Abstract
In 2005, we reported the infection status of 438 stray cats with various species of intestinal helminths, including nematodes (4 species), trematodes (23 species), and cestodes (5 species) in the Republic of Korea. However, morphologic details of each helminth species have not been provided. In the present study, we intended to describe morphologic details of 13 trematode species which were either new fauna of cats (10 species) or new fauna of not only cats but also all animal hosts (3 species). The worms were fixed in 10% neutral buffered formalin under a cover slip pressure, stained with Semichon's acetocarmine, and then observed using a light microscope equipped with a micrometer. The 13 subjected species included members of the Heterophyidae (Stellantchasmus falcatus, Stictodora fuscata, Stictodora lari, Centrocestus armatus, Procerovum varium, and Cryptocotyle concava), Echinostomatidae (Echinostoma hortense, Echinostoma revolutum, Echinochasmus japonicus, and Stephanoprora sp.), Diplostomidae (Neodiplostomum seoulense), Plagiorchiidae (Plagiorchis muris), and Dicrocoeliidae (Eurytrema pancreaticum). By the present study, Cryptocotyle sp. and Neodiplostomum sp. recored in our previous study were identified as C. concava and N. seoulense, respectively. Three species, P. varium, C. concava, and Stephanoprora sp., are new trematode fauna in Korea.
Keywords: Procerovum varium, Cryptocotyle concava, Stephanoprora sp., intestinal trematode, fauna, stray cat
INTRODUCTION
The stray cat, Felis catus, is well established as a powerful predator in the wild natural ecosystem of the Republic of Korea. This highly adaptable predator actively consume the wide-ranged food stuff originated from the mammalian, avian, reptilian, amphibian, and piscine preys as well as invertebrates. Consequently, it has been known that the stray cat is infected with numerous parasites and act as an important reservoir host for parasites of medical or veterinary importance. On the other hand, surveys on the intestinal parasites of stray cats have been conducted in various regions of the world [1-6]. In the Republic of Korea, studies on helminthic infections of cats have been performed by several workers [7-13].
As trematodes of cats, a total of 10 species, i.e., Clonorchis sinensis, Paragonimus sp., Heterophyes nocens, Heterophyopsis continua, Pygidiopsis summa, Metagonimus yokogawai, Centrocestus sp., Pharyngostomum cordatum, Echinochasmus perfoliatus, and Echinoparyphium sp., were reported in the Republic of Korea until 2000 [7-10]. Subsequently, in 2005, more than 15 species were added to the trematode fauna of felines by Sohn and Chai [12], and Gymnophalloides seoi was found in the small intestines of 2 feral cats from Shinan-gun, Jeollanam-do in 2009 [13]. Among the trematode fauna reported by Sohn and Chai [12], 13 species (Procerovum varium, Stellantchasmus falcatus, Stictodora fuscata, Stictodora lari, Centrocestus armatus, Cryptocotyle sp., Echinostoma hortense, Echinostoma revolutum, Echinochasmus japonicus, Stephanoprora sp., Neodiplostomum sp., Plagiorchis muris, and Eurytrema pancreatitum) were new for cat parasites in Korea. However, no morphologic descriptions were made on these trematode species. Therefore, in the present study, we intended to provide morphologic details and characteristics of these 13 trematode species infecting cats.
MATERIALS AND METHODS
The trematode specimens were collected as previously described [12]. The collected worms were fixed with 10% neutral buffered formalin under a cover glass pressure, stained with Semichon's acetocarmine, and observed using a light microscope equipped with a micrometer (OSM-4, Olympus Co., Tokyo, Japan). All measurements are given in µm unless stated otherwise.
RESULTS
Morphology of worms first recorded in Korea [12]
Procerovum varium (Fig. 1)
Fig. 1.
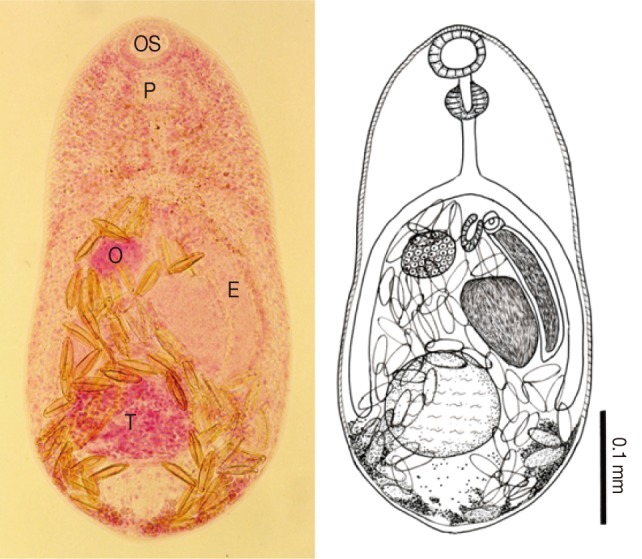
Procerovum varium adult (Semichon's acetocarmine stained and a drawing) recovered from the small intestine of a cat. Body small, 435×235 µm in average size, and having a muscular oral sucker (OS), a pharynx (P), a small ventral sucker with a long and thick-walled expulsor (E), a spherical ovary (O), single globular testis (T), and follicular vitellaria distributed in the post-ovarian fields.
Body small, pear-shaped, 435 (425-445 in range) long and 235 (230-240) wide (n=2), with greatest width at posterior 1/3 of the body. Oral sucker subterminal, 44 (41-46) by 47 (44-51). Prepharynx very short. Pharynx subglobular or elliptical, 37 (36-38) by 35 (33-36). Esophagus short, 53 (51-54) in length. Ceca bifurcating anterior 1/3 and terminating posterior 1/4, middle level of testis. Ventral sucker very small, 25 by 38, embedded in ventrogenital sac. Expulsor long and thick-walled, 156 (153-159) by 29 (26-33). Seminal vesicle saccular, 87 (82-92) by 51. Ovary spherical or subspherical, 44 (41-46) by 47 (46-49), preequatorial and slightly dextral to midline. One testis globular or subglobular, 102 (90-115) by 118 (102-133), lying in middle of hind-body. Vitellaria follicular, distributing in post-testicular fields near posterior extremity. Uterus with eggs occufying from anterior 1/3 to posterior end, most of hind-body. Eggs small, yellow, and 26 (24-28) by 13 (11-14).
Cryptocotyle concava (Fig. 2)
Fig. 2.
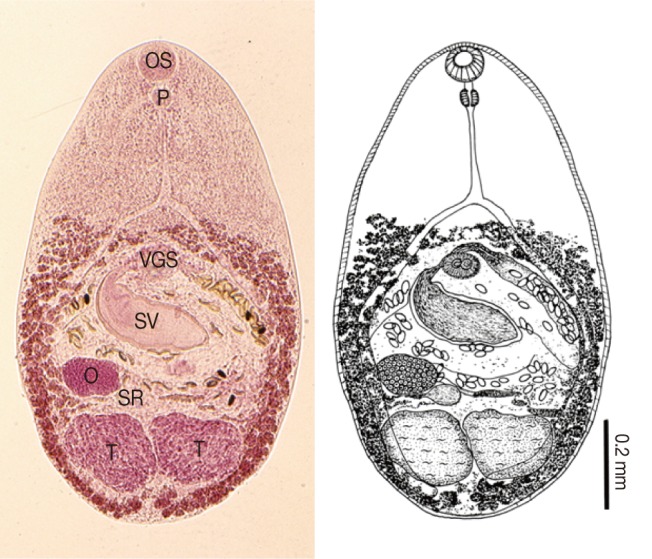
Cryptocotyle concava adult (Semichon's acetocarmine stained and a drawing) recovered from the small intestine of a cat. Body small, 778×576 µm in average size, and having a muscular oral sucker (OS), a pharynx (P), a ventrogenital sac (VGS) with a small ventral sucker, a thick-walled seminal vesicle (SV), a spherical ovary (O), slightly lobulated 2 testes (T), and follicular vitellaria distributed along the extracecal margin from the preacetabular level to the posterior end of the body.
Body small, oval or spatulate, 778 (500-1,170) long and 576 (468-732) wide (n=20). Oral sucker subterminal, 75 (50-84) by 89 (70-105). Pharynx subglobular, 36 (28-49) by 43 (25-55). Esophagus slender, 114 (63-230) in length. Ventrogenital sac elliptical, 52 (35-88) by 65 (48-88), with small ventral sucker and genital pore. Seminal vesicle saccate, thick-walled, 270 (150-332) by 114 (75-153). Ovary spherical, 70 (38-133) by 76 (50-120), lyingy dextral to midline. Seminal receptacle elliptical and small, just behind of the left margin of ovary. Two testes slightly lobulated, closely located in posterior portion; right 182 (108-332) by 85 (50-161); left 169 (110-307) by 89 (50-197). Vitellaria follicular, distributing along extracecal margin from pre-acetabular level to posterior end of body. Eggs small, yellow, and 25.6 (23.0-28.1) by 13.2 (11.0-16.6).
Stephanoprora sp. (Fig. 3)
Fig. 3.
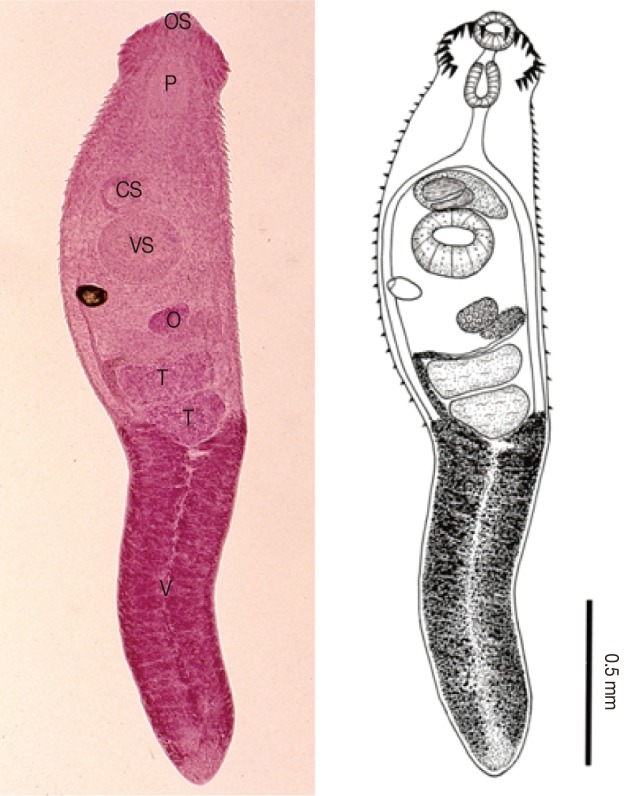
Stephanoprora sp. adult (Semichon's acetocarmine stained and a drawing) recovered from the small intestine of a cat. Body elongated and 2,317×474 µm in average size, having a characteristic head crown with 22 collar spines, a muscular oral sucker (OS), a pharynx (P), a well developed ventral sucker (VS), a cirrus sac (CS), a spherical ovary (O), transversely elliptical 2 testes (T), and follicular vitellaria (V) distributed in the post-testicular fields.
Body elongated, somewhat attenuated at both ends, 2,317 (2,253-2,381) long and 474 (461-486) wide (n=2). BW (body width) to BL (body length) ratio about 20%. Head crown distinct, bearing total 22 collar spines in 1 row, and interrupted at the dorsal side of oral sucker, 135 (125-145) by 305 (300-310). Dorsal spines 45 (43-48) long and 14 broad at base, lateral spines 40 (39-41) by 12, the last one (33 by 11) of 2 end group spines smaller than the second one (38 by 12). Oral sucker subterminal, 105 by 113 (100-125). Prepharynx short. Pharynx subglobular, 133 (130-135) by 105 (100-110). Esophagus slender, 190 (175-205) in length. Ceca bifurcated at 513 (21-24% of body length) from anterior end. Ventral sucker large and well developed, 215 (205-225) by 230 (225-235), located at 713 (27-32% of body length) from anterior end. Cirrus sac well developed and contained saccular seminal vesicle, 225 (215-235) by 123 (110-135). Ovary spherical, submedian, 95 (80-110) by 120 (115-125). Two testes transversely elliptical, directly tandem near the middle portion of the body; anterior 125 (100-150) by 245 (215-275); posterior 140 (130-150) by 218 (200-235). Total testicular region 274 (about 12% of body length). Vitellaria follicular, occupying from 73 from posterior end of body to about middle of posterior testis. Eggs, a few in the uterus, operculated, yellow, 82 (77-92) by 65 (61-69).
Morphology of worms first recorded in cats in Korea [12]
Stellantchasmus falcatus
Body small, 500 (390-555) long and 294 (255-325) wide (n=20). Oral sucker subterminal, 42 (38-48) by 55 (50-60). Pharynx subglobular, 29 (25-33) by 29 (20-40). Esophagus slender, 86 (63-100) in length. Ventral sucker small, 38 (33-43) by 39 (33-45). Expulsor long and thick-walled, 115 (83-138) by 47 (43-55). Seminal vesicle saccate, 81 (63-100) by 28 (25-33). Ovary spherical, 49 (33-63) by 61 (50-73). Two testes ovoid or globular, slightly oblique and widely separated; right 111 (93-126) by 72 (63-83); left 106 (70-125) by 69 (50-88). Vitellaria follicular and distributed in post-ovarian fields. Eggs small, yellow, and 24 (23-25) by 12 (11-13).
Stictodora fuscata
Body small, leaf-like, 657 (490-950) long, and 304 (250-440) wide (n=10). Oral sucker subterminal, 61 (53-70) by 71 (55-95). Prepharynx very short, 28 (10-48) in length. Pharynx subglobular, 59 (50-70) by 40 (30-60). Esophagus short, 31 (15-58) in length. Gonotyl elliptical, 73 (63-100) by 42 (38-53), armed with 13-18 spines. Seminal vesicle constircted into 3 or 4 saccules. Ovary semilunar, 42 (30-55) by 75 (50-110). Two testes bean-shaped, obliquely tandem in middle part of the body; right 49 (38-65) by 103 (80-138), left 54 (43-63) by 101 (75-130). Vitellaria follicular and distributed in post-testicular fields. Eggs small, yellow, and 34 (33-39) by 21 (20-23).
Stictodora lari
Body small, long slender, 628 (540-750) long, and 229 (200-260) wide (n=10). Oral sucker round and subterminal, 47 (40-53) by 59 (53-63). Prepharynx short. Pharynx well developed, 49 (43-53) by 38 (33-45). Esophagus short, 38 (28-55) in length. Gonotyl elliptical, 74 (65-80) by 60 (50-75), armed with numerous spines. Seminal vesicle constricted into 3 or 4 parts, thin-walled, located between ventrogenital sac and ovary. Ovary elliptical, 42 (33-60) by 60 (50-70). Two testes subglobular, obliquely tandem in middle part of the body; right 59 (45-80) by 80 (58-100), left 58 (45-75) by 74 (53-90). Vitellaria follicular and distributed in post-testicular fields. Eggs small, yellow, and 28 (28-29) by 15 (15-16).
Centrocestus armatus
Body very small, 336 (285-420) long, and 167 (155-185) wide (n=10). Oral sucker subterminal, 55 (50-70) by 61 (55-75), armed with about 42 circumoral spines. Prepharynx very short. Pharynx globular, 33 (30-38) by 33 (30-43). Esophagus short, 25 in length. Ventral sucker round or elliptical, 39 (30-45) by 47 (43-50). Seminal vesicle large and saccular, constircted into two parts, locating transversely behind ventral sucker. Ovary elliptical, 55 (45-63) by 39 (30-50). Two testes ellipsoidal, side by side near the posterior end; right 56 (50-65) by 34 (25-40), left 60 (50-80) by 34 (28-40). Vitellaria follicular and distributed along extracecal margins from pharyngeal level to posterior end. Eggs small, yellow, and 28 by 16.
Echinostoma revolutum
Body elongated, somewhat attenuated at both end, 6,488 (6,375-6,600) long and 1,275 (1,000-1,550) wide (n=5). Head crown disinct, bearing about 37 collar spines with 5 end group spines, 290 (285-295) by 553 (550-555). Oral sucker subterminal, 228 (215-240) by 233 (230-225). Prepharynx very short, 125 (100-150) in length. Pharynx subglobular, 200 by 205 (190-220). Esophagus short, 633 (565-700) in length. Ventral sucker large and protruded ventrally, 705 (700-710) in diameter. Cirrus sac well developed and contained saccular seminal vesicle, 475 (450-500) by 258 (250-265). Ovary spherical and on the median line of the body, 198 (195-200) by 353 (350-355). Two testes slightly lobed and tandem; anterior 448 (400-495) by 585 (485-685); posterior 550 by 550 (475-625). Vitellaria follicular, distributing laterally from the anterior level of uterus to posterior end of the body. Eggs operculated, golden yellow, 103 (93-113) by 57 (50-63).
Echinostoma hortense
Body elongated, somewhat attenuated anteriorly, 6,485 (5,625-8,450) long and 1,110 (900-1,725) wide (n=6). Head crown disinct, bearing about 27 collar spines with 4 end group spines, 214 (190-230) by 405 (375-440). Oral sucker subterminal, 193 (170-215) by 210 (185-250). Prepharynx very short, 41 (15-55) in length. Pharynx subglobular, 182 (165-215) by 163 (135-215). Esophagus short, 275 (150-340) in length. Ventral sucker large and protruded ventrally, 566 (490-700) by 580 (500-700). Cirrus sac well developed and contained saccular seminal vesicle, 495 (380-700) by 258 (210-340). Ovary spherical, at right lateral portion of anterior 1/3 of the body, 242 (200-350) by 292 (210-350). Two testes slightly lobed and tandem; anterior 588 (450-750) by 638 (470-1,080); posterior 728 (580-960) by 532 (400-800). Vitellaria follicular, distributing all of the lateral field from the anterior 1/3 level to posterior end of the body. Eggs operculated, golden yellow, 102 (93-128) by 57 (45-68).
Echinochasmus japonicus
Body small, somewhat attenuated anteriorly, 534 (450-640) long and 328 (300-360) wide (n=10). Head crown disinct, bearing total 24 collar spines in one row, and interrupted at the dorsal side of oral sucker, 154 (128-175) by 89 (75-100). Oral sucker subterminal, 48 (38-60) by 55 (50-60). Prepharynx very short. Pharynx subglobular, 52 (50-63) by 43 (35-55). Esophagus slender, 63 (50-83) in length. Ventral sucker large and well developed, 90 (85-108) by 110 (100-118). Cirrus sac well developed and contained saccular seminal vesicle, 123 (105-150) by 61 (50-75). Ovary spherical, submedian, 40 (28-50) by 57 (50-73). Two testes transversely elliptical, directly tandem near the middle portion of the posterior body; anterior 33 (20-45) by 139 (100-175); posterior 50 (38-63) by 123 (93-150). Vitellaria follicular, distributing laterally from the level of ventral sucker down to the posterior end of the body. Eggs operculated, yellow, 83 (75-86) by 58 (51-62).
Neodiplostomum seoulense
Body small, 1,603 (1,290-2,030) in length (n=6), bisegmented into two region, fore-body round, ventrally concave and tapering anteriorly, 835 (710-1,000) by 783 (650-920), hind-body elongate, 768 (520-1,030) by 505 (440-580). Oral sucker subterminal, 73 (68-83) by 83 (75-88). Prepharynx absent. Pharynx subglobular, 74 (70-75) by 83 (58-55). Esophagus almost absent. Ventral sucker small, 85 (65-95) by 102 (88-118). Tribocytic organ slightly elliptical with a median slit, 319 (225-400) by 311 (200-400). Ovary spherical, 113 (90-150) by 180 (150-225). Two testes dumbel-shaped; anterior 150 by 275; posterior 222 (165-300) by 390 (370-425). Vitellaria follicular, mostly distributing from just anterior to the ventral sucker to junctional portion of the 2 bodies. Eggs large, yellow, 90 (83-95) by 53 (50-56).
Plagiorchis muris
Body elongated, tapering at both end, 1,380 long and 460 wide (n=1). Oral sucker subterminal, 170 by 173. Pharynx subglobular, 70 by 78. Esophagus short, 75 in length. Ventral sucker slightly smaller than oral sucker, 138 by 138. Seminal vesicle elongated and saccate, terminating between ventral sucker and ovary. Ovary spherical, preequatorial, located to the right of the median line, 133 by 138. Two testes slightly spherical, postequatorial, obliquely tandem; anterior 200 by 188; posterior 270 by 195. Vitellaria follicular, distributing laterally from the level of pharynx to the posterior end of body. Uterus passing posteriorly between 2 testes. Eggs conspicuously operculated, golden yellow, 33.5 (32.5-35.0) by 19.5 (18.8-20.0).
Eurytrema pancreaticum
Body leaf-like with a terminal projection, appoximately 10,170 (from the broken anterior margin to the posterior end: 7,250) long and 3,900 wide (n=1). Ceca slightly convulute, terminate at the posterior 1/6 of body length. Ventral sucker large and well developed, 1,110 by 960. Cirrus sac well developed and contained saccular seminal vesicle, 1,080 by 350. Two testes lobed, locate the both side of ceca at the posterior level of ventral sucker, right 470 by 450; left 460 by 370. Ovary lobed, submedian, 450 by 350. Vitellaria follicular, distributing laterally at submedian level. Eggs operculated, dark brown and thick shelled, 44.6 (43.8-45.0) by 29.9 (28.8-30.0).
DISCUSSION
Trematode infections in cats have been reported by several workers in Korea. In 1967, Kang [7] detected 2 trematode species, i.e., C. sinensis and Paragonimus sp., in cats from a western region of Gyeongsangnam-do. In 1979, Lee [8] reported 6 trematode species, namely, C. sinensis, H. nocens, M. yokogawai, Centrocestus sp., E. perfoliatus, and Echinoparyphium sp., in cats from Gyeongsangbuk-do [8]. Eom et al. [9] described heterophyid flukes, including H. continua, H. nocens, and P. summa, from cats purchased at Jungang Market in Seoul in 1985 [9]. Huh et al. [10] in 1993 found 2 trematode species, i.e., C. sinensis and P. cordatum, in cats purchased at a market in Seoul. Therefore, before 2005, a total of 10 trematode species (C. sinensis, Paragonimus sp., M. yokogawai, H. nocens. H. continua, P. summa, Centrocestus sp., E. perfoliatus, Echinoparyphium sp., and P. cordatum) were known among the trematode fauna of Korean cats [12].
Subsequently, in 2005, Sohn and Chai [12] detected 23 trematode species which included C. sinensis, Paragonimus westermani, Eurytrema pancreatitum, P. cordatum, Metagonimus spp., H. nocens, P. summa, H. continua, Stictodora fuscata, S. lari, Acanthotrema felis, Stellantchasmus falcatus, Centrocestus armatus, Procerovum varium, Cryptocotyle sp., Echinostoma revolutum, Echinostoma hortense, Echinochasmus japonicus, Stephanoprora sp., Plagiorchis muris, Neodiplostomum sp., unidentified echinostome larvae, and a diplostomulum (mesocercaria of Diplostomum sp.) in 438 stray cats from a wholesale house of animals located in Gupo, Buk-gu, Busan Metropolitan City, Korea. Among them, 13 species were newly recorded as cat intestinal flukes [12]; 10 species (S. falcatus, S. fuscata, S. lari, C. armatus, E. hortense, E. revolutum, E. japonicus, N. seoulense, P. muris, and E. pancreatitum) were new fauna of cats and 3 species (P. varium, C. concava, and Stephanoprora sp.) were new fauna of not only cats but also all animal hosts in Korea. There was a necessity to provide morphologic details of these trematodes. Therefore, in the present study, we intended to record the morphologic details of these 13 new fauna of cats.
With regard to cat trematode parasites, Scholz et al. [1] reported 5 species (Opisthorchis viverrini, Haplorchis pumilio, Haplorchis taichui, Haplorchis yokogawai, and S. falcatus) in domestic cats from Vientiane province, Lao PDR. In several regions of Egypt, Kuntz and Chandler [14] detected 14 trematode species (Heterophyes heterophyes, Heterophyes aequalis, H. pumilio, H. taichui, H. yokogawai, S. falcatus, Pygidiopsis genata, Phagicola longicollis, P. ascolonga, Stictodora sawakinensis, Echinochasmus liliputanus, Stephanoprora denticulatoides, Mesostephanus appendiculatus, and Cynodiplostomum namrui) from domestic cats. Most of these trematode species detected from cats are zoonotic and transmitted via eating raw flesh of freshwater or brackish water fish. Therefore, it is suggested that stray cats may play an important role as a reservoir host in endemic areas of zoonotic trematodes.
The genus Procerovum is a small trematode group of the family Heterophyidae, and characterized by possessing a single testis and a large prominent seminal vesicle modified into an expulsor. Infections by these flukes have been recorded mainly in birds and occasionally in mammals, including humans from China, Japan, the Philippines, Vietnam, India, and Australia [15,16]. Only 3 species, namely P. varium, P. calderoni, and P. cheni, are recognized to be valid by morphologic differences, including the extent of ceca and size of the expulsor. Our specimens are characterized by the presence of a saccular seminal vesicle with thick-walled chambers and an expulsor less than 160 µm long. Meanwhile, P. calderoni has a very long expulsor measuring longer than 200 µm, and P. cheni has a bipartite seminal vesicle with thin-walled chambers and an expulsor shorter than 100 µm long [15]. Moreover, our specimens were morphometrically very similar with P. varium from Vietnam which was described by Chai et al. [16]. Accordingly, it is confirmed that our specimen is identical to P. varium, and this species is indigenously distributed in Korea.
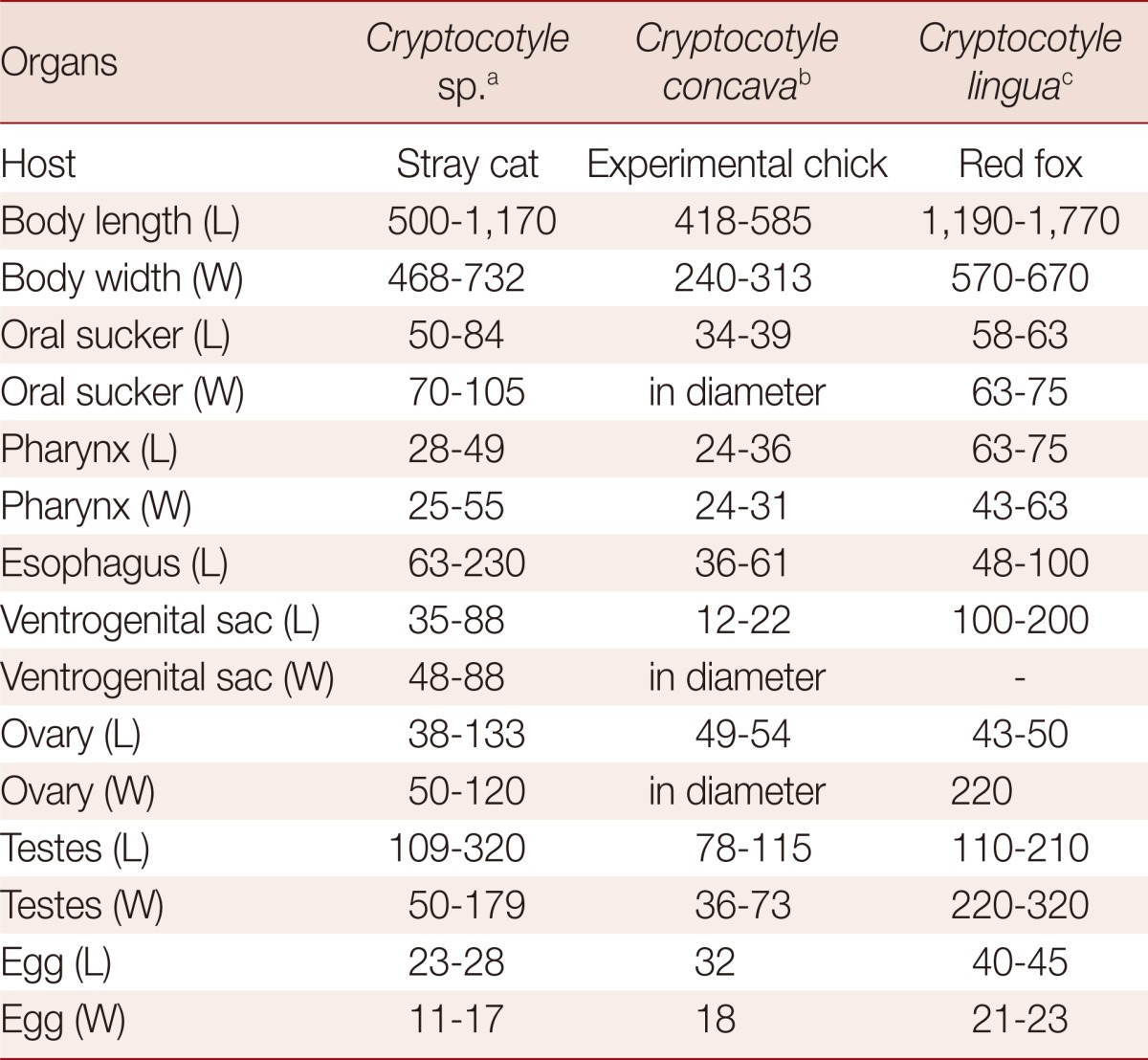
Trematodes of the genus Cryptocotyle (Heterophyidae) are intestinal parasites of fish-eating birds and mammals, and they are widely distributed throughout the world. Among 11 species recorded, Cryptocotyle lingua is known from various species of birds and mammals, including cats, Felis catus. This species was also found from herring gulls (Larus argentatus vegae), stray dogs (Canis familiaris), and red foxes (Vulpes vulpes schrencki) in Japan [17]. However, our specimens were morphologically compatible with C. concava, rather than C. lingua. The body shape is usually elongated in C. lingua, while it is somewhat oval or spatulated in C. concava which was consistent with our specimens [18]. The shape of testes, slightly lobed or severely lobed, is somewhat obscure as a differential point between C. lingua and C. concava. The location of 2 testes is side by side in C. concava and in our specimens, while it is slightly oblique in C. lingua. The distribution of vitellaria, extending anteriorly to mid-point between the ventral sucker and intestinal bifurcation, is commonly revealed in C. lingua, C. concava, and also our specimens, unlike in C. jejuna in which vitellaria extending to the level of the anterior edge of the ventral sucker. The egg size in our specimens was more or less smaller than those of C. lingua and C. concava (Table 1). Based on the aforementioned morphologic characters, our specimens were almost idendical with C. concava except in the size of eggs.
Table 1.
Comparison of Cryptocotyle sp. morphometrics with those of previous studies
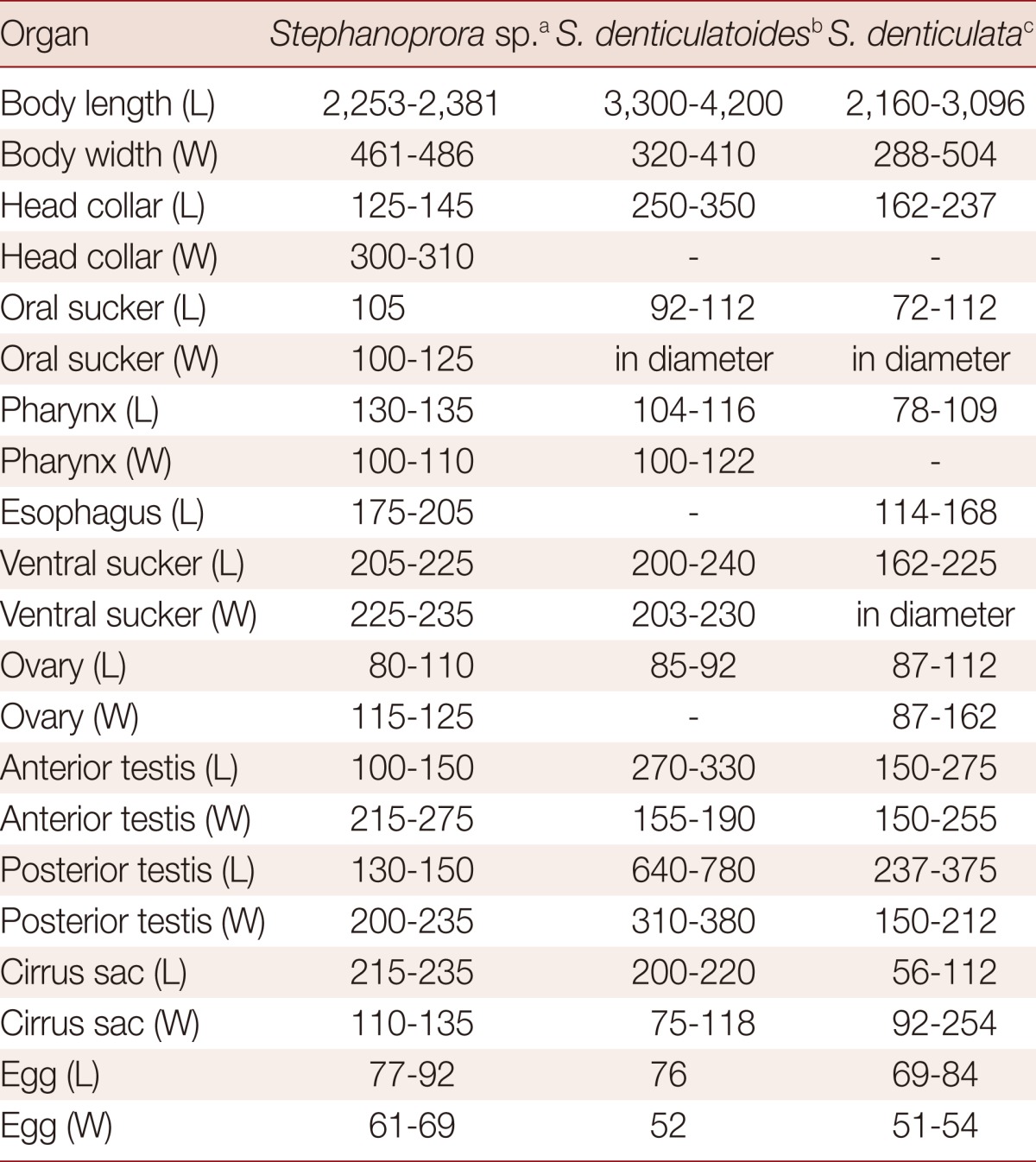
The genus Stephanoprora (Echinostomatidae) is a group of echinostomatid flukes, and mainly found in birds and rarely in mammals. Among the species described, S. denticulatoides was found in dogs from Crimea and cats from the Mediterranean coast of Egypt [14,19]. Isaichikoff [19] recorded this echinostome as a new species based on some morphological differences from a close species, S. denticulata. He regarded following characters as differential points from S. denticulata; large collar spines, larger body, suckers, ovary, and testes, and more anterior extending of the vitellaria [19]. However, Stephanoprora species are difficult to distinguish on the basis of adult morphology alone. Since this fluke groups have been described from birds and mammals of wide geographical ranges, they can differ in important morphologic characters, i.e., measurements of the body and internal organs, shape and location of internal organs, shape and arrangement of collar spines, and distribution of vitelline glands, according to the age (maturity) of worms recovered and host species. Moreover, the quality of worm specimens can affect the species identification. Accordingly, the more solid index is needed for the definitive identification of Stephanoprora species.
It has been known that more than 13 species have the following morphologic characters like S. denticulata; a total of 22 collar spines, an unlobed ovary, and the anterior limits of the vitelline glands fluctuating between the anterior limits of the anterior testis and the testicular junction [20]. Among them, 2 species recovered from mammalian hosts, S. denticulatoides and S. denticulata, were compared with our specimens from cats (Table 2) [14,20]. Our specimens (about 20% of BW/BL ratio) are somewhat stouter than S. denticulatoides (about 10%) and S. denticulata (about 15%). However, the sucker ratio, diameter of the oral sucker to the ventral sucker, 1:2.07-2.13, is nearly equal with those of 2 species. The arrangement pattern of 22 collar spines is nearly the same, but the size of each spines is more or less smaller than those of the 2 species. The egg size was somewhat larger in our specimens. On the other hand, from the standpoint of some morphologic characters, such as the anterior limits of the vitelline glands, smaller size of the eggs than the ovary, uterine extent, diameter of suckers, sucker ratio, and having 2 corner spines on each side of the pharynx, about 10 species, including S. denticulatoides are regarded as the synonyms of S. denticulata [20]. Conclusively, based on the aforementioned comparative morphologies, it is suggested that our specimens, tentatively called Stephanoprora sp., should be considered identical with S. denticulata although there are minor differences between them and lacking enough number of our specimens.
Table 2.
Comparison of Stephanoprora sp. morphometrics with those of previous studies
The remaining 10 species (S. falcatus, S. fuscata, S. lari, C. armatus, E. revolutum, E. hortense, E. japonicus, N. seoulense, P. muris, and E. pancreatitum), newly recorded as cat flukes in Korea, are all zoonotic trematodes, and their infections to humans and animals have been sporadically reported [21]. With the exception of humans and cats, a few animal species have been recorded as natural definitive hosts of these flukes in Korea. For example, adults of C. armatus were recovered from egrets [22], E. revolutum from rats [23], E. hortense from rats, dogs, and mice [24-27], E. japonicus from egrets, ducks, and shrews [22,27,28], and N. seoulense and P. muris from rats and mice. Before the present study, N. seoulense and P. muris were described only from rodent hosts (Rattus norvegicus and Apodemus agrarius), and they were somewhat smaller than our specimens [24,25,29,30].
E. pancreaticum normally parasitizes the biliary and/or pancreatic duct of ruminants, such as cattle, sheep, goat, and rabbit. However, sometimes this fluke accidentally infects humans. In Korea, human infections by this fluke were reported by detecting eggs in stool examinations [31,32]. Adult worms were found at a human autopsy case in Japan [33]. The specimens from this human were about 10.5 mm in length and 6 mm in width, having the oral sucker (2.0 mm in diameter) larger than the ventral sucker (1.5 mm in diameter) and characteristic dark brown eggs (av. 47 by 30 µm in size) [33]. Although our specimen is partly broken and somewhat smaller than those from a human case, morphologic characteristics, including convoluted ceca, lobed ovary, 2 lobed testes, and operculated dark brown eggs, were identical with that of the previous study [33].
ACKNOWLEDGMENTS
This work was financially supported by a grant from the National Institute of Biological Resources (NIBR), Korea, as a part of the Discovery of Korean Indigenous Species project. We thank Jung-A Kim, Department of Parasitology, Gyeongsang National University School of Medicine, Jinju, Korea, for her help in the recovery of worms.
References
- 1.Scholz T, Uhlírová M, Ditrich O. Helminth parasites of cats from the Vientiane province, Laos, as indicators of the occurrence of causative agents of human parasitoses. Parasite. 2003;10:343–350. doi: 10.1051/parasite/2003104343. [DOI] [PubMed] [Google Scholar]
- 2.Borthakur SK, Mukharjee SN. Gastrointestinal helminthes in stray cats (Felis catus) from Aizawl, Mizoram, India. Southeast Asian J Trop Med Public Health. 2011;42:255–258. [PubMed] [Google Scholar]
- 3.Khalafalla RE. A survey study on gastrointestinal parasites of stray cats in northern region of Nile delta, Egypt. PLoS One. 2011;6:e20283. doi: 10.1371/journal.pone.0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millán J, Casanova JC. High prevalence of helminth parasites in feral cats in Majorca Island (Spain) Parasitol Res. 2009;106(1):183–188. doi: 10.1007/s00436-009-1647-y. [DOI] [PubMed] [Google Scholar]
- 5.Labarthe N, Serrão ML, Ferreira AM, Almeida NK, Guerrero J. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Vet Parasitol. 2004;123:133–139. doi: 10.1016/j.vetpar.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Adams PJ, Elliot AD, Algar D, Brazell RI. Gastrointestinal parasites of feral cats from Christmas Island. Aust Vet J. 2008;86:60–63. doi: 10.1111/j.1751-0813.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 7.Kang HJ. Studies on the parasitic helminths of the cats in western province of Kyung Sang Nam-do. Res Bull Chinju Agric Coll. 1967;6:91–96. (in Korean) [Google Scholar]
- 8.Lee HS. A survey on helminth parasites of cats in Gyeongbuk Area. Korean J Vet Res. 1979;19:57–61. (in Korean) [Google Scholar]
- 9.Eom KS, Son SY, Lee JS, Rim HJ. Heterophyid trematodes (Heterophyopsis continua, Pygidiopsis summa and Heterophyes heterophyes nocens) from domestic cats in Korea. Korean J Parasitol. 1985;23:197–202. doi: 10.3347/kjp.1985.23.2.197. [DOI] [PubMed] [Google Scholar]
- 10.Huh S, Sohn WM, Chai JY. Intestinal parasites of cats purchased in Seoul. Korean J Parasitol. 1993;31:371–373. doi: 10.3347/kjp.1993.31.4.371. [DOI] [PubMed] [Google Scholar]
- 11.Yang HJ, Park TW, Cheon SJ, Yoon YB, Kim NJ, Park BK, Kim CS. Internal parasites of cats in Iri and its vicinity. Korean J Vet Serv. 1995;18:33–40. (in Korean) [Google Scholar]
- 12.Sohn WM, Chai JY. Infection status with helminthes in feral cats purchased from a market in Busan, Republic of Korea. Korean J Parasitol. 2005;43:93–100. doi: 10.3347/kjp.2005.43.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin EH, Park JH, Guk SM, Kim JL, Chai JY. Intestinal helminth infections in feral cats and a raccoon dog on Aphaedo Island, Shinan-gun, with a special note on Gymnophalloides seoi Infection in cats. Korean J Parasitol. 2009;47:189–191. doi: 10.3347/kjp.2009.47.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuntz RE, Chandler AC. Studies on Egyptian trematodes with special reference to the heterophyids of mammals. I. Adult flukes, with descriptions of Phagicola longicollis n. sp., Cynodiplostomum namrui n. sp., and a Stephanoprora from cats. J Parasitol. 1956;42(4 Section 1):445–459. [PubMed] [Google Scholar]
- 15.Umadevi K, Madhavi R. Observations on the morphology and life-cycle of Procerovum varium (Onji & Nishio, 1916) (Trematoda: Heterophyidae) Syst Parasitol. 2000;46:215. doi: 10.1023/a:1006398205390. [DOI] [PubMed] [Google Scholar]
- 16.Chai JY, De NV, Sohn WM. Foodborne trematode metacercariae in fish from northern Vietnam and their adults recovered from experimental hamsters. Korean J Parasitol. 2012;50:317–325. doi: 10.3347/kjp.2012.50.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura Y. Cryptocotyle lingua (Creplin) from the red fox, Vulpes vulpes schrencki Kishida. Res Bull Meguro Parasit Mus. 1973;7:15–16. [Google Scholar]
- 18.Hoffman GL. Studies on the life cycle of Cryptocotyle concavum from the common sucker and experimentally in the chick. Proc North Dakota Acad Sci. 1957;11:2. [Google Scholar]
- 19.Isaichikoff IM. Parasitic worms of domestic carnivores of Crimea. Uchen Trudy Sibirsk Vet. 1925;6:47–104. (in Russian) [Google Scholar]
- 20.Nasir P, Scorza JV. Studies on freshwater larval trematodes. 18. The life cycle of Stephanoprora denticulata (Rudolphi, 1802) Odhner, 1910 (Trematoda: Digenea: Echinostomatidae) Z Parasitenkd. 1968;30:134–148. doi: 10.1007/BF00259722. [DOI] [PubMed] [Google Scholar]
- 21.Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 22.Ryang YS, Ahn YK, Yoon MB. Trematode infections in the small intestine of Egretta alba medesta in Kangwon-do. Korean J Parasitol. 1991;29:227–233. doi: 10.3347/kjp.1991.29.3.227. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Sohn WM, Chai JY. Echinostoma revolutum and Echinoparyphium recurvatum recovered from house rats in Yangyang-gun, Kangwon-do. Korean J Parasitol. 1990;28:235–240. doi: 10.3347/kjp.1990.28.4.235. [DOI] [PubMed] [Google Scholar]
- 24.Seo BS, Rim HJ, Lee CW. Studies on the parasitic helminths of Korea I. Trematodes of rodents. Korean J Parasitol. 1964;2:20–26. doi: 10.3347/kjp.1964.2.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Seo BS, Cho SY, Hong ST, Hong SJ, Lee SH. Studies on parasitic helminths of Korea V. Survey on intestinal trematodes of house rats. Korean J Parasitol. 1981;19:131–136. doi: 10.3347/kjp.1981.19.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Cho SY, Kang SY, Ryang YS. Helminthes infections in the small intestine of stray dogs in Ejungbu City, Kyunggi Do, Korea. Korean J Parasitol. 1981;19:55–59. doi: 10.3347/kjp.1981.19.1.55. [DOI] [PubMed] [Google Scholar]
- 27.Chai JY, Park JH, Jung BK, Guk SM, Kim JL, Shin EH, Klein TA, Kim HC, Chong ST, Baek LJ, Song JW. Echinostome infections in the striped-field mouse, Apodemus agrarius, and the Ussuri white-toothed shrew, Crocidura lasiura, caught near the demilitarized zone, Gyonggi-do (province), republic of Korea. Korean J Parasitol. 2009;47:311–314. doi: 10.3347/kjp.2009.47.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eom KS, Rim HJ. A study on the parasitic helminths of domestic duck (Anas platyrhynchos var. domestica Linnaeus) in Korea. Korean J Parasitol. 1984;22:215–221. doi: 10.3347/kjp.1984.22.2.215. [DOI] [PubMed] [Google Scholar]
- 29.Chai JY, Park JH, Guk SM, Kim JL, Kim HJ, Kim WH, Shin EH, Klein TA, Kim HC, Chong ST, Song JW, Baek LJ. Apodemus agrarius as a new definitive host for Neodiplostomum seoulense. Korean J Parasitol. 2007;45:157–161. doi: 10.3347/kjp.2007.45.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai JY, Park JH, Guk SM, Kim JL, Kim HJ, Kim WH, Shin EH, Klein TA, Kim HC, Chong ST, Song JW, Baek LJ. Plagiorchis muris infection in Apodemus agrarius from northern Gyeonggi-do (Province) near the demilitarized zone. Korean J Parasitol. 2007;45:153–156. doi: 10.3347/kjp.2007.45.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Im KI, Koh TY. One case of Dicrocoeliidae infection. Korean J Parasitol. 1971;9:58–60. doi: 10.3347/kjp.1971.9.2.58. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Park SL, Sohn WM, Lim YJ. A case of Dicrocoeliidae ova. Dong-A J Med. 1993;5:135–137. (in Korean) [Google Scholar]
- 33.Ishii Y, Koga M, Fujino T, Higo H, Ishibashi J, Oka K, Saito S. Human infection with the pancreas fluke, Eurytrema pancreaticum. Am J Trop Med Hyg. 1983;32:1019–1022. doi: 10.4269/ajtmh.1983.32.1019. [DOI] [PubMed] [Google Scholar]


