Abstract
Background & Aims
Pancreatic adenocarcinoma, among the most lethal human malignancies, is resistant to current chemotherapies. We have previously shown that triptolide inhibits the growth of pancreatic cancer cells in vitro and prevents tumor growth in vivo. This study investigates the mechanism by which triptolide kills pancreatic cancer cells, which has not been previously studied.
Methods
Cells were treated with triptolide and viability and caspase-3 activity were measured using colorimetric assays. Annexin V, propidium iodide and acridine orange staining were measured by flow cytometry. Immunofluorescence was used to monitor the localization of cytochrome c and LC3 proteins. Caspase-3, Atg5 and Beclin1 levels were downregulated by exposing cells to their respective siRNA.
Results
We show that triptolide induces apoptosis in MiaPaCa-2, Capan-1 and BxPC-3 cells and autophagy in S2-013, S2-VP10 and Hs766T cells. Triptolide-induced autophagy has a pro-death effect, requires autophagy-specific genes, atg5 or beclin1, and is associated with the inactivation of the Akt/mTOR/p70S6K pathway and the upregulation of the ERK1/2 pathway. Inhibition of autophagy in S2-013 and S2-VP10 cells results in cell death via the apoptotic pathway whereas inhibition of both autophagy and apoptosis rescues cell death.
Conclusions
This study shows, for the first time, that triptolide kills pancreatic cancer cells by two different pathways. It induces caspase-dependent apoptotic death in MiaPaCa-2, Capan-1 and BxPC-3 and caspase-independent autophagic death in metastatic cell lines, S2-013, S2-VP10 and Hs766T, thereby making it an attractive chemotherapeutic agent against a broad spectrum of pancreatic cancers.
Keywords: Autophagy, apoptosis, pancreatic cancer, cell deathf
Introduction
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States. The 5-year survival rate for pancreatic cancer is estimated to be < 5% due to its aggressive growth, metastasis and resistance to most chemotherapies.1 Efforts are ongoing to understand the pathobiology of pancreatic cancer and to develop innovative and effective therapies. An important part of this process is to understand the mechanism of cell death induced by potential chemotherapeutic agents.
Autophagy is responsible for the removal and breakdown of cellular materials.2 A basal level of constitutive autophagy is essential for the maintenance of cellular homeostasis. Autophagy is activated during environmental stress, such as nutrient starvation, thereby promoting cell survival;3 however, the occurrence of autophagic structures in dying cells has led to the hypothesis that autophagy may have a role in cell death. In contrast to apoptosis, cell death associated with autophagy is caspase-independent and does not involve nuclear fragmentation. Recent reports suggest that autophagy and apoptosis are often induced by the same stimuli, they share similar effectors and regulators and there exists a complex cross-talk between the two processes.4
Although many signaling pathways regulate autophagy, signaling from the cytoplasm to the autophagy machinery is mainly controlled, in a negative manner, through the serine/threonine kinase, mammalian target of Rapamycin (mTOR).5 Akt, a positive regulator of mTOR, suppresses the formation of autophagosomes and inhibits autophagy.6 In addition, autophagy is known to stimulate the Raf-1/MEK1/ERK1/2 pathway.7 While there is increasing evidence implicating the importance of autophagy in cancer and tumor development, the fundamental question, whether autophagy kills cancer cells or protects them from unfavorable conditions, remains controversial.
Triptolide, a diterpene triepoxide extracted from the Chinese herb Tripterygium wilfordii has been shown to inhibit the proliferation of cancer cells in vitro and reduce the growth and metastases of tumors in vivo. To date, in vivo studies have shown that triptolide inhibits the growth of cholangiocarcinoma cells in hamsters8 and xenografts of human melanoma, breast cancer, bladder cancer and gastric carcinoma in nude mice.9 Previous data from our lab has shown that triptolide inhibits the growth of both pancreatic cancer and neuroblastoma cells in vitro and prevents tumor growth in vivo.10, 11
Neither the role of autophagy in pancreatic cancer nor the effect of triptolide on autophagy has been investigated. Hence, we decided to investigate: (i) whether triptolide modulates autophagy in pancreatic cancer cells and (ii) whether induction of autophagy in pancreatic cancer cells has a pro-survival or pro-death effect. We show that triptolide decreases the viability of several pancreatic cancer cell lines and mediates cell death in pancreatic cancer cells by two different mechanisms: via the apoptotic pathway in MiaPaCa-2, Capan-1 and BxPC-3 cells and by inducing autophagy in S2-013, S2-VP10 and Hs766T cells. Amongst the genes frequently altered in pancreatic adenocarcinoma, all the cell lines used in this study have a mutation in p53, p16 and DPC4 genes and all except BxPC-3 and Hs766T cells have a mutation in the K-ras gene.12, 13 Also, inhibition of autophagy in S2-013 and S2-VP10 cells results in apoptotic cell death, indicating a cross-talk between apoptosis and autophagy in pancreatic cancer cells.
Results
Triptolide decreases viability of pancreatic cancer cells but has no effect on the cell cycle
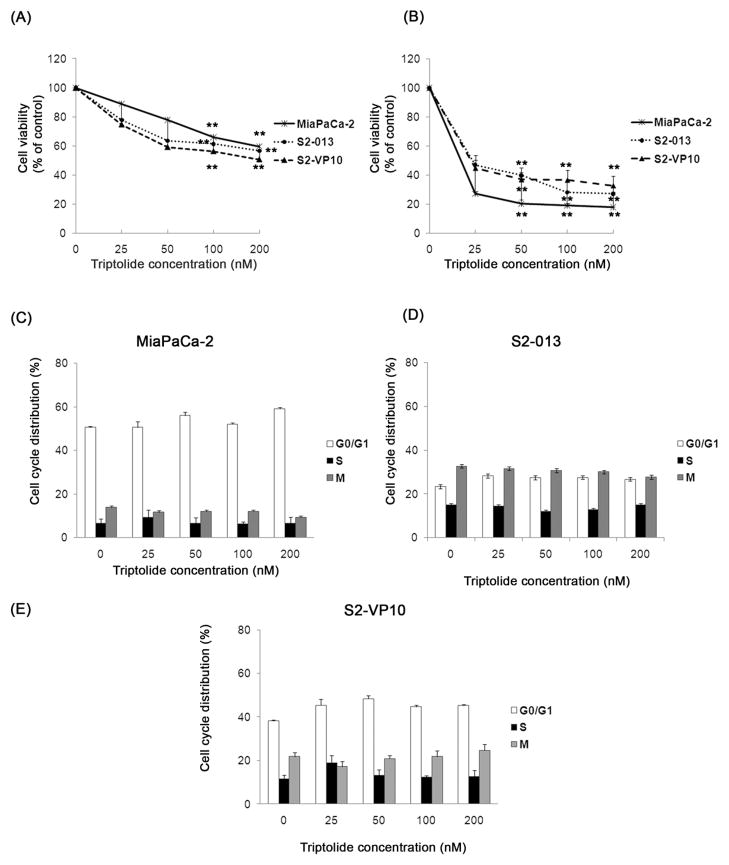
Previous data from our laboratory have shown that triptolide decreases viability of pancreatic cancer cells (Panc-1 and MiaPaCa-2) in vitro and inhibits tumor growth of MiaPaCa-2 in vivo.10 In this study, we have continued to explore the effect of triptolide on MiaPaCa-2, and have evaluated its effect on other pancreatic cancer cell lines: S2-013, S2-VP10, Bx-PC3, Capan-1 and Hs766T. Pancreatic cancer cells were exposed to increasing concentrations of triptolide for 24 h or 48 h and cell viability was monitored. All the cell lines tested show a significant dose- and time-dependent decrease in viability after triptolide treatment (Figures 1A, 1B and S1). To test the effect of triptolide on the cell cycle, cells were treated with increasing concentrations of triptolide for 24 h, stained with propidium iodide and assayed by flow cytometry. The distribution of the phases of the cell cycle is similar in the absence or presence of triptolide in MiaPaCa-2, S2-013 and S2-VP10 cells. (Figure 1C). We therefore conclude that triptolide does not affect the cell cycle in the cell lines tested.
Figure 1. Effect of triptolide on viability and cell cycle distribution of pancreatic cancer cells.
Treatment of MiaPaCa-2, S2-013 and S2-VP10 cells with triptolide shows a significant decrease in cell viability after 24 h (A) and 48 h (B) of treatment and no change in the percentage of the total cell population in each phase of the cell cycle after 24 h of treatment (C–E). The bars represent mean ± SEM, n≥4, **p <0.01 (t test).
Triptolide induces both apoptotic and non-apoptotic cell death in pancreatic cancer cells
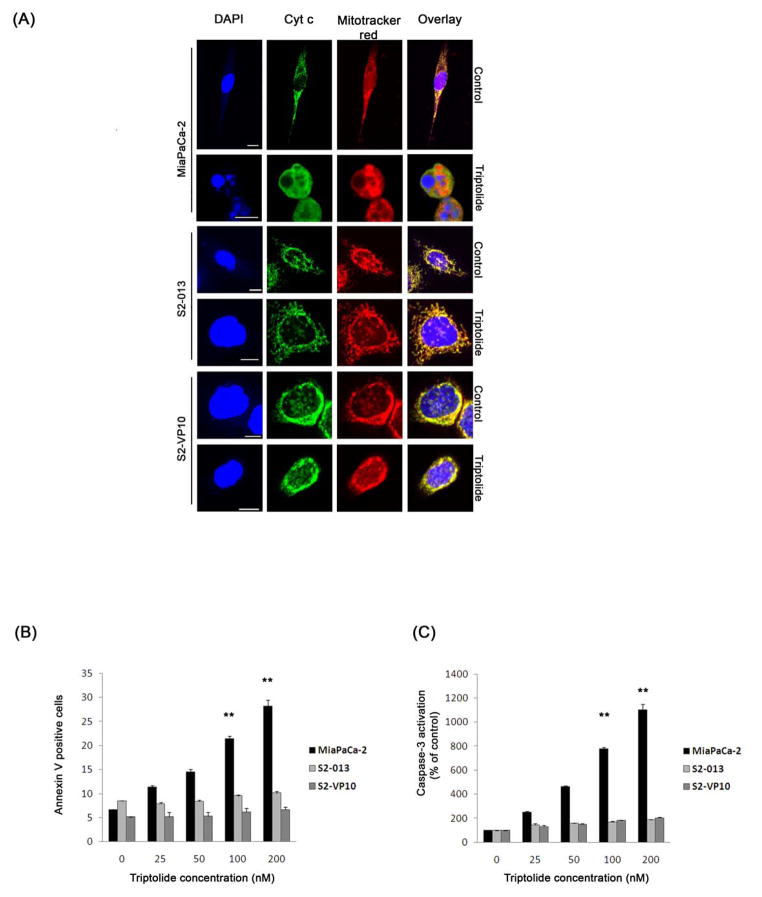
To elucidate the mechanism by which triptolide causes cell death in pancreatic cancer cells, we monitored three different markers of apoptosis: cytochrome c release, Annexin V staining and caspase-3 activation. The release of cytochrome c from the mitochondria into the cytosol was monitored by confocal microscopy. Untreated cells show punctate staining and colocalization of cytochrome c (green) and mitochondria (red) (Figure 2A). The Pearson’s correlation coefficient, a measure of the extent of overlap between the two colors, is 0.888, 0.941 and 0.888 respectively (Pearson’s coefficient: 1 = complete overlap; 0 = no overlap). After triptolide treatment, MiaPaCa-2 but neither S2-013 nor S2-VP10 cells show diffused staining for cytochrome c, while the mitochondria remain punctate (Figure 2A, triptolide). The Pearson’s correlation coefficient for MiaPaCa-2 is 0.101, indicating a release of cytochrome c, while that for S2-013 and S2-VP10 cells is 0.927 and 0.914 respectively. Also, nuclear staining by DAPI shows the presence of fragmented nuclei only in MiaPaCa-2 cells after triptolide treatment. Likewise, treatment of MiaPaCa-2 cells with triptolide shows a time- and dose-dependent increase in Annexin V staining, a marker for early stages of apoptosis, which is not observed in S2-013 or S2-VP10 cells (Figure 2B, S2A). In addition, the effect of triptolide on the activation of the effector caspase, caspase-3 was assayed. A significant time- and dose-dependent increase in caspase-3 activation is observed after treatment with triptolide in MiaPaCa-2, Bx-PC3 and Capan-1 cells but not in S2-013, S2-VP10 and Hs766T cells (Figure 2C, S2 B and S2 C). Our data show that although all the cell lines tested undergo cell death in response to triptolide, the mechanism through which this occurs varies.
Figure 2. Effect of triptolide treatment on the markers of apoptosis in pancreatic cancer cells.
(A) Treatment of MiaPaCa-2, but not S2-013 and S2-VP10 cells, shows a release of cytochrome c into the cytosol. Cytochrome c (green, punctate) colocalizes with mitotracker red (red, punctate) in untreated cells. The nuclei have been stained with DAPI (blue). Scale bar is 10 μM. (B, C) Treatment with triptolide for 24 h shows a significant increase in Annexin V staining and caspase-3 activation in MiaPaCa-2 cells but not in S2-013 and S2-VP10 as compared to the control. The bars represent mean ± SEM, n≥3, **p<0.01 (t test).
Triptolide induces autophagy in pancreatic cancer cells
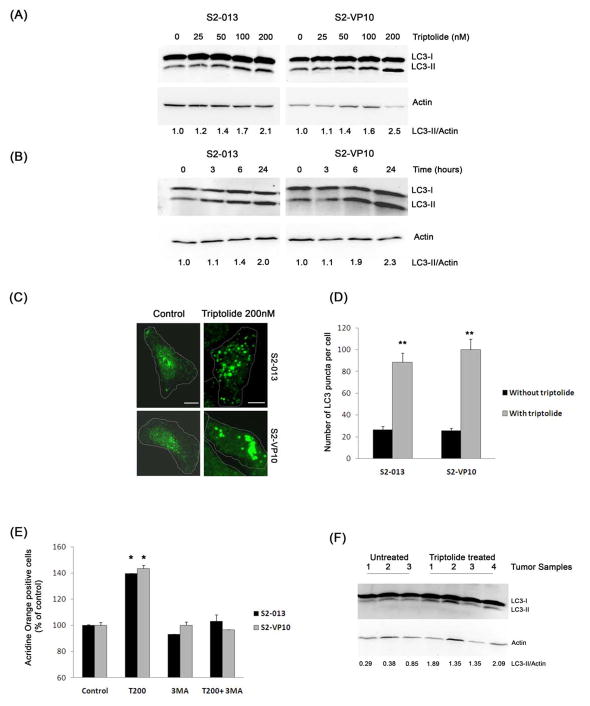
Since it has been shown that cancer cells undergo autophagy in response to various anticancer therapies, we examined whether triptolide induces autophagy in cells which show non-apoptotic cell death. First, we determined the induction of autophagy by monitoring the formation of autophagososme-specific protein LC3. LC3 is present in two forms: LC3-I, the cyostolic form and LC3-II, the membrane bound form. When autophagy is induced LC3-I is conjugated to phosphatidylethanolamine to form LC3-II, seen as a faster migrating band by western blotting. Since the amount of LC3-II correlates with the number of autophagosomes, it is a good indicator of autophagosome formation and hence the status of autophagy.14 LC3 can also be detected by immunofluoresence; LC3-II shows punctate staining whereas LC3-I show a diffused staining pattern. Treatment of S2-013 and S2-VP10 cells with triptolide shows a dose- and time-dependent increase in the autophagy-specific LC3-II form, compared to the control, as seen by western blotting (Figure 3A, 3B). The induction of LC3-II is first seen after 6 h of treatment with triptolide. In support of these data, immunostaining for LC3 shows a homogenous cytosolic distribution of LC3 in the untreated S2-013 and S2-VP10 cells (Figure 3C, left panel, 3D) which shifts to a punctate pattern following triptolide treatment (Figure 3C, right panel, 3D). Also, triptolide-treated S2-013 orthotopic tumor samples show a 3-fold increase in LC3-II compared to the control (saline-treated tumors) (Figure 3F). A similar study done in MiaPaCa-2 shows a decrease, whereas Hs766T cells show an increase, in the LC3-II form as seen by both western blotting and immunofluoresence (Figure S3, S4).
Figure 3. Effect of triptolide on autophagy in pancreatic cancer cells.
≥S2-013 and S2-VP10 exposed to increasing concentrations on triptolide for 24 h (A) or to triptolide 200 nM for indicated times (B) show a time- and dose-dependent increase in the autophagy-specific-LC3-II protein. The relative levels of LC3-II to Actin (loading control) are indicated below the corresponding bands. (C and D) Treatment of S2-013 and S2-VP10 cells with triptolide 200 nM for 24 h shows a significant increase in the LC3 punctate staining pattern when compared with untreated cells. The white dotted line indicates the outline of the cells. Results shown are representative of four independent experiments. Scale bar is 10 μM. (E) S2-013 and S2-VP10 cells exposed to triptolide 200 nM but neither 3-methyl adenine (3-MA) 2 mM alone nor the combination for 24 h shows a significant increase in red fluorescence compared to untreated cells. (F) Triptolide-treated S2-013 orthotopic tumor samples show an increase in LC3-II levels compared to untreated tumor samples. The bars represent mean ± SEM, n=3, *p <0.05, **p<0.01 (t test).
The induction of autophagy causes the formation of acidic autophagosomes which can be detected by staining cells with a lysomotropic agent, acridine orange, which displays a red fluorescence at an acidic pH.15 S2-013 and S2-VP10 cells were stained with acridine orange after treatment with triptolide and the appearance of red fluorescence was monitored by flow cytometry. Staining of both S2-013 and S2-VP10 cells with acridine orange shows a significant increase in red fluorescence after treatment with triptolide for 24 h as compared to the control (Figure 3E). Furthermore, treatment of cells with a combination of triptolide and 3-methyl adenine (2 mM), a specific inhibitor of autophagy, decreases the number of acridine orange positive cells (Figure 3E). A similar increase in acridine orange positive cells is observed in Hs766T cells in response to triptolide treatment (Figure S3D). Our results demonstrate that triptolide induces autophagy in S2-013, S2-VP10 and Hs766T cells, but not in MiaPaCa-2 cells.
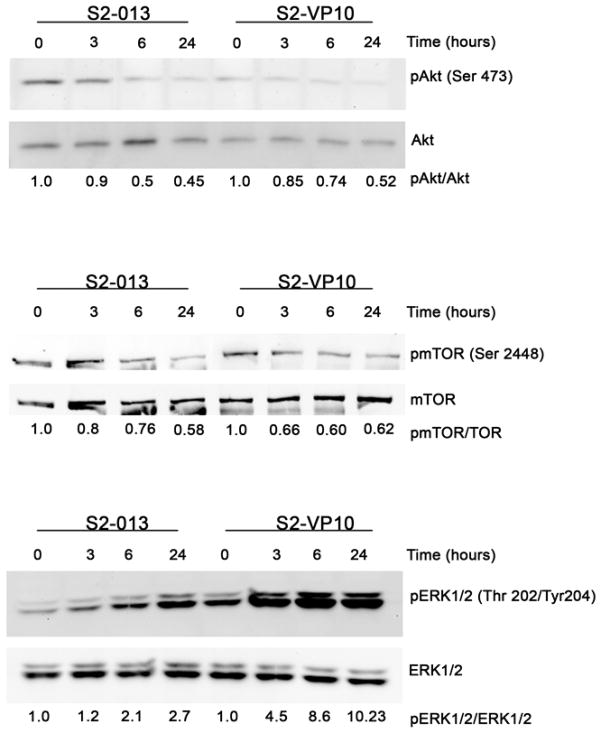
Triptolide inhibits the Akt/mTOR/p70S6K pathway and activates the ERK1/2 pathway in S2-013 and S2-VP10 cells
It has been previously reported that autophagy is negatively regulated by the Akt/mTOR/p70S6K pathway and positively regulated by the ERK1/2 pathway.16, 17 In order to confirm that triptolide induces autophagy in S2-013 and S2-VP10 cells, we examined its effect on these pathways. Treatment of both S2-013 and S2-VP10 cells with triptolide shows a downregulation of phosphorylated Akt and phosphorylated mTOR, the active forms, 3 h post- treatment (Figure 4). In contrast to this, triptolide treatment shows a sustained increase in the levels of phosphorylated ERK1/2, the active form, visible 3 h post-treatment in S2-013 and S2-VP10 cells (Figure 4). These results demonstrate that triptolide induces autophagy in both S2-013 and S2-VP10 by inhibiting the Akt/mTOR/p70S6K pathway and upregulating of ERK1/2 pathway.
Figure 4. Effect of triptolide on Akt/mTOR/p70S6kinase pathway and ERK pathway in S2-013 and S2-VP10 cells.
Cells exposed to 200 nM triptolide for 3 h, 6 h and 24 h show a decrease in the levels of phosphorylated Akt and mTOR and an increase in ERK1/2 phosphorylation after 3 h of treatment. The relative levels of pAkt to total Akt, pmTOR to total mTOR and pERK1/2 to total ERK1/2 are indicated below the corresponding bands.
Previous data from our laboratory showed that triptolide causes apoptotic cell death of pancreatic cancer cells by decreasing the levels of heat shock protein 70 (Hsp70) and increasing the levels of cytosolic calcium.10, 18 We therefore investigated if these events also occur in cells that show triptolide-induced autophagy. Both S2-013 and S2-VP10 cells show a dose-dependent decrease in the levels of Hsp70 post triptolide treatment for 48 h (Figure S5A). Furthermore, a knockdown of Hsp70 by siRNA did not affect the sensitivity of either S2-013 or S2-VP10 cells to triptolide (Figure S5B, C) nor did it have an effect on triptolide-induced autophagy in these cell lines (Figure S5D, E). Furthermore, treatment of S2-013 and S2-VP10 cells with triptolide shows a significant increase in the Ca2+cytosolic levels which are comparable to our previously published observations in MiaPaCa-2 (Figure S6).
Triptolide causes caspase-dependent cell death in MiaPaCa-2 but not in S2-013 and S2-VP10 cells
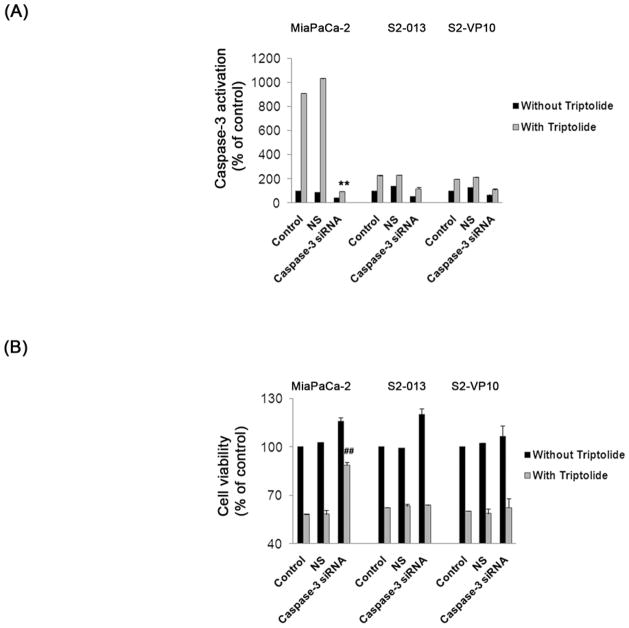
To further investigate the mechanism by which triptolide induces cell death in pancreatic cancer cells we selected MiaPaCa-2, cells which show apoptotic cell death and S2-013 and S2-VP10, cells which show non-apoptotic cell death in response to triptolide. Caspase-3, essential for apoptosis but not autophagy, was down regulated using a caspase-3-specific siRNA pool prior to triptolide treatment. Treatment of all the three cell lines with triptolide following caspase-3 siRNA transfection shows a decrease in caspase-3 activation, relative to control (cells treated with lipofectamine alone or with non-silencing siRNA and triptolide), indicating a knock-down of caspase-3 (Figure 5A). In the absence of caspase-3, only MiaPaCa-2 but not S2-013 or S2-VP10 cells show a significant rescue of cell viability after triptolide treatment as compared to control (Figure 5B). These results show that MiaPaCa-2 cells undergo caspase-dependent apoptotic cell death whereas S2-013 and S2-VP10 cells undergo caspase-independent, non-apoptotic cell death in response to triptolide.
Figure 5. Effect of inhibition of caspase-3 on triptolide response in pancreatic cancer cells.
Knockdown of caspase-3 by siRNA pool for 24 h followed by 200 nM triptolide treatment for 24 h decreases capase-3 activation in MiaPaCa-2, S2-013 and S2-VP10 cells when compared to cells treated with lipofectamine alone (control) or non-silencing siRNA (NS) and 200 nM triptolide (A). The viability of MiaPaCa-2 but not S2-013 and S2-VP10 cells is significantly rescued after the knock-down of caspase-3 followed by triptolide treatment when compared with control or NS cells treated with triptolide (B). The bars represent mean ± SEM, n=3, **p <0.01 (t test), ## p< 0.01 (t test).
Non-apoptotic cell death pathways include necrosis, autophagy and mitotic catastrophe.19 Since necrotic cell death is characterized by the release of Lactate Dehydrogenase (LDH) in the medium, we tested the effect of triptolide on the induction of necrosis in by measuring LDH release. The amount of LDH released in all the three cell lines after treatment with triptolide for 24 h is comparable to their respective controls, untreated cells (Figure S7). These data show that triptolide induces cell death in S2-013 and S2-VP10 cells via a non-apoptotic, non-necrotic pathway.
Inhibition of autophagy causes apoptotic cell death whereas inhibition of both apoptosis and autophagy rescues triptolide-mediated cell death in S2-013 and S2-VP10 cells
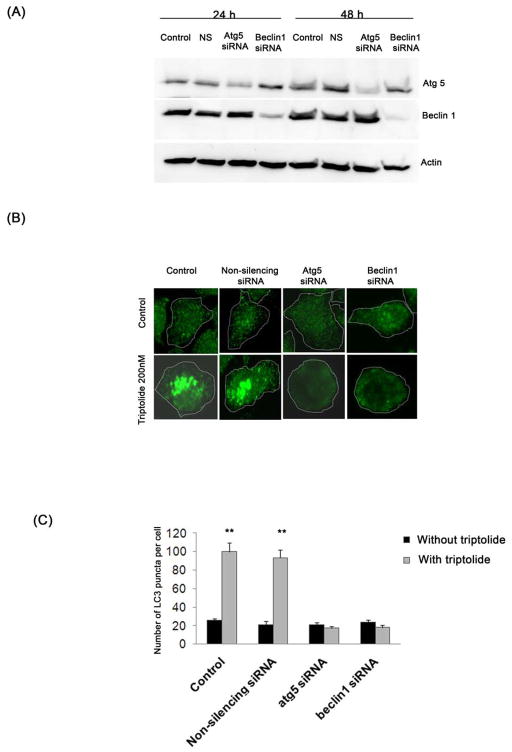
Since cell death in S2-013 and S2-VP10 cells was associated with autophagy we tested if there was a link between the induction of autophagy and cell death. Autophagy was inhibited using siRNA pool against Beclin1, a protein involved in the initial steps of vesicle nucleation and Atg5, a protein essential in vesicle elongation.20 Figure 6A shows a significant reduction in the levels of either Atg5 or Beclin1 after treatment with their respective siRNA relative to control.
Figure 6. Effect of triptolide on S2-013 and S2-VP10 cells after a knock-down of autophagy-specific genes.
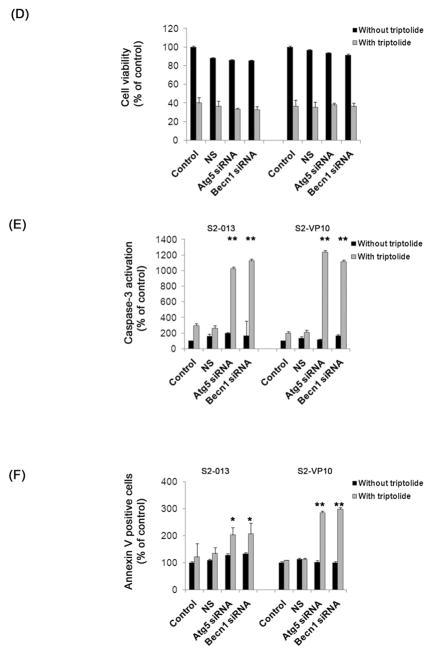
(A) Treatment of S2-013 cells with a pool of either atg5 siRNA or beclin1 siRNA shows a significant decrease in Atg5 or Beclin1 levels after 24 h and 48 h, which is absent in cells treated with lipofectamine alone (control) or with non-silencing siRNA (NS). Actin is used as a loading control. (B, C) Treatment of S2-013 with 200 nM triptolide for 24 h following a knock-down of atg5 or beclin1 genes with siRNA pool for 48 h shows a significant decrease in LC3 punctate pattern as compared to cells treated with non-silencing siRNA or lipofectamine alone followed by triptolide. The white dotted line indicates the outline of the cells. S2-013 and S2-VP10 cells treated with triptolide 200 nM for 48 h following atg5 or beclin1 knock-down shows a decrease in cell viability which is comparable to control or NS cells treated with triptolide (D). Knock-down of atg5 or beclin1 followed by triptolide 200 nM treatment for 48 h (E) or 24 h (F) shows a significant increase in caspase-3 activation and Annexin V positive cells when compared to control or NS cells treated with triptolide. The data shown are representative of three independent experiments. The bars represent mean ± SEM, n=3, * p<0.05, **p <0.01 (t test).
We next tested the effect of triptolide, in the absence of either atg5 or beclin1, on autophagy in S2-013, by monitoring the localization of LC3. In the absence of atg5 or beclin1, treatment with triptolide shows a diffused LC3 staining, indicating cytosolic localization, whereas triptolide-treated control cells show punctate LC3 staining pattern, indicating membrane localization (Figure 6B, 6C). These data show that triptolide is unable to induce autophagy in the absence of autophagy-specific genes. Next we monitored the effect of triptolide on viability in the absence of autophagy genes in S2-013 and S2-VP10 cells. Surprisingly, in the absence of atg5 or beclin1, treatment with triptolide for 48 h shows a significant decline in cell viability in both S2-013 and S2-VP10 cells, which is comparable to control (Figure 6D). Thus downregulation of autophagy is unable to provide protection against triptolide-mediated cell death, suggesting an alternate mechanism of cell death. Since several reports suggest that there is a cross-talk between apoptosis and autophagy,4, 21 we tested the role of apoptosis in triptolide-mediated cell death in the absence of autophagy. In the absence of atg5 or beclin1, S2-013 and S2-VP10 cells treated with triptolide show a significant increase in caspase-3 activation and Annexin V staining when compared with triptolide-treated control cells (Figures 6E, 6F). These results demonstrate that in the absence of autophagy, triptolide induces apoptotic cell death in S2-013 and S2-VP10 cells. A similar experiment done in MiaPaCa-2 cells shows no change triptolide sensitivity in the absence of autophagy genes (Figure S8), indicating that triptolide-mediated cell death in MiaPaCa-2 cells is independent of autophagy.
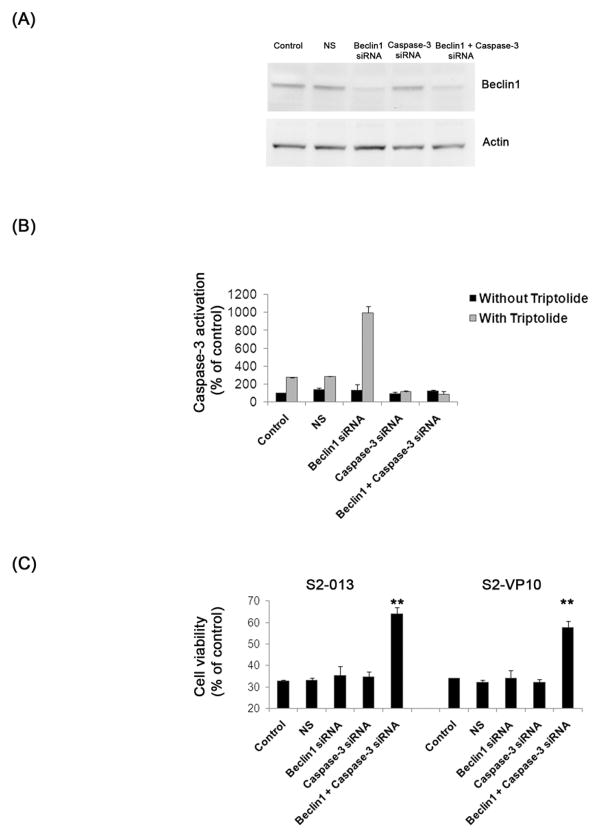
Further, we investigated whether dual silencing of autophagy-specific gene, beclin1 and apoptotic–specific gene, caspase-3 could rescue triptolide-mediated cell death in S2-013 and S2-VP10 cells. Figures 7A, B show a knock-down of both Caspase-3 and Beclin1 after treatment with their respective siRNA but not with the non-silencing siRNA. In the absence of both beclin1 and caspase-3, treatment with triptolide shows a significant rescue in cell viability in both S2-013 and S2-VP10 cells (Figure 7C, D). The decrease in viability of both the cell lines following triptolide treatment after a knock-down of either beclin1 or caspase-3 is comparable to the control cells and agrees with our earlier findings (Figures 5B, 6C). Taken together our results indicate that triptolide causes cell death in S2-013 and S2-VP10 cells by induction of autophagy, which can only be prevented by inhibition of both autophagy and apoptosis.
Figure 7. Effect of triptolide after inhibition of apoptosis and autophagy on cell viability in S2-013 and S2-VP10 cells.
(A) Treatment of S2-013 cells with a pool of beclin1 alone or beclin1 and caspase-3 siRNA shows a significant decrease of Beclin1 levels after 48 h, which is absent in cells treated with lipofectamine alone (control), with non-silencing siRNA (NS) or with caspase-3 siRNA alone. Actin is used as a loading control. (B) S2-013 cells treated with triptolide 200 nM for 48 h after caspase-3 siRNA alone or beclin1 and caspase-3 dual knockdown shows absence of caspase-3 activation. (C) S2-013 and S2-VP10 cells treated with triptolide 200 nM 48 h following beclin1 and caspase-3 dual silencing shows a significant rescue of cell viability when compared to control, NS, or cells treated with either beclin1 siRNA or caspase-3 siRNA alone along with triptolide. The bars represent mean ± SEM, n=3, **p <0.01 (t test).
Discussion
In pancreatic adenocarcinoma, K-ras, p53, p16 and DPC4 genes are most frequently altered. Amongst the cell lines used in this study, MiaPaCa-2, Capan-1, S2-013 and S2-VP10 cells show a mutation in all the four genes mentioned above while BxPC-3 and Hs766T cells have mutated p53, p16 and DPC4 genes but wild type K-ras gene12, 13 and the S2-013 and S2-VP10 cell lines are derived from the same parent line, SUIT-2.22 In this study we have shown that triptolide induces apoptotic cell death in MiaPaCa-2, Bx-PC3 and Capan-1 cells whereas it causes non-apoptotic cell death in S2-013, S2-VP10 and Hs766T. It induces autophagy in S2-013, S2-VP10 and Hs766T cells and also inhibits the Akt/mTOR/p70S6K pathway and activates the ERK pathway. There exists a cross-talk between autophagy and apoptosis, as triptolide induces apoptotic cell death in S2-013 and S2-VP10 cells in the absence of autophagy. The absence of both apoptosis- and autophagy-specific genes in S2-013 and S2-VP10 cells rescues triptolide-mediated cell death, thus proving convincingly that triptolide induces autophagic cell death in some pancreatic cancer cells.
It has been previously reported that triptolide induces cell cycle arrest in the S phase of human fibrosarcoma HT-1080 cells 23 and MDA-MB-231 breast cancer cells.24 However, our study does not show that triptolide exerts any effect on the cell cycle of pancreatic cancer cell lines (Figure 1C, D, and E). Hence, triptolide causes cell death in the pancreatic cancer cells by a mechanism which is independent of cell cycle arrest.
Triptolide induces apoptosis in MiaPaCa-2, BxPC-3 and Capan-1 cells as evidenced by a dose-dependent increase in caspase-3 activation (Figures 2C, S2C), and Annexin V staining and nuclear fragmentation (Figures 2A, 2B). The release of cytochrome c from the mitochondria into the cytosol suggests that triptolide kills MiaPaCa-2 cells via the ‘intrinsic or mitochondrial’ pathway (Figure 2A), as shown by our group10, 11 and others.25 On the other hand, the above mentioned apoptotic markers are absent in S2-013, S2-VP10 and Hs766T cells treated with triptolide (Figure 2A, 2B, 2C and S2C). Furthermore, S2-013 and S2-VP10 cells do not show markers of apoptosis even after prolonged incubation (48 h) with triptolide confirming the utilization of a non-apoptotic cell death pathway (Figure S2A, B).
The present study confirms that triptolide induces autophagy in S2-013 S2-VP10 and Hs766T cells. The induction of autophagy is a specific response to triptolide because: (i) the increase in LC3-II form is both time- and dose-dependent (Figures 3, S3), (ii) triptolide-treated S2-013 tumors also exhibit an increase in LC3-II in vivo (Figure 3F), (iii) the increase in acridine orange staining in response to triptolide can be reversed by the addition of an autophagy-specific inhibitor, 3-methyl adenine (Figure 3E) and (iv) triptolide fails to induce the formation of membrane bound LC3 after a knock-down of autophagy-specific genes (Figures 6B,6C). This study clearly demonstrates that triptolide inhibits the Akt/mTOR/p70S6K pathway and activates ERK signaling pathway, resulting in the induction of autophagy (Figure 4), a finding which corroborates previously published data that a combination of Akt inhibition and ERK activation are common mechanisms by which anticancer agents induce autophagy.16, 17, 26
We have previously shown that one of the mechanisms of triptolide-mediated cell death is to decrease the levels of Hsp70.10 Although our present study confirms that triptolide decreases Hsp70 levels in S2-013 and S2-VP10 cells (Figure S5A) triptolide-mediated cell death remains unaffected in the absence of hsp70 in these cells. This protective effect could be due to the presence of residual amount of Hsp70 after siRNA treatment (Figure S5B) which decreases following triptolide treatment and this corroborates the observed decrease in viability and induction of autophagy (Figure S5C–F). Although, Hsp70 has been shown to be involved in a distinct type of autophagy known as chaperone-mediated autophagy27 our results do not show the involvement of Hsp70 in triptolide mediated autophagy in S2-013 and S2-VP10 cells (Figure S5D, E). Recent studies have shown that cytosolic calcium, a known mediator of apoptosis, is also induces autophagy.28 In accordance with this and our previously published work18 triptolide causes an increase in Ca2+cytosolic levels in both S2-013 and S2-VP10 cells (Figure S6).
A knock-down of caspase-3 in S2-013 and S2-VP10 cells does not affect cell viability after triptolide treatment (Figure 5B), suggesting the involvement of a non-apoptotic and caspase-independent cell death pathway in these cells. The maintenance of plasma membrane integrity, measured by LDH release, after treatment with triptolide rules out the possibility of induction of necrotic cell death (Figure S7). Surprisingly, a knock-down of atg5 or beclin1, genes essential in autophagy, did not prevent triptolide-mediated cell death in S2-013 and S2-VP10 cells but instead triggered apoptosis (Figure 6), whereas dual silencing of beclin1 and caspase-3 rescued triptolide-mediated cell death (Figure 7). This clearly shows that triptolide induces autophagic cell death in S2-013 and S2-VP10 cells, there exists a cross-talk between the autophagic and apoptotic pathways and that these two pathways are not mutually exclusive. These results also confirm that S2-013 and S2-VP10 cells harbor intact apoptotic machinery, but that they preferentially activate the autophagic pathway in response to triptolide. In contrast to this, MiaPaCa-2 cells respond to triptolide by inducing apoptosis (Figure 2) as triptolide-induced cell death is rescued by caspase-3 knock-down (Figure 5B) but remains unaffected after atg5 or beclin1 knock-down (Figure S8), thus confirming that triptolide-mediated cell death occurs independent of autophagy in these cells.
Studies show that the decision between autophagy and apoptosis might be determined by a few key molecular players that are common to both the pathways: (i) ER localization of BCL2 or BCL-XL inhibits autophagy,21 (ii) nuclear localization of p53 induces autophagy, whereas cytoplasmic accumulation inhibits autophagy29 and (iii) mitochondrial translocation of the shorter isoform of the tumor suppressor protein p14ARF, smARF stimulates autophagy.30 Evaluating the effect of triptolide on these regulators of the autophagic and apoptotic pathway will aid in explaining why different pancreatic cancer cells have a differential response to triptolide.
In conclusion, this study shows for the first time that triptolide induces autophagy in pancreatic cancer cells. Our study sheds light on the fundamental question- whether autophagy is protective or causes cell death- proving convincingly that triptolide-mediated induction of autophagy causes cell death of pancreatic cancer cells. Although a basal level of autophagy is necessary to maintain cellular homeostasis it may become a cell death mechanism if the amplitude of autophagy increases above a threshold level, leading to a loss of viability as seen in S2-013, S2-VP10 and Hs766T cells after triptolide treatment. Furthermore, there exists a cross-talk between apoptosis and autophagy in S2-013 and S2-VP10 cells, either both pathways function independently to kill the cells, with autophagy being the preferred pathway or autophagy antagonizes apoptosis and hence apoptosis is seen only after inhibiting autophagy. This relationship merits further investigation. The ability of triptolide to induce either mechanism of cell death both in vitro and in vivo and in several pancreatic cancer cell lines, makes it an attractive chemotherapeutic agent against a broad spectrum of pancreatic cancers.
Materials and Methods
Cell Culture and Cell viability Assay
Pancreatic cancer cells were grown and treated with triptolide and viability was measured as previously described.10, 31
Cell cycle Analysis
Cell cycle was analysed using the Guava cell cycle reagent and Guava PCA flow cytometery. according to the manufacturer’s instructions.
Measurement of Annexin V-Positive Cells and caspase-3 activation
Externalization of phosphatidylserine and caspase-3 activity were measured as previously described.10
Transfection with siRNA
ON-TARGET plus SMART Pool Human caspase-3 siRNA (Cat # L-004307-00), Human beclin1 siRNA (L-010552-00) and Human atg5 siRNA (Cat # L-004374-00) were used as per the manufacturer’s instructions. Transfections were carried out as previously described.32
Acridine Orange staining for acidic vacuole quantification
To detect the formation of acidic vacuoles, cells were stained with acridine orange and analysed by flow cytometer as previously described.15
Western Blot
Western blot on protein samples was performed as previously described.18
Immunofluorescence
Cells were grown, treated, fixed as previously described,18 stained using mouse monoclonal anti-cytochrome c antibody (BD Pharmingen) for cytochrome c staining or rabbit polyclonal anti-LC3B antibody for LC3 staining. Mitotracker Red (Molecular Probes) was used to stain mitochondria prior to fixing. The slides were mounted and images obtained as previously described.18 The LC3 dots were quantified using the Image J software command ‘Analyze Particles’, which counts and measures objects in binary or thresholded images.
Statistical Analysis
Values are expressed as the mean ± SEM. All experiments with cells were repeated at least thrice. The significance of the difference between the control and each experimental test condition was analyzed by unpaired Student’s t-test and a value of p<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
Grant Support: National Institutes of Health grants DK58694, CA124723, CA131663 (to A.K.S.), grants from the Hirshberg Foundation for Pancreatic Cancer Research and Robert and Katherine Goodale Foundation (to A.K.S.), and by intramural funding from the University of Minnesota’s Surgery Department.
We thank Jerry Sedgeweick for help with the analysis of the LC3 confocal images.
Abbreviations
- siRNA
Short interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 5.Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–65. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 6.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Pattingre S, Bauvy C, Codogno P. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem. 2003;278:16667–74. doi: 10.1074/jbc.M210998200. [DOI] [PubMed] [Google Scholar]
- 8.Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998;133:169–75. doi: 10.1016/s0304-3835(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 10.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–16. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 11.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, Sorenson BS, Saltzman DA, Vickers SM, Saluja AK. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–90. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Monti P, Marchesi F, Reni M, Mercalli A, Sordi V, Zerbi A, Balzano G, Di Carlo V, Allavena P, Piemonti L. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004;445:236–47. doi: 10.1007/s00428-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 13.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Kloppel G, Kalthoff H, Ungefroren H, Lohr M, Scarpa A. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 15.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–8. [PubMed] [Google Scholar]
- 16.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 17.Newman RA, Kondo Y, Yokoyama T, Dixon S, Cartwright C, Chan D, Johansen M, Yang P. Autophagic cell death of human pancreatic tumor cells mediated by oleandrin, a lipid-soluble cardiac glycoside. Integr Cancer Ther. 2007;6:354–64. doi: 10.1177/1534735407309623. [DOI] [PubMed] [Google Scholar]
- 18.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–82. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bruin EC, Medema JP. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat Rev. 2008;34:737–49. doi: 10.1016/j.ctrv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GR. Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 2009;116:697–712. doi: 10.1042/CS20080508. [DOI] [PubMed] [Google Scholar]
- 21.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 22.Iwamura T, Caffrey TC, Kitamura N, Yamanari H, Setoguchi T, Hollingsworth MA. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2) Cancer Res. 1997;57:1206–12. [PubMed] [Google Scholar]
- 23.Fidler JM, Li K, Chung C, Wei K, Ross JA, Gao M, Rosen GD. PG490–88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther. 2003;2:855–62. [PubMed] [Google Scholar]
- 24.Liu J, Jiang Z, Xiao J, Zhang Y, Lin S, Duan W, Yao J, Liu C, Huang X, Wang T, Liang Z, Wang R, Zhang S, Zhang L. Effects of triptolide from Tripterygium wilfordii on ERalpha and p53 expression in two human breast cancer cell lines. Phytomedicine. 2009 doi: 10.1016/j.phymed.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Choi YJ, Kim TG, Kim YH, Lee SH, Kwon YK, Suh SI, Park JW, Kwon TK. Immunosuppressant PG490 (triptolide) induces apoptosis through the activation of caspase-3 and down-regulation of XIAP in U937 cells. Biochem Pharmacol. 2003;66:273–80. doi: 10.1016/s0006-2952(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 26.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–6. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 27.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, Vicencio JM, Soussi T, Kroemer G. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle. 2008;7:3056–61. doi: 10.4161/cc.7.19.6751. [DOI] [PubMed] [Google Scholar]
- 30.Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, Kimchi A. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22:463–75. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Borja-Cacho D, Yokoyama Y, Chugh RK, Mujumdar NR, Dudeja V, Clawson KA, Dawra RK, Saluja AK, Vickers SM. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. J Gastrointest Surg. 14:252–60. doi: 10.1007/s11605-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–25. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.