ, Loh IK, Lee SH, Cho SY, Park SC, Bae JW, Kim JH, Lee JS, Koo BY, Kim KS. Study on the status of helminthic infection in Koreans. Korean J Parasitol. 1969;7:53–70. doi: 10.3347/kjp.1969.7.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Social Affairs and Korean Association for Parasite Eradication. Prevalence of intestinal parasitic infections in Korea. The 1th report. Seoul, Korea: 1971. pp. 1–26. (Monographic series in Korean) [Google Scholar]
- 18.Ministry of Health and Social Affairs and Korean Association for Parasite Eradication. Prevalence of intestinal parasitic infections in Korea. The 2nd report. Seoul, Korea: 1976. pp. 1–247. (Monographic series in Korean) [Google Scholar]
- 19.Ministry of Health and Social Affairs and Korean Association for Parasite Eradication. Prevalence of intestinal parasitic infections in Korea. The 3rd report. Seoul, Korea: 1981. pp. 1–275. (Monographic series in Korean) [Google Scholar]
- 20.Seo BS, Lee SH, Cho SY, Chai JY, Hong ST. An epidemiologic survey on clonorchiasis and metagonimiasis in riverside areas in Korea. Korean J Parasitol. 1981;19:137–150. doi: 10.3347/kjp.1981.19.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health and Social Affairs and Korean Association of Health. Prevalence of intestinal parasitic infections in Korea. The 4th report. Seoul, Korea: 1986. pp. 1–417. (Monographic series in Korean) [Google Scholar]
- 22.Ministry of Health and Social Affairs and Korean Association of Health. Prevalence of intestinal parasitic infections in Korea. The 5th report. Seoul, Korea: 1992. pp. 1–215. (Monographic series in Korean) [Google Scholar]
- 23.Lee SK, Shin BM, Chung NS, Chai JY, Lee SH. Second report on intestinal parasites among the patients of Seoul Paik Hospital (1984-1992) Korean J Parasitol. 1994;32:27–33. doi: 10.3347/kjp.1994.32.1.27. (in Korean) [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health and Social Affairs and Korean Association of Health. Prevalence of intestinal parasitic infections in Korea. The 6th report. Seoul, Korea: 1997. pp. 1–287. (Monographic series in Korean) [Google Scholar]
- 25.Korean Association of Health Promotion. Prevalence of intestinal parasitic infections in Korea. The 7th report. Seoul, Korea: 2004. pp. 1–275. (Monographic series in Korean) [Google Scholar]
- 26.Chai JY, Park JH, Guk SM, Kim HJ, Kim WH, Kim JL, Gu YS, Shin EH, Park HM, Hong KS, Kim SD, Lee SH. Status of intestinal parasite infections among 4,137 residents from provinces nationwide and metropolitan areas in the Republic of Korea (2004) Infect Chemother. 2006;38:198–203. [Google Scholar]
- 27.Cho SH, Lee KY, Lee BC, Cho PY, Cheun HI, Hong ST, Sohn WM, Kim TS. Prevalence of clonorchiasis in southern endemic areas of Korea in 2006. Korean J Parasitol. 2008;46:133–137. doi: 10.3347/kjp.2008.46.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang SY, Loh IK, Park YH, Lim DB. Survey on the helminth ova in the stools of inhabitants in Cheju Province, Korea. Korean J Parasitol. 1964;2(2):131. (6th Annual Meeting abstract) [Google Scholar]
- 29.Kang SY, Kim BC, Loh IK, Park YH, Lim DB. An investigation on taeniasis in Cheju-do Prefecture (Quelpart Island), Korea. Part. II. A study on chemotherapy of taeniasis, the treatment of Taenia saginata and T. solium with bithionol. Korean J Intern Med. 1965;8:23–30. (in Korean) [Google Scholar]
- 30.Cho KM, Hong SO, Kim CH, Soh CT, Kim SH, Kim ON, Kim SH, Yun HC. Studies on taeniasis in Cheju-do. J Korean Mod Med. 1967;7:455–461. (in Korean) [Google Scholar]
- 31.Seo BS, Rim HJ, Cho SY, Ahn JH, Kwak JW, Lee JW, Kang SC. The prevalence of intestinal helminthes in inhabitants of Cheju Do. Korean J Parasitol. 1972;10:100–108. doi: 10.3347/kjp.1972.10.2.100. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH. Survey on taeniasis and eating habits of meat in Jeju-do. Korean J Vet Public Health. 1980;4:52–56. (in Korean) [Google Scholar]
- 33.Joo KH, Seong DR, Cho YJ. An epidemiological survey on the taeniasis in Seoul City and Cheju Do, Korea. Korean J Rural Med. 1985;10:26–35. (in Korean) [Google Scholar]
- 34.Soh CT, Lee KT, Kim SH, Fan PC. Studies on the epidemiology and chemotherapy of taeniasis on Cheju Island, Korea. Yonsei Rep Trop Med. 1988;19:25–31. [Google Scholar]
- 35.Fan PC, Chan CH, Chao D, Chung WC. Studies on some problems of taeniasis among aborigines in Taiwan; Program and Summary of Sino-Japanese Seminar on Parasitic Zoonoses; 28-29 August 1982; Hirosaki, Japan. 1982. pp. 110–115. [Google Scholar]
- 36.Fan PC. Taiwan Taenia and taeniasis. Parasitol Today. 1988;4:86–88. doi: 10.1016/0169-4758(88)90204-9. [DOI] [PubMed] [Google Scholar]
- 37.Chao D, Fan PC. Larval stage of a possible new species of tapeworm from Taiwan aborigines. Chin Biosci. 1986;27:1–6. [Google Scholar]
- 38.Chan CH, Fan PC, Chung WV, Chen YA, Wu CC, Hsu MC, Chao D. Studies on taeniasis in Taiwan. I. Prevalence of taeniasis among aborigines in Wulai District, Taipei County, northern Taiwan; Proceed 1st Sino-American Symp; 1987. pp. 65–77. [Google Scholar]
- 39.Fan PC, Chung WC, Chan CH, Wong MM, Wu CC, Hsu MC, Huang SH, Chen YA. Studies on taeniasis in Taiwan. III. Preliminary report on experimental infection of Taiwan Taenia in domestic animals; Proceed 1st Sino-American Symp; 1987. pp. 119–125. [Google Scholar]
- 40.Fan PC, Chung WC, Lin CY, Soh CT, Lee KT, Kosman ML, Kosin E, Depari AA, Napitupulu T. Studies on taeniasis in Taiwan. VI. Is Taenia saginata from Taiwan, Korea and Indonesia a new species. Chin J Parasitol. 1988;1:56–70. [Google Scholar]
- 41.Ito A, Fan PC, Chung WC, Suzuki M. Cross protection against Taenia taeniaeformis in rats vaccinated with non-viable oncosphere of Asian Taenia or T. saginata. J Helminthol. 1994;68:83–85. doi: 10.1017/s0022149x00013535. [DOI] [PubMed] [Google Scholar]
- 42.Fan PC. Review of taeniasis in Asia. Chin J Microbiol Immunol. 1995;28:79–94. [PubMed] [Google Scholar]
- 43.Fan PC, Lin CY, Chen CC, Chung WC. Morphological description of Taenia saginata asiatica (Cyclophyllidea: Taeniidae) from man in Asia. J Helminthol. 1995;69:299–303. doi: 10.1017/s0022149x00014863. [DOI] [PubMed] [Google Scholar]
- 44.Han HR. A survey on Cysticercus cellulosae infection in swine of Cheju-do. Korean J Public Health. 1969;6:23–28. (in Korean) [Google Scholar]
- 45.Lee KT, Kim CH, Park CT, Lee MY. Cysticercosis and taeniasis in Chollabukdo Province. Korean J Parasitol. 1966;4:39–45. doi: 10.3347/kjp.1966.4.1.39. (in Korean) [DOI] [PubMed] [Google Scholar]
- 46.Hong ST, Hong SJ, Lee SH, Kim IS, Shin JS. A study on the intestinal helminths of the patients in a leprosarium in Korea. Korean J Parasitol. 1983;21:102–104. doi: 10.3347/kjp.1983.21.1.102. (in Korean) [DOI] [PubMed] [Google Scholar]
- 47.Min DY. Cestode infections in Korea. Korean J Parasitol. 1990;28(suppl):123–144. doi: 10.3347/kjp.1990.28.suppl.123. [DOI] [PubMed] [Google Scholar]
- 48.Eom KS, Rim HJ. Natural infections of Asian Taenia saginata metacestodes in the livers of Korean domestic pigs. Korean J Parasitol. 1992;30:15–20. doi: 10.3347/kjp.1992.30.1.15. [DOI] [PubMed] [Google Scholar]
- 49.Eom KS, Rim HJ. Experimental human infection with Asian Taenia saginata metacestodes obtained from naturally infected Korean domestic pigs. Korean J Parasitol. 1992;30:11–24. doi: 10.3347/kjp.1992.30.1.21. [DOI] [PubMed] [Google Scholar]
- 50.Eom KS, Rim HJ, Geerts S. Experimental infection of pigs and cattle with eggs of Asian Taenia saginata with special reference to its extrahepatic viscerotropism. Korean J Parasitol. 1992;30:269–275. doi: 10.3347/kjp.1992.30.4.269. [DOI] [PubMed] [Google Scholar]
- 51.Rim HJ, Song KW, Joo KH, Lee JS, Kim JJ. An epidemiological note on the taeniasis in Korea. Korean J Parasitol. 1980;18:235–240. doi: 10.3347/kjp.1980.18.2.235. (in Korean) [DOI] [PubMed] [Google Scholar]
- 52.Joo KH, Seong DR, Cho YJ. An epidemiological survey on the taeniasis in Seoul city and Cheju-do, Korea. Korean J Rural Med. 1985;10:26–35. (in Korean) [Google Scholar]
- 53.Jeon HK, Kim KH, Chai JY, Yang HJ, Rim HJ, Eom KS. Sympatric distribution of three human Taenia tapeworms collected between 1935 and 2005 in Korea. Korean J Parasitol. 2008;46:235–241. doi: 10.3347/kjp.2008.46.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galan-Puchades MT, Mas-Coma S. Considering Taenia asiatica at species level. Parasitol Today. 1996;12:123. doi: 10.1016/0169-4758(96)80674-0. [DOI] [PubMed] [Google Scholar]
- 55.Galan-Puchades MT, Fuentes MV. The Asian Taenia and the possibility of cysticercosis. Korean J Parasitol. 2000;38:1–7. doi: 10.3347/kjp.2000.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chi JG, Sung RH, Cho SY. Tissue parasitic diseases in Korea. J Korean Med Sci. 1988;3:51–62. doi: 10.3346/jkms.1988.3.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho YJ, Chu JP, Yang MH, Lee JH, Jeong GS. Analysis of parasitic diseases in biopsy in Kunghee Medical center (1972-1983) Korean J Infect Dis. 1998;30:173–179. (in Korean) [Google Scholar]
- 58.Kim J, Chung WS, Cho KH. Status of parasitic infection diagnosed by surgical biopsy in Kwangju and Chollanam-do. Korean J Parasitol. 1994;32:93–100. doi: 10.3347/kjp.1994.32.2.93. (in Korean) [DOI] [PubMed] [Google Scholar]
- 59.Choi WH, Chu JP, Jiang M, Lee YS, Kim BS, Kim DG, Park YK. Analysis of parasitic diseases diagnosed by tissue biopsy specimens at Kyunghee Medical Center (198402005) in Seoul, Korea. Korean J Parasitol. 2010;48:85–88. doi: 10.3347/kjp.2010.48.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong Y, Cho SY, Cho MS, Kwon OS, Kang WS. Seroepidemiological observation of Taenia solium cysticercosis in epileptic patients in Korea. J Korean Med Sci. 1993;8:145–152. doi: 10.3346/jkms.1993.8.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SH, Kim MN, Back BY, Chai JY, Kim TH, Hwang YS. Analysis of parasite-specific antibody positive patients for Clonorchis sinensis, Paragonimus westermani, cysticercus and sparganum using ELISA. Korean J Lab Med. 2003;23:126–131. [Google Scholar]
- 62.Lee MK, Hong SJ, Kim HR. Seroprevalence of tissue invading parasitic infections diagnosed by ELISA in Korea. J Korean Med Sci. 2010;25:1272–1276. doi: 10.3346/jkms.2010.25.9.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chai JY, Hong ST, Choi MH, Shin EH, Bae YM, Hong SJ, Sohn WM, Yu JR, Kho WG, Seo M, Park YK, Han ET. Clinical Parasitology. Seoul, Korea: Seoul National University Press; 2011. pp. 439–442. (Textbook in Korean) [Google Scholar]
- 64.Flisser A. Where are the tapeworms? Parasitol Int. 2006;55:S117–S120. doi: 10.1016/j.parint.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Hong ST, Chai JY, Choi MH, Huh S, Rim HJ, Lee SH. A successful experience of soil-transmitted helminth control in the Republic of Korea. Korean J Parasitol. 2006;44:177–185. doi: 10.3347/kjp.2006.44.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheun HI, Kong Y, Cho SH, Lee JS, Chai JY, Lee JS, Lee JK, Kim TS. Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol. 2009;47:323–335. doi: 10.3347/kjp.2009.47.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen C, Li S, Zheng S, Choi MH, Bae YM, Hong ST. Tissue parasitic helminthiases are prevalent at Cheongjin, North Korea. Korean J Parasitol. 2007;45:139–144. doi: 10.3347/kjp.2007.45.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]