Abstract
Background
Pancreatic acinar cells are commonly co-transplanted along with islets during auto-and allo-transplantations. The aims of this study were to identify how acinar cell proteases cause human islet cell loss before and after transplantation of impure islet preparations and to prevent islet loss and function with supplementation of alpha-1 antitrypsin (A1AT).
Methods
Acinar cell protease activity, insulin levels, and percent islet loss were measured after culture of pure and impure clinical islet preparations. The effect of proteases on ultra-structure of islets and beta cell insulin granules were examined by transmission electron microscopy (TEM). The number of insulin granules and insulin-labeled immune-gold particles were counted. The in vivo effect of proteases on islet function was studied by transplanting acinar cells adjacent to islet grafts in diabetic mice. The effects of A1AT culture supplementation on protease activity, insulin levels, and islet function were assessed in pure and impure islets.
Results
Islet loss after culture was significantly higher in impure relative to pure preparations (30 vs. 14%, p<0.04). Lower islet purity was associated with increased protease activity and decreased insulin levels in culture supernatants. Reduced beta cell insulin granules and insulin degradation by proteases were confirmed by TEM. Transplantation results showed delayed islet graft function when acinar cells were transplanted adjacent to the islets under the kidney capsule. Supplementation of A1AT to impure islet cultures maintained islet mass, restored insulin levels, and preserved islet functional integrity.
Conclusions
Culture of impure islets in the presence of A1AT prevents insulin degradation and improves islet recovery.
Keywords: Islet cell transplantation, Insulin degradation, Acinar cell protease, Impure islet culture, Protease inhibitor and Alpha-1 antitrypsin
INTRODUCTION
The process of human islet isolation yields pure and impure (islet and acinar cells) islet cell preparations (1-3). Both pure and impure islets (down to 30% purity) are commonly transplanted in order to maximize the islet dose in auto- and allo-transplantation. According to the collaborative islet transplant registry 2008 report, the average islet purity from allo-transplantation preparations (n=489) was only 63.4% ± 17.5% (4). In our center, we have performed over 375 islet auto-transplantations as well as several allo-transplantations (5-8) and in many of these cases the transplantation of unpurified / impure islets was unavoidable.
Islet culture before clinical allo-transplantation is widely considered to be advantageous for transplant recipients; however, loss of islet cell mass throughout the culture period (9), especially in impure islet cultures, is frequently observed and has been a major impediment to successful transplantation in many centers. In our preliminary studies we reported that when islets are cultured in the presence of acinar cells (impure preparations) they are exposed to various proteolytic enzymes–especially trypsin, chymotrypsin, and elastase (TCE)—released from dying acinar cells that are potentially harmful to islets (10). Interestingly, we observed that insulin in the culture milieu (secreted by islets and present in supplements of culture media) is degraded in the presence of acinar cell proteases. This is a strong indication that the release of proteases in impure islet cultures creates an inappropriate culture environment for optimal survival and function of islets. Furthermore, we anticipate a similar islet cell loss may occur after transplantation of impure islets because proteases affect beta cell insulin granules. During transplantation, islets and acinar cells are forced to be closely packed in the capillaries of the portal vein, and as acinar cells dye, the released proteases (TCE) may be deleterious to nearby islets. Similarly in past research studies, Grey et al. (11) have reported that a delay in islet graft function in mouse models is due to the detrimental effect of acinar cell proteases.
In this study, we further evaluated the effect of proteases on intact insulin, islet cell structure, and beta cell insulin granules by insulin ELISA, SDS-PAGE, and TEM. Additionally, the in vivo effect of acinar cells on islet graft function was studied in diabetic nude mice by transplanting islets adjacent to acinar cells under the kidney capsule. Then, in an attempt to optimize the survival and functional capacity of islets co-cultured with acinar cells, we supplemented culture media with a clinical grade protease inhibitor, alpha-1 antitrypsin (A1AT).
A1AT is a serine protease inhibitor naturally secreted by the liver to protect body tissues from enzymes of inflammatory cells. Commercial clinical grade human A1AT is commonly used to treat emphysema, and has shown potential in islet research by protecting islet beta cells against apoptosis (12) as well as improving islet graft survival in mice (13-17). In our preliminary study, we have observed A1AT blocks wide spectrum of acinar cell proteases. The main goal of this study was to determine whether islet grafts are depreciated by acinar cell proteases and if islet cultures could benefit from A1AT supplementation. This study demonstrated that supplementation of A1AT in islet culture reduces islet cell loss and protects insulin from degradation by inhibiting the activity of acinar cell proteases.
RESULTS
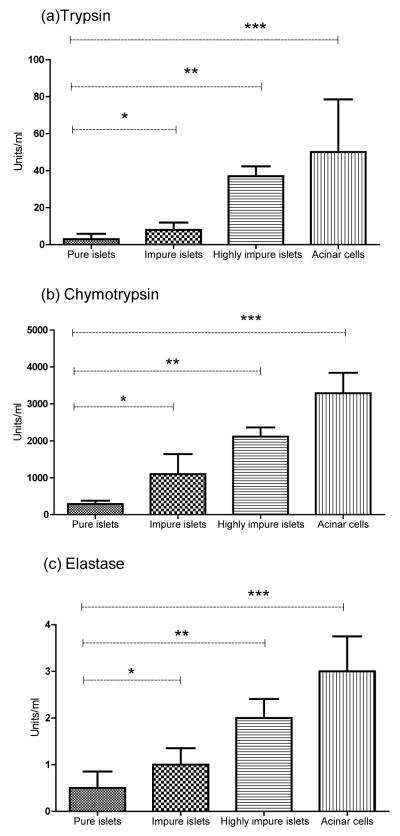
Islet cell death during culture is frequently observed with the percent islet loss generally more in impure islet cultures than pure islet cultures. In our clinical islet preparations, on the day of transplant the average islet cell loss after culture for 36-72 hrs was 14% in pure preparations (n=10), and 30% in impure preparations (n=8)(p=0.04; Fig.1a) resulting in fewer islets available for transplantation. Proteolytic enzymes (TCE) are released as exocrine cells die throughout the islet isolation process. Exocrine contamination persisting after purification and carrying into culture in varying degrees may be detrimental to co-cultured islets. Measuring the levels of TCE in supernatants of pure (80-90% purity), impure (50-60% purity), and highly impure (30-40% purity) islet cultures and acinar cell cultures by fluorometric spectroscopy, a significant increase in each protease activity was observed to be directly proportional to the level of exocrine contamination (Fig. 2a, b, c), agreeing with previous findings published in our preliminary report (10).
Figure 1.
A: In human clinical islet preparations, pure (83% ± 8.2% purity) and impure islets (40% ± 10.6% purity) were cultured in identical culture medium. Loss of islet cell mass during culture was calculated as the % difference between pre-culture IEQ and post-culture IEQ. The mean islet cell loss was found to be 14% in pure cultures (n=10) and 30% in impure cultures (n=8), *p=0.04.
B: In research preparations (n=8), insulin levels were measured from the culture supernatant of pure (80-90% purity), impure (50-60% purity), and highly-impure (30-40% purity) islets. Islets were cultured overnight at 37°C in identical culture conditions. Low levels of insulin were measured from the culture supernatant of impure and highly impure islets compare to pure islets, even though 2000 islets (IEQ) were uniformly cultured in all three conditions.
C: Impure human islets (50-60% purity) were cultured in CMRL-1099 supplemented media with the presence or absence of A1AT to mimic the clinical islet culture (n=8). Islet cell loss was higher in non-treated control group (B) when compared to (A) A1AT treated group. The islet culture recovery was significantly high in A1AT treated group (27%).
D: In human islet research preparations (n=8), insulin levels were measured from culture supernatants of pure (80-90% purity), impure (50-60% purity), and highly-impure islets (30-40% purity). All three conditions uniformly contained 2000 islets (IEQ) and were cultured overnight at 37°C in the presence or absence of A1AT supplementation. The insulin levels measured from A1AT supplemented groups (*pure-A1AT, p<0.0001; ** impure-A1AT, p=0.02; and highly-impure-A1AT, ***p=0.01) were significantly higher when compared to non-treated groups.
Figure 2.
Measurement of activated proteases, (A) trypsin, (B) chymotrypsin, and (C) elastase, in cultures supernatant of pure islets (80-90% purity), impure islets (50-60% purity), highly-impure islets (30-40% purity), and acinar cells in research preparation (n=8). The culture supernatants were taken within 24 hrs for protease measurement. The TCE activity is statistically significant across different levels of purity. (A) Trypsin activity in pure islets were compared to impure (*p=0.005), highly-impure (**p=0.003), and acinar cells (***p=0.02). (B) Chymotrypsin activity in pure islets were compared to impure (*p=0.001), highly impure (**p=0.002), and acinar cells (***p=0.0084) and (C) elastase activity in pure islets were compared to impure (*p=0.014), highly impure (**p=0.002), and acinar cells (***p=0.008).
Insulin measurements of islet culture supernatants revealed significantly lower levels of insulin in lower purity groups. Insulin levels measured in impure (n=8) and highly impure (n=8) islet cultures were 30% (p<0.01) and 16% (p<0.01) of the insulin level achieved in pure cultures, respectively, even though 2000 islets (IEQ) were uniformly present across purity groups (Fig. 1b). This assay in correlation with the previous results obtained from protease quantitation; therefore, reveal a significant inverse relationship between protease activity and insulin level in the context of islet culture purity.
In our preliminary report (10) we confirmed the correlation between acinar cell proteases and the decreased insulin levels observed in impure islet cultures. Visualized by SDS-PAGE, the incubation of insulin with commercial TCE proteases or acinar cell supernatant resulted in the degradation of insulin protein. This result in combination with decreased islet culture recoveries in impure islet cultures shows a strong indication that acinar cell proteases decrease islet survival.
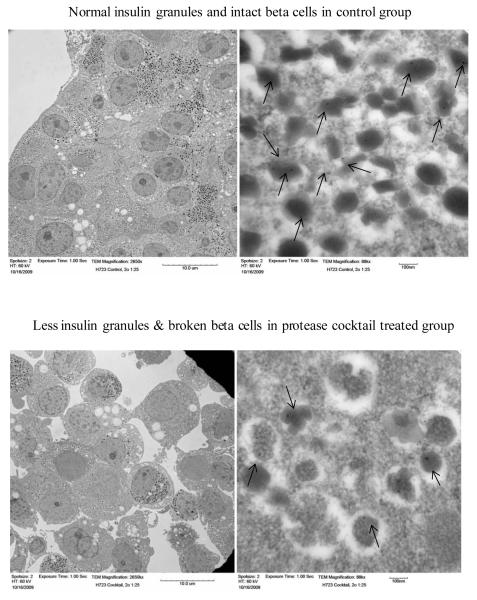
After determining the in vitro effect of proteases on insulin, electron microscopy was used to further examine protease action at the cellular level within beta cells. Quantification of insulin granules and immuno-gold particles in protease treated groups relative to untreated control groups revealed a reduction of intracellular insulin granules and gold particles of 65% and 64%, respectively, when treated with trypsin, 14% and 10% with chymotrypsin, 32% and 31% with elastase, 42% and 41% with a TCE cocktail, and 22% and 19% with acinar cell culture supernatant. Figure 3 represents ultrastructural changes of beta cell morphology after treatment of pure islets with a protease cocktail, depicting the disruption of beta cell membranes as well as a reduction of immuno-gold labeled insulin granules compared to the intact beta cells and number of insulin granules observed in untreated control islets.
Figure 3.
The effects of proteases on human islet integrity and insulin granules in beta cells were studied using transmission electron microscopic (TEM) immuno-gold labeling technique. The insulin secretory granules are positively marked with gold particles. Control (non-treated) islet cells show normal insulin granules and intact beta cells. Protease (cocktail) treated islet cells clearly show a reduction of insulin granules and broken beta cells. Immuno gold labeling of insulin granule was observed at 88kx magnification. Arrows indicate immuno-gold particles in beta cells.
The effect of acinar cells was observed with in vivo study of diabetic nude mice (n=15) which received 2,000 islets (IEQ) of either handpicked pure islets, impure islets, or pure islets transplanted adjacent to acinar cells. Normoglycemia (reversal of diabetes) with a blood-glucose level <200mg/dL was observed for 30 days post-transplantation in all mice receiving pure islets and hyperglycemia (failed reversal of diabetes) was observed in all mice receiving impure islets, whereas all mice receiving pure islets adjacent to acinar cells exhibited a delayed reversal of diabetes (Fig. 4 a,b,c). Blood glucose levels reached to normal level only after 7-10 days from the day of transplantation. It indicates that acinar cell proteases degrade insulin which comes from transplanted islets.
Figure 4.
The effect of proteases on human islet function was studied at the in vivo level by transplanting 2000 human islets (IEQ) under the left kidney capsules of streptozotocin-induced diabetic nude mice (n=15). Blood glucose level was monitored after transplantation of pure, impure, and pure islets/acinar cells under the renal capsule of diabetic nude mice.(A) Transplantation of 2000 handpicked pure islets (IEQ) reversed the diabetes condition, (B) impure islets failed to reverse the graft function even though 2000 human islets (IEQ) were transplanted (C) equal volume of acinar cells were transplanted adjacent to 2000 pure islets (IEQ) (without mixing) showed a delayed islet graft function (about one week) to reverse the diabetic condition. After nephrectomy, reversal of diabetic condition was observed in groups A and C.
In human research islet preparations (n=8), A1AT supplementation in the islet culture media significantly inhibited the action of acinar cell proteases to prevent islet loss, and maintained 45% higher average islet culture recovery in treated, impure groups compared to untreated, impure groups (p<0.01) (Fig.1c). A1AT protease inhibition was confirmed by measuring TCE activity levels in pure and impure islet cultures supplemented with A1AT (Fig.5). Trypsin activity decreased by 97% and 98% (p<0.01), chymotrypsin was reduced by 95% (p<0.01) and 99% (p<0.01), and elastase was reduced by 99% (p<0.01) and 99% (p<0.01) in pure and impure cultures, respectively.
Figure 5.
In human islet research preparations (n=8), protease activity was measured from the culture supernatant of pure (80-90% purity) and impure islets (50-60% purity) cultured overnight at 37°C in the presence or absence of A1AT supplementation. Addition of A1AT significantly decreased the activity levels of (A) trypsin (pure-A1AT, *p<0.001; impure-A1AT, **p=0.007), (B) chymotrypsin (pure-A1AT, *p=0.006; impure-A1AT, **p<0.0001) and (c) elastase (pure-A1AT, *p<0.001; impure-A1AT, **p<0.001) compare to non-treated control.
Although 2000 islets (IEQ) were uniformly cultured in all preparations (Fig.1d), the insulin concentration in culture supernatants of untreated, control islet cultures was significantly lower than A1AT treated culture supernatants at all purity levels. The respective average insulin values for the control and treated groups were: 68,344 ± 84,688 and 104,240 ± 52,039 μU/ml for pure cultures (n=5, p<0.0001); 39,435 ± 56,485 and 86,315 ± 63,975 μU/ml for impure cultures (n=5, p=0.02); and 21,892 ± 39,776 and 54,001 ± 71,591 for highly impure cultures (n=5, p=0.01) (Fig.1d). A1AT supplementation restored normal insulin levels by blocking insulin degradation.
Islet cell viability tests revealed no significant differences between the control and the A1AT treated groups as assessed by FDA/PI microfluorometric assay (99 ± 1% and 99 ± 1.5 %, respectively) and OCR (95 ± 12 and 85 ± 5 OCR/DNA, respectively).
DISCUSSION
In general, human islet isolation process yields pure and impure (islet/acinar) islet cell fractions. Immediately after isolation from a donor pancreas, human islets may either be transplanted (islet autograft preparations) or cultured for a period of 36-72 hours before transplantation (islet allograft preparations) (2, 18). In both cases, both pure and impure islets (down to 30% purity) are commonly transplanted in order to maximize the islet dose and, therefore, increase the graft function and the probability of insulin independence for the patient. Currently, the major disadvantage of islet culture is a loss of islet cell mass that occurs over time (9, 21) and is more frequently observed in impure islet cultures relative to pure islet cultures (Fig. 1a).
In a continued effort to study the effects of acinar cell contamination, we show that acinar cell proteases create an inappropriate environment for islet survival and function in vitro and after transplantation. Activated exocrine proteases released by dying acinar cells significantly degraded insulin in the culture medium, damaged beta cell membranes and diminished intracellular insulin granules. Eventhough culture medium contains serum which is not sufficient to inhibit the high level of proteases released by acinar cells. We then demonstrated the inhibition of these effects by supplementing A1AT to the culture medium and significantly insulin degradation and reduced islet cell loss from impure preparations.
The optimal culture medium for human islets has been extensively studied by Holmes et al (19). While there is general consensus amongst islet transplant centers for the use of CMRL as the optimal base medium (19), the benefits of additives such as albumin (20), prolactin (21) and FBS are still under investigation, and their use is varied amongst isolation centers. The clinical islet transplantation consortium currently utilizes IGF (7) for islet culture supplementation and evidence from reports suggests that IGF, insulin, or insulin in synergy with transferin and selenium (ITS) is beneficial for promoting cellular growth and repair pathways (22, 23). Insulin supplementation may also help preserve islet function by inhibiting excess insulin release through negative feedback mechanisms which prevent metabolic burnout (24). Work by Llievia et al. (25) also showed decreased islet mantle disintegration when islets are cultured in media conditioned with IGF, suggesting that sustaining this important structural component is necessary to prevent islet death during culture (26-28). Interestingly, our results clearly show the deleterious effects of acinar cell proteases on insulin concentration in cultures of impure islets (Fig.1b). Inhibiting this degradation, therefore, is critical for protecting islets from structural degradation and excess metabolic demand, and promoting cellular repair and growth pathways.
The detrimental effects of activated acinar cell proteases (TCE) have been a concern for islet biologists in the past; however, previous research has focused on inhibiting activated exocrine proteases during organ preservation (29, 30) and the isolation process in attempt to improve the pre-culture islet yield (31, 32). Pefabloc, a serine protease inhibitor, is one commercial product that has been successfully used in animal and human islet isolations to decrease the measured activity of proteases (32), increase islet yield (33), and improve islet functional viability (34). Similarly, ulinastatin has also been shown to inhibit chymotrypsin and elastase activity during pancreas digestion (35). While protease activity has been beneficially decreased at the early stages of islet isolation, until this report, there has been a lack of information regarding the impact of TCE during islet culture, specifically impure islet culture. Observing a two-fold increase in islet loss in impure cultures relative to pure cultures, the present study highlights the apparent detrimental effects of acinar cell proteases on impure islet culture recovery (Fig.1c).
By supplementing a clinical grade protease inhibitor, A1AT, to the impure islet culture medium, this study successfully increased culture recoveries of impure islet preparations by 27%. The use of A1AT is advantageous over other protease inhibitors because its clinical use is already established for other medical conditions such as lung disease (36, 37). Showing potential in islet research, recent investigation of monotherapy treatments of human A1AT in diabetic nude mice have shown improved islet graft survival in allogenic transplantation (13-17). Moreover, novel findings indicate that A1AT significantly reduces cytokine and streptozotocin-induced β-cell apoptosis (12) and can enhance insulin secretion (38). A1AT treatment of islet graft recipients in animal models has already provided conclusive evidence to support the future translation of A1AT supplementation to clinical islet research. One key limitation of this study is the lack of pre-clinical data investigating the treatment of impure islet graft recipients with A1AT to improve in vivo islet functional capacity. However, our study showed that insulin breakdown by proteases (inside and outside of beta cell) can be prevented by A1AT supplementation.
Results from this study clearly demonstrate the deleterious effect of acinar cell proteases on in vivo islet function as assessed by transplantation in streptozotocin-induced diabetic nude mice. In previous studies, Gray et al. have reported a similar type of observation in rat models, concluding exocrine contamination is detrimental based on his histological evidence (11), and Hesse et al. produced similar findings in canine models (39). In human islet graft recipients, however, though we believe that acinar cell proteases degrade insulin systemically after transplantation, it is difficult to monitor this degradation because exogenous insulin is generally administered to patients over the subsequent few weeks. In our islet auto-transplant experience, there is an apparent relationship between insulin independence and the purity of the transplanted islets (unpublished data: insulin independent group purity was 59±11% and insulin dependent group purity was 30±4%, n=32, p=0.0053). Because impure islet preparations are often unavoidably transplanted along with pure preparations to increase the total quantity of islets delivered, we conclude that A1AT may have therapeutic potential for islet graft recipients because of its anti-inflammatory and protease inhibitory effects.
Materials and Methods
Human islet isolation and islet culture
Human islet isolations were done in the University of Minnesota Molecular and Cellular Therapeutics GMP Facility in compliance with FDA regulations. The islet isolation method has been previously reported (7, 40). Clinical islet preparations (n=10) were cultured according to the study protocol (30,000 IEQ/flask) in CMRL-1066 (Connaught Medical Research Laboratories, Mediatech, Inc. Manassas, VA) supplemented culture medium (40). Culture supernatants were taken from pure and impure islet fractions within 24 hrs of culture for protease measurements, and islet cell loss was calculated on the day of transplantation. In human research preparations (n=8), pure (80-90% purity), impure (50-60% purity), and highly-impure (30-40% purity) islets were cultured in the presence or absence of A1AT (0.5mg/ml). The culture supernatants were sampled within 24 hrs for insulin and protease measurements. The islets were subjected to recovery and viability assessments.
Alpha-1 antitrypsin preparation
PROLASTIN-C Alpha-1 Proteinase Inhibitor (Human) is a clinical grade, sterile, lyophilized preparation of purified human alpha-1 proteinase inhibitor (alpha-1 PI), also known as alpha-1 antitrypsin (Talecris products, USA). Alpha-1 PI was aseptically reconstituted according to the manufacturer’s instructions.
Measurement of protease (TCE) activity
Trypsin, chymotrypsin, and elastase activities were measured by fluorometric assay (excitation 380 nM and emission 440 nM) using specific substrates for each enzyme (Tryspin- Boc-Gln-Ala-Arg-MCA; chymotrypsin- Suc-Ala-Ala-Pro-Phe-MCA; and elastase- Suc-Ala-Ala-Ala-MCA; Peptides International Inc, Louisville, Kentucky, USA) according to the method of Kawabata et al. (41). The measurement was done in trypsin assay buffer containing Tris (50 mM), NaCl (150 mM), CaCl2 (1 mM) and BSA (0.1 mg/ml just before use). The pH was adjusted to 8.1 after adding BSA. The results are indicated in slope per minute and were converted into units by using standards.
Insulin measurement
Using an insulin ELISA kit (Mercodia, NC,USA), insulin levels were measured from the culture supernatant of 2000 handpicked islets (IEQ) placed uniformly amongst acinar cells in petri dishes to create pure (80-90% purity), impure (50-60% purity), and highly impure (30-40% purity) cultures; measurements were taken within 24 hrs after culture. Using Tris-Tricine gel insulin degradation by acinar cell proteases was documented in our preliminary report (10).
The degradation of insulin was determined by the disappearance of the intact insulin protein band on a Tris/Tricine gel (n=5). 5μg of recombinant human insulin (Roche Applied Science, Mannheim, Germany) was incubated with either 100μl exocrine acinar cell supernatant (known amount of pure exocrine acinar cells were cultured for 24-48 hrs using CMRL-1066 supplemented media), commercial trypsin (250μg/100μl), chymotrypsin (250ug/100ul), elastase (250ug/100ul) or no treatment (control) for overnight at 37°C. The samples were analyzed by using a 16.5% polyacrylamide, Tris-Tricine ready precast Gel (Bio-Rad, USA). To confirm that the protease enzymes (trypsin, chymotrypsin, and elastase) are responsible for the degradation of intact insulin molecule, some of the samples were also treated with A1AT.
Immuno-gold labeling and transmission electron microscopy of insulin granules
Transmission electron microscopic (TEM) immuno-gold labeling technique was used to study the effect of proteases on insulin granules in beta cells. Insulin granules can be visualized when immuno-gold particles bind to insulin and are examined by electron microscopy. Handpicked human islets (2000 IEQ) were either treated with commercial trypsin (250μg/100μl), chymotrypsin (250ug/100ul), elastase (250ug/100ul) or TCE cocktail, or 100μl acinar cell culture supernatant (known amount of pure exocrine acinar cells were cultured for 24-48 hrs using CMRL-1066 supplemented media), or received no treatment (control). These samples were cultured overnight and fixed with a 2% gluteraldehyde. Samples were then embedded in Embed 812 resin (Electron Microscopy Sciences, Hatfield, Pennsylvania). Ultrathin sections 80–100 nm thick were cut on a Leica Ultracut UCT microtome using a diamond knife and post-stained with 3% uranyl acetate followed by Sato’s triple-lead stain. Sections were examined with an FEI Phillips CM 12 transmission electron microscope operating at 60 kV. Images of insulin granules were recorded with a Maxim DL digital capture system. This technique allowed for the visual detection and quantification of insulin granules in control and treated β-cells; if proteases degraded insulin, fewer granules were detected.
Quantification of insulin granules and gold particles
Ultra structural quantification of the total number of insulin granules and gold particles in 30 cells was imaged by TEM following the immuno-gold labeling procedure. The cellular granule distribution of cultured islets after overnight exposure to various proteases was compared to the control (untreated) group. The digital image was measured using Image Pro plus Software, version 6.2 (Media Cybernetics, Inc.).
In vivo effect of acinar cell proteases in mouse model
To study the effect of acinar cells in impure islet transplantations, diabetic mice (n=15) received subrenal capsule transplantations of either 2000 IEQ of handpicked pure islets (n=5), 2000 IEQ of impure islets (40-50% purity) (n=5), or 2000 handpicked pure islets (IEQ) transplanted directly adjacent to pure acinar cells (n=5) with this approach pure islets and acinar cells were in two different regions without mixing. Male athymic nude mice were used in compliance with guidelines from the Institutional Animal Care Committee at the University of Minnesota. Diabetes was induced by intravenous injection of 240mg/kg streptozotocin. Mice were considered diabetic when their blood glucose levels were ≤350 mg/dl for 2 consecutive days. After transplantation, blood glucose levels and mouse body weight were measured every day for the first week, then 3 days per week. The return of normoglycemia in cured mice was identified by two consecutive blood glucose levels ≥200 mg/dl. At 30 days posttransplant, left nephrectomy was performed in the cured mice to confirm a return to hyperglycemia.
Islet quality assessments
In human research islet preparations (n=5), islets treated with A1AT were subjected to viability assays [FDA/PI (fluorescent diacetate and propidium iodide)] and oxygen consumption rate (OCR) as previously described (42).
Statistical analysis
Results were calculated as mean ± standard deviation (SD). Statistical significance was evaluated among groups by using Kruskal–Wallis one-way ANOVA followed by the Mann Whitney t-test. A P value <0.05 was considered statistically significant.
Fig. 6.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Brian Flanagan, Tom Gilmore, Jeff Ansite, Josh Wilheim, Muhammad Abdulla, Brian Perrault, Kate Mueller, Lei Tian, and Jie Gao for excellent technical support.
Funding Source: This project was supported partially by grants from the National Center for Research Resources (NCRR) Human Pancreatic Islet Cell Resources (ICRs) (U42 RR16598), the Juvenile Diabetes Research Foundation grants (#31-2008-336, #4-2004-372 and #4-2008-386, #6-2008-1033), and the Schulze Family Foundation. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, the Juvenile Diabetes Research Foundation, or the Schulze Family Foundation.
Abbreviations
- A1AT
Alpha-1 antitrypsin
- ELISA
Enzyme linked immunosorbent assay
- FDA/PI
Fluorescent diacetate and propidium iodide
- IEQ
Islet equivalent
- OCR
Oxygen consumption rate
- SD
Standard deviation
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCE
Trypsin, chymotrypsin, elastase
- TEM
Transmission electron microscopy
Footnotes
Contribution: Authors, Gopalakrishnan Loganathan, Rajinder K. Dawra, Mark A. Sanders, Klearchos K. papas, David E.R. Sutherland, Bernhard J. Hering and A.N. Balamurugan participated in design and performance of research and data analysis
Authors, Subbiah Pugazhenthi, Zhiguang Guo, Alexander Wiseman, V. Kumaravel and Ashok K. Saluja contributed reagents, analytical tools for the study
Authors, Gopalakrishnan Loganathan, Sajjad M. Soltani, and A.N.Balamurugan contributed to writing of the paper
All authors declare that there is no duality of interest associated with this manuscript.
REFERENCES
- 1.Murdoch TB, McGhee-Wilson D, Shapiro AM, Lakey JR. Methods of human islet culture for transplantation. Cell Transplant. 2004;13(6):605. [PubMed] [Google Scholar]
- 2.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 3.Toso C, McCall M, Emamaullee J, et al. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int. 2010;23(3):259. doi: 10.1111/j.1432-2277.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 4.Alejandro R, Barton FB, Hering BJ, Wease S. Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783. doi: 10.1097/TP.0b013e3181913f6a. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Najarian JS, Sutherland DE, Matas AJ, Goetz FC. Human islet autotransplantation following pancreatectomy. Transplant Proc. 1979;11(1):336. [PubMed] [Google Scholar]
- 6.Sutherland DE, Gores PF, Hering BJ, Wahoff D, McKeehen DA, Gruessner RW. Islet transplantation: an update. Diabetes Metab Rev. 1996;12(2):137. doi: 10.1002/(SICI)1099-0895(199607)12:2<137::AID-DMR159>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 8.Bellin MD, Blondet JJ, Beilman GJ, et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatr Diabetes. 2009 doi: 10.1111/j.1399-5448.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kin T, Senior P, O’Gorman D, Richer B, Salam A, Shapiro AM. Risk factors for islet loss during culture prior to transplantation. Transpl Int. 2008;21(11):1029. doi: 10.1111/j.1432-2277.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 10.Loganathan G, Dawra RK, Pugazhenthi S, et al. Culture of impure human islet fractions in the presence of alpha-1 antitrypsin prevents insulin cleavage and improves islet recovery. Transplant Proc. 2010;42(6):2055. doi: 10.1016/j.transproceed.2010.05.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray DW, Sutton R, McShane P, Peters M, Morris PJ. Exocrine contamination impairs implantation of pancreatic islets transplanted beneath the kidney capsule. J Surg Res. 1988;45(5):432. doi: 10.1016/0022-4804(88)90193-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Lu Y, Campbell-Thompson M, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56(5):1316. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A. 2005;102(34):12153. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis EC, Mizrahi M, Toledano M, et al. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A. 2008;105(42):16236. doi: 10.1073/pnas.0807627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koulmanda M, Bhasin M, Hoffman L, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105(42):16242. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strom TB. Saving islets from allograft rejection. Proc Natl Acad Sci U S A. 2005;102(36):12651. doi: 10.1073/pnas.0506079102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir GC, Koulamnda M. Control of inflammation with alpha1-antitrypsin: a potential treatment for islet transplantation and new-onset type 1 diabetes. Curr Diab Rep. 2009;9(2):100. doi: 10.1007/s11892-009-0018-5. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Tanaka K. Pancreatic islet cell transplantation using non-heart-beating donors (NHBDs) J Hepatobiliary Pancreat Surg. 2005;12(3):227. doi: 10.1007/s00534-005-0978-z. [DOI] [PubMed] [Google Scholar]
- 19.Holmes MA, Clayton HA, Chadwick DR, Bell PR, London NJ, James RF. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation. 1995;60(8):854. [PubMed] [Google Scholar]
- 20.Lee RH, Carter J, Szot GL, Posselt A, Stock P. Human albumin preserves islet mass and function better than whole serum during pretransplantation islet culture. Transplant Proc. 2008;40(2):384. doi: 10.1016/j.transproceed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Mita A, Ricordi C, et al. Prolactin supplementation to culture medium improves beta-cell survival. Transplantation. 89(11):1328. doi: 10.1097/TP.0b013e3181d98af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraga DW, Sabek O, Hathaway DK, Gaber AO. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation. 1998;65(8):1060. doi: 10.1097/00007890-199804270-00009. [DOI] [PubMed] [Google Scholar]
- 23.Froesch ER, Zapf J. Insulin-like growth factors and insulin: comparative aspects. Diabetologia. 1985;28(8):485. doi: 10.1007/BF00281982. [DOI] [PubMed] [Google Scholar]
- 24.Gaber AO, Fraga D. Advances in long-term islet culture: the Memphis experience. Cell Biochem Biophys. 2004;40(3 Suppl):49. doi: 10.1385/cbb:40:3:49. [DOI] [PubMed] [Google Scholar]
- 25.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol. 1999;161(3):357. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn C, Hurwitz SA, Kumar MG, Cotton J, Spandau DF. Activation of the insulin-like growth factor-1 receptor promotes the survival of human keratinocytes following ultraviolet B irradiation. Int J Cancer. 1999;80(3):431. doi: 10.1002/(sici)1097-0215(19990129)80:3<431::aid-ijc16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Sawada M, Yoshida S, Hanaoka F, Marunouchi T. Insulin prevents apoptosis of external granular layer neurons in rat cerebellar slice cultures. Neurosci Lett. 1995;199(1):37. doi: 10.1016/0304-3940(95)12009-s. [DOI] [PubMed] [Google Scholar]
- 29.Al-Abdullah IH, Bentsi-Barnes K, Valiente L, et al. Testing combinations of protease inhibitor and preservation solution to improve islet quality and yield. Transplant Proc. 2008;40(2):390. doi: 10.1016/j.transproceed.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 30.Lu WT, Lakey JR, Juang JH, Hsu BR, Rajotte RV. Effect of pefabloc on islet isolation from cold preserved rat pancreas. Transplant Proc. 2002;34(7):2700. doi: 10.1016/s0041-1345(02)03381-x. [DOI] [PubMed] [Google Scholar]
- 31.Lakey JR, Helms LM, Kin T, et al. Serine-protease inhibition during islet isolation increases islet yield from human pancreases with prolonged ischemia. Transplantation. 2001;72(4):565. doi: 10.1097/00007890-200108270-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rose NL, Palcic MM, Lakey JR. An evaluation of endogenous pancreatic enzyme levels after human islet isolation. Pancreas. 2003;27(2):167. doi: 10.1097/00006676-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto S, Rigley TH, Reems JA, Kuroda Y, Stevens RB. Improved islet yields from Macaca nemestrina and marginal human pancreata after two-layer method preservation and endogenous trypsin inhibition. Am J Transplant. 2003;3(1):53. doi: 10.1034/j.1600-6143.2003.30110.x. [DOI] [PubMed] [Google Scholar]
- 34.Rose NL, Palcic MM, Helms LM, Lakey JR. Evaluation of Pefabloc as a serine protease inhibitor during human-islet isolation. Transplantation. 2003;75(4):462. doi: 10.1097/01.TP.0000046537.47139.CE. [DOI] [PubMed] [Google Scholar]
- 35.Umeadi C, Bentsi-Barnes K, Kandeel F, Al-Abdullah IH. Endogenous pancreatic protease activity and methods for impeding their function. Transplant Proc. 2008;40(2):355. doi: 10.1016/j.transproceed.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Casolaro MA, Fells G, Wewers M, et al. Augmentation of lung antineutrophil elastase capacity with recombinant human alpha-1-antitrypsin. J Appl Physiol. 1987;63(5):2015. doi: 10.1152/jappl.1987.63.5.2015. [DOI] [PubMed] [Google Scholar]
- 37.Wewers MD, Casolaro MA, Sellers SE, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 38.Kalis M, Kumar R, Janciauskiene S, Salehi A, Cilio CM. alpha 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic beta-cells. Islets. 2010;2(3):185. doi: 10.4161/isl.2.3.11654. [DOI] [PubMed] [Google Scholar]
- 39.Hesse UJ, Sutherland DE, Gores PF, Sitges-Serra A, Najarian JS. Comparison of splenic and renal subcapsular islet autografting in dogs. Transplantation. 1986;41(2):271. doi: 10.1097/00007890-198602000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Balamurugan AN, Breite AG, Anazawa T, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89(8):954. doi: 10.1097/TP.0b013e3181d21e9a. [DOI] [PubMed] [Google Scholar]
- 41.Kawabata S, Miura T, Morita T, et al. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172(1):17. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- 42.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98(5):1071. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]