Abstract
The dopamine D3 receptor has received attention over the last two decades as a target for medications development for substance abuse disorders. Results have remained mixed. Despite emergence of more D3-selective ligands, possible attribution of observed effects to D2 receptors remains a concern. Knockout mice may help shed light on mechanisms. Here we evaluated the effect of constitutive D3 receptor inactivation (“knockout”) on the reinforcing effects of cocaine. We tested D3 wild-type (WT), heterozygous (), and knockout (), mice in acquisition and maintenance of intravenous self-administration across a broad range of cocaine doses, using a fixed ratio (FR) 1 and a progressive ratio (PR) schedule of reinforcement, along with parallel food-reinforced studies. Generally, mice showed cocaine self-administration comparable to WT controls across assays. Moderate and nonsignificant trends toward lesser reinforcing effects of a low cocaine dose (0.32 mg/kg) were apparent in acquisition and PR studies, consistent with the idea that the D3 receptor may play a subtle role in the reinforcing effects of low cocaine doses under low FR conditions. However, those effects with cocaine self-administration were more subtle than the lower responding of D3 knockout mice observed with food-maintained behavior. In addition, the D3 antagonist PG01037 failed to affect cocaine self-administration under an FR 1 schedule in WT mice. The present data do not support a necessary role for the D3 receptor in the direct reinforcing effects of cocaine.
Keywords: cocaine, dopamine receptors, knockout mice, progressive ratio, food-maintained behavior
Ligands acting at dopamine D2-like (D2, D3, D4) receptors modulate cocaine self-administration behavior, whether studied in nonhuman primates, rats, or mice. D2-like antagonists produce rightward shifts in the cocaine dose-effect function, consistent with surmountable antagonism (Bergman, Kamien, & Spealman, 1990; Caine & Koob, 1994; Caine et al., 2002). Conversely D2-like agonists can maintain self-administration behavior in animals trained to self-administer cocaine, and produce leftward shifts of the cocaine dose-effect function (Barrett, Miller, Dohrmann, & Caine, 2004; Caine, Negus, Mello, & Bergman, 1999; Caine et al., 2002; Collins & Woods, 2007; Nader & Mach, 1996; Wise, Murray, & Bozarth, 1990; Woolverton, Goldberg, & Ginos, 1984). However, it remains unclear which receptor subtypes mediate those effects. We previously reported that mice with a genetic mutation of the D2 receptor (knockout; mice) self-administered cocaine as much as wild type (WT) mice, indeed with higher intake at high unit doses, suggesting D2 receptors are not necessary for cocaine’s reinforcing effects in mice, but rather are involved in factors limiting cocaine intake (Caine et al., 2002). While the D3 receptor is expressed at low levels in the brain relative to the D1 or D2 subtypes, its enriched localization in the nucleus accumbens suggested a possible involvement in reward (Caine & Koob, 1993; Hall et al., 1996; for review, see Heidbreder & Newman, 2010; Lévesque et al., 1992; Pilla et al., 1999; Sokoloff, Giros, Martres, Bouthenet, & Schwartz, 1990). Further supporting a potential role in reward, there is evidence D3 receptor stimulation may modulate striatal dopaminergic activity especially in ventral striatum (Gobert et al., 1995; Kreiss et al., 1995; Parsons et al., 1996; Pugsley et al., 1995; Zapata, Witkin & Shippenberg, 2001). Despite much research dedicated to elucidating the role of D3 receptors in the abuse-related effects of cocaine, and the potential of D3 antagonists as addiction medications, results remain perplexing and no clear consensus has been reached.
Putative D3-selective antagonists have repeatedly been reported to block the acquisition and/or expression of cocaine-, nicotine- or heroin-conditioned place preferences (CPPs; Ashby, Paul, Gardner, Heidbreder, & Hagan, 2003; Cervo, Burbassi, Colovic, & Caccia, 2005; Le Foll, Sokoloff, Stark, & Goldberg, 2005; Micheli et al., 2007; Vorel et al., 2002). However in self-administration paradigms, D3 antagonists appeared largely ineffective at decreasing cocaine intake, doing so only under schedules of high response requirement (e.g., second order, FR 10, progressive ratio [PR]) and often at doses that decreased food-maintained behavior as well (Di Ciano, Underwood, Hagan, & Everitt, 2003; Gál & Gyertyan, 2003; Higley et al., 2011; Martelle et al., 2007; Thanos et al., 2008; Xi et al., 2005, 2006). Further, in nonhuman primates, D3 antagonism failed to reduce cocaine self-administration under a second order schedule of reinforcement (Achat-Mendes, Platt, Newman, & Spealman, 2009; Achat-Mendes et al., 2010). As putative D3 antagonists have been shown to inhibit the reinstatement of responding on a manipulandum previously reinforced with cocaine under extinction conditions, it has been proposed that they modulated the “motivation to self-administer” cocaine (Achat-Mendes et al., 2010; Vorel et al., 2002; Xi et al., 2004). Other studies using the reinstatement paradigm in nonhuman primates or rats were consistent with an involvement of D2 receptors rather than, or in addition to, D3 receptors (Achat-Mendes et al., 2010; Khroyan, Barrett-Larimore, Rowlett, & Spealman, 2000; Schmidt, Anderson, & Pierce, 2006). To the extent that reinstatement paradigms may be sensitive to interoceptive cues produced by cocaine or test compounds, it is worth noting that D3 preferential agonists partially or fully substituted for the cocaine discriminative stimulus in many studies and species, and conversely putative D3-selective antagonists or partial agonists sometimes attenuated cocaine discrimination (Achat-Mendes et al., 2009; Acri et al., 1995; Beardsley, Sokoloff, Balster, & Schwartz, 2001; Lamas, Negus, Nader, & Mello, 1996; Martelle et al., 2007). Nevertheless, the relative role of D2 versus D3 receptors in those effects also remains controversial (Achat-Mendez et al., 2010; Baker, Hood, & Heidema, 1999; Baker, Svensson Garner, & Goodwin, 1998; Christian, Goodwin, & Baker, 2001). The controversy is further complicated by the lack of highly selective D2 ligands, as well as the possibility that some of the effects of D3-selective or preferential ligands may have been mediated by D2 receptors when higher doses were used in vivo. The use of genetically engineered mice lacking the D3 receptor (knockout, mice) has provided a complementary approach to investigate D3 receptor involvement in cocaine’s abuse-related effects as well as the in vivo selectivity of putative D3 selective drugs.
Results from studies in mice on the effects of cocaine (or other abused substances) have been somewhat contradictory. Investigations have indicated either increased, decreased, or unchanged psychomotor activating effects of cocaine or amphetamine in mice relative to WT mice (Carta, Gerfen, & Steiner, 2000; Karasinska, George, Cheng, & O’Dowd, 2005; McNamara et al., 2006; Xu et al., 1997). To further complicate matters, where a genotype effect was observed, ambulatory activity and stereotyped behaviors appeared to be affected in opposite directions by the mutation (Carta, Gerfen, & Steiner, 2000; Mc-Namara et al., 2006). There are three separate lines of mice (Accili et al., 1996; Jung et al., 1999; Xu et al., 1997), but the discrepancies between studies show no obvious relation to the use of a specific line or another. At the neurochemical level (e.g., c-fos induction), mice have shown exaggerated or prolonged responses to cocaine administration (Chen & Xu, 2010; Jiao et al., 2007; Zhang et al., 2004; Zhou et al., 2007). Other endpoints that may be more directly related to cocaine’s abuse showed no effect of the D3 null mutation. Indeed, cocaine increased extracellular dopamine levels in the ventral striatum to a comparable degree in and WT mice (Zapata, Witkin, & Shippenberg, 2001). Cocaine CPP was also comparable between and WT mice (Chen&Xu, 2010; Karasinska, George, Cheng, & O’Dowd, 2005). A few investigations found amphetamine or cocaine to be more potent in mice relative to WT mice (Kong, Kuang, Li, & Xu, 2011; Xu et al., 1997). Thus CPP findings in mice are in apparent contrast to the D3 antagonist data from rats, and it should be noted that profound species differences in the effects of D2/D3 agonists have been reported between mice and rats (Halberda, Middaugh, Gard, & Jackson, 1997; Li et al., 2010; Ralph & Caine, 2005; Thomsen, Ralph, & Caine, 2011). Similarly, some data suggested mice showed altered abuse-related effects of ethanol, but subsequent studies failed to corroborate those findings (Boyce-Rustay & Risinger, 2003; McQuade, Xu, Woods, Seeley, & Benoit, 2003; Narita, Soma, Tamaki, & Suzuki, 2002). Finally, mice were recently reported to self-administer cocaine at the same level as WT mice under an FR 1 schedule of reinforcement, although only one cocaine dose was tested (Song et al., 2012).
The goal of the present study was to investigate the role of the dopamine D3 receptor subtype in the reinforcing effects of cocaine using knockout mice that lack the D3 receptor but express D2 and D4 receptors. Acquisition of cocaine self-administration as well as food-maintained behavior was compared in experimentally naïve , , and WT mice. Dose–effect functions for cocaine self-administration were then determined under an FR 1 and a PR schedule of reinforcement to compare the potency and efficacy of cocaine as a reinforcer in mutant and WT mice. Finally, we tested whether the D3 antagonist PG01037 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-trans-but-2-enyl)-4-pyridine-2-ylbenzamide)] (Grundt et al., 2005 could modulate cocaine self-administration behavior in WT mice.
Methods
Animals and Housing
Male and female WT, , and mice were generated and backcrossed to a C57BL/6J background as described previously (Xu et al., 1997). The mice were genotyped by Southern blotting of tail DNA (Xu et al., 1997). Animals were transferred to McLean Hospital at 10–18 weeks of age and were acclimated to the housing facilities at least 7 days before experiments were initiated. During this time they were also handled, and were anesthetized briefly once for subcutaneous implantation of an identification microchip. Animals were group housed up to five per cage at ~22 °C and ~55% humidity, under a 12-hr light/dark cycle. Water was accessible ad libitum and mouse chow (rodent diet 5015, PMI Feeds, Inc., St. Louis, MO) was provided once daily after training/testing sessions, approximately 4 g/mouse/day. This feeding regimen was adopted so that a small amount of food remained the following day in nearly all cages, and was replaced with 4 g per mouse of fresh chow, and thus did not represent food deprivation while curtailing the few mice that tended toward hyperphagia and obesity. For enrichment, rodent “treats,” nesting material and hiding/nesting devices were provided. Running wheels were available, although before catheter implantation only in the self-administration groups, to avoid potential injuries caused by the protruding catheter base. All testing was conducted during the light phase of the circadian cycle. All procedures were carried out in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Operant Conditioning Apparatus
Operant conditioning chambers as well as training and evaluation of food-maintained behavior under an FR 1 schedule have been described in detail (Caine, Negus & Mello, 1999; Thomsen et al., 2005). Briefly, each operant conditioning chamber contained two nose-poke holes equipped with a photocell and a yellow cue light, 10 mm above the grid floor. Centered between the nose-poke holes was a plate into which liquid food could be delivered. A liquid swivel mounted on a balance arm was used for intravenous (IV) drug delivery in the freely moving animals. Responding in the right hole resulted in delivery of a reinforcer and illumination of the cue light for a 20-s timeout (TO) period during which no reinforcer could be earned (timeout responses were recorded but had no scheduled consequences). Responses in the left hole were counted but had no scheduled consequences. The house light was illuminated after delivery of a single noncontingent reinforcer (with the exception of acquisition sessions for cocaine self-administration, in which noncontingent reinforcers were never delivered) and stayed on until the session ended.
Catheter Implantation Surgery and Maintenance
An indwelling catheter was implanted into the right or left external jugular vein under oxygen/isoflurane or oxygen/sevoflurane vapor anesthesia. The surgical procedure has been described in great detail elsewhere (Thomsen & Caine, 2005). Briefly, a catheter was inserted 1.2 cm into the jugular vein and anchored to the vein with sutures. The catheter ran subcutaneously to the base located above the midscapular region. The mice were allowed 7 days recovery, during which 0.02 ml of 0.9% saline containing heparin (30 USP units/ml) and antibiotic (Ticarcillin disodium or cefazoline, 67 mg/ml) was infused daily through the catheter to forestall clotting and infection. Outside self-administration sessions, the free end of the cannula guide was kept closed at all times. Catheter patency was confirmed after completion of an experimental phase by the infusion of 0.02–0.03 ml of 15 mg/ml ketamine plus 0.75 mg/ml midazolam in saline. Loss of muscle tone and clear signs of anesthesia within 3 s of infusion indicated catheter patency.
Overview of Experiments
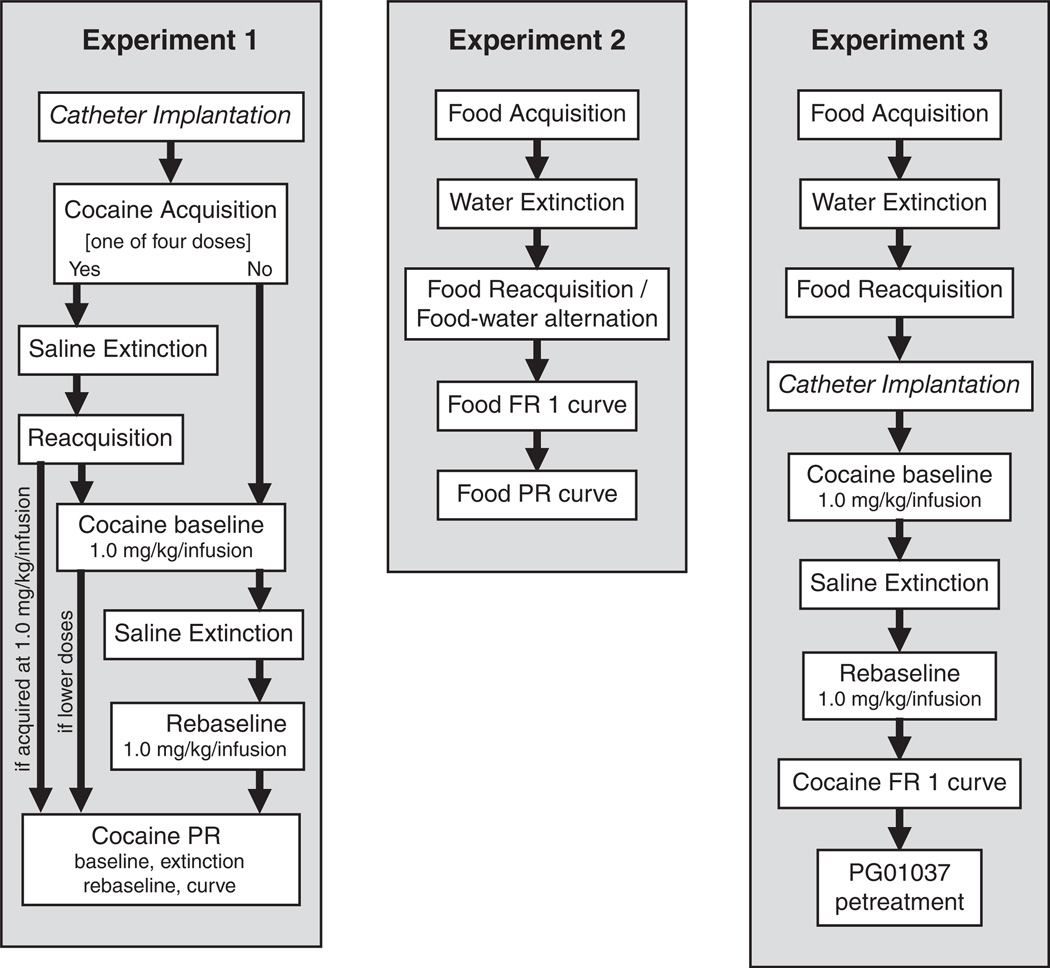
Mice were assigned to different experimental groups as follows. In Experiment 1, mice were allowed to acquire nose poking maintained by IV cocaine (one of four doses), followed by extinction and reacquisition of self-administration. The mice were also tested under a PR schedule of cocaine reinforcement. In Experiment 2, mice were allowed to acquire nose poking maintained by liquid food, and concentration-effect functions were evaluated under an FR 1 then a PR schedule of reinforcement. In Experiment 3, mice were trained with liquid food as under Experiment 2 (acquisition, extinction, and reacquisition phases only—data were comparable to those of Experiment 2 and are not shown), then underwent catheter implantation surgery and self-administered IV cocaine under an FR 1 schedule of reinforcement. Figure 1 shows a diagram of the groups and sequence of training/testing phases.
Figure 1.
Diagram of experimental groups (1, 2, 3) and the sequence of training/testing phases in each experiment. In Experiment 1, note that mice which were assigned the highest cocaine dose (1.0 mg/kg/infusion) proceeded directly from reacquisition to the PR training. Mice assigned to lower doses were first required to meet baseline, extinction, and rebaseline criteria at the 1.0 mg/kg/infusion dose (to provide a similar starting point for all mice). Regardless of dose, mice which failed to acquire cocaine self-administration were similarly required to meet criteria at the 1.0 mg/kg/infusion dose before proceeding to the PR training. Some attrition due to catheter failure or health complications occurred throughout experimental phases.
Experiment 1: Acquisition of Cocaine Self-Administration Behavior in Experimentally Naïve Mice
Following jugular catheter implantation and recovery (see above), experimentally naïve mice were introduced to the operant chamber with 0.0032, 0.032, 0.32, or 1.0 mg/kg/infusion intravenous cocaine available under an FR 1 (TO) 20-s schedule of reinforcement, for daily 3-hr sessions, 5 days per week. Because no mice acquired self-administration at the two lower doses, data from those two groups were pooled for presentation and analysis. The sessions were started immediately before introducing the animal into the chamber to ensure that the first nose-pokes were reinforced. Mice were allowed to self-administer cocaine for at least seven and up to 10 sessions (if criteria were not met within seven sessions). Mice were considered to have acquired cocaine self-administration when they met the following criteria: 1) at least two consecutive sessions of ≥15 reinforcers earned per session (“acquisition level”) and ≥75% responses in the active hole; 2) extinction of responding when saline was substituted for cocaine (i.e., <50% of the acquisition level); 3) mice were required to show nose-poking levels comparable to acquisition levels when cocaine was again made available after extinction. Extinction and reacquisition phases are now routinely included in our acquisition criteria because we have observed that false positives can occur (e.g., in strains with high baseline activity) when only the initial phase of self-administration is considered (Thomsen, Hall, Uhl & Caine, 2009; Thomsen & Caine, 2011).To prevent overdose, the total drug intake was limited to 30 mg/kg/session (reacquisition). Catheter patency was verified at the end of the acquisition phase and again after the extinction and reacquisition phases; animals in which catheter patency could not be demonstrated were excluded from the data set (11 mice). Cocaine acquisition data were collected from the following numbers of mice (group sizes): at 0.0032/0.032 mg/kg/infusion, nine WT mice (seven females, two males), 13 mice (six females, seven males), and eight mice (females). At 0.32 mg/kg/infusion, 20 WT mice (five females, 15 males), 11 mice (four females, seven males), and 12 mice (one female, 11 males). At 1.0 mg/kg/infusion, 10 WT mice (five females, five males), six mice (males), and five mice (males).
After completion of the acquisition experiment, mice with patent catheters were allowed to self-administer 1.0 mg/kg/infusion cocaine until intake was stable (at least 15 reinforcers earned per session and <20% variations over two consecutive sessions) under the FR 1 schedule, then behavior was trained and evaluated under the PR schedule as described below.
Experiment 2: Acquisition and Evaluation of Food-Maintained Behavior Under the FR 1 and PR Schedules
To obviate potential effects of food neophobia, experimentally naïve mice were first acclimated to the liquid food (5 ml of Ensure protein drink) in the operant conditioning chamber, as follows. The mice were food-deprived exclusively before the first presentation of the Ensure by removing food from the home cage, previously available ad libitum, 18–21 hours before the session (water remained available). Mice were then placed in the operant chamber with a cup of Ensure in daily 2-hr sessions. When a minimum of 1.5 ml were consumed per session (typically within one or two sessions), the acquisition phase began, and mice were placed in the operant conditioning chamber for one 2-hr session daily, 5 days per week. During the acquisition phase, liquid food (25 µl per reinforcer) was presented according to an FR 1 (TO) 20-s schedule of reinforcement. The mice were allowed at least seven sessions to acquire responding, and as long as needed until criteria were met (criteria: two consecutive sessions with a minimum of 30 reinforcers earned per session and at least 75% responses on the active manipulandum). After acquisition criteria were met, water was substituted for at least three sessions and until responding was extinguished to <50% of food responding. A series of six sessions alternating 100% food and water were then presented, to facilitate rapid extinction when responding was not reinforced. Finally, a range of liquid food dilutions (water, 3, 10, 32, and 100%) was then presented consecutively for one session each within subjects according to a Latin square design, followed by a second determination in the same sequence. The two determinations were averaged in each mouse, and the average was used for analysis and graphic representation. Then 100% Ensure was made available under an FR 1 then FR 3 session before introduction of the PR schedule (see below), and food concentration-effect functions were again determined. Food acquisition data were collected from 16 WT mice (14 females, two males), 10 mice (six females, four males), and 11 mice (six females, five males).
Experiment 3: Cocaine Self-Administration Behavior Under the FR 1 Schedule
Mice were trained with liquid food as described under Experiment 2 (using a separate cohort of mice) and underwent jugular catheter implantation and recovery (see above). Intravenous cocaine was then made available as the reinforcer (1.0 mg/kg/infusion) under an FR 1 TO 20-s schedule of reinforcement, until baseline criteria were met (two consecutive sessions with ≥15 reinforcers earned per session, <20% variation in number of reinforcers earned between the two sessions, and ≥75% responses in the active hole). Thereafter saline was substituted for cocaine until extinction to criteria (<80% of baseline self-administration level), then baseline behavior was reestablished with 1.0 mg/kg/infusion, followed by dose-effect functions determined within subjects (saline, 0.032, 0.1, 0.32, 1.0, and 3.2 mg/kg/infusion, tested consecutively for one session each in a pseudorandom order counterbalanced across genotypes). To prevent overdose, the total drug intake was limited to 30 mg/kg/session. Sessions were as described for food, except that session length was 3 hr. For these and all other experiments, catheter patency was verified periodically and data from animals in which catheter patency could not be demonstrated after a dose-effect determination were excluded from the data set.
A subset of these WT mice were then used to test PG01037 pretreatments to cocaine self-administration. Following the dose-effect function determination, self-administration was allowed to stabilize using 0.32 mg/kg/infusion cocaine under the FR 1 schedule of reinforcement in WT mice. Then PG01037 (10–56 mg/kg, Latin square design) was tested as a pretreatment to this cocaine dose, administered intraperiteneally, 10 min before the self-administration session.
Progressive Ratio Schedule of Reinforcement
Cocaine self-administration behavior and food-maintained behavior was also evaluated under a PR schedule of reinforcement. Mice from Experiment 1 were used to collect cocaine dose-effect functions under the PR schedule of reinforcement, along with some mice which were previously trained to press levers for liquid food reinforcement before being switched over to nose-poke holes (contributions were balanced across and WT, while mice were all from Experiment 1). The food-trained mice had been tested with one or more D3-preferring ligands under an FR 10 schedule of food reinforcement prior to being implanted with a catheter. All mice were allowed to nose-poke under an FR 1 schedule of reinforcement reinforced by 1.0 mg/kg/infusion cocaine until stable intake level (i.e., less than 20% variation and at least 15 reinforcers earned per session), before proceeding to the PR schedule (typically 2–3 days). An FR 3 schedule was used as a transition from the FR 1 schedule prior to introduction of the PR schedule, until the reinforcers earned were comparable to the FR 1 level (typically less than five sessions).
For the PR schedule, the ratio for successive reinforcers was incremented in steps according to the logit equation: ratio = 19 × [1 + log(step/(7−0.3 × step))], resulting in ratios of 3, 9, 13, 16, 18, 20, 22, 24, 25, 27, 28, 29, 31, 32, 34, 35, 37, 39, 41, 44, 47, 52, 64, and so forth (see Thomsen et al., 2005). The breaking point was defined as the number of reinforcers earned (last completed ratio) after a 60-min limited hold (i.e., period with no reinforcer earned). If a breaking point had not been reached within 6 hr, the session was terminated to prevent health hazard (e.g., hypothermia, dehydration; less than 5% of the “breaking points” were recorded due to session expiration without a full 1-hr limited hold). After a stable baseline had been achieved (two consecutive sessions with breaking points >10 reinforcers and with less than 20% variation), saline or water was substituted until responding extinguished to breaking points <10 reinforcers and ≤50% of the baseline. Cocaine dose-effect curves (saline, 0.03, 0.32, and 1.0 mg/kg/infusion cocaine) and liquid food concentration-effect curves (water, 3, 10, 32, 100% food in water) were determined within-subjects according to a Latin square design, with each dose tested for two or three consecutive sessions (i.e., if the breaking points reached in the two first determinations varied by more than 20%, a third determination was made). For each dose/concentration, breaking points were averaged over test days, and the average was used for data presentation and analysis. Doses/concentrations were tested consecutively (not separated by baseline days).
Drugs
Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (NIH, Bethesda, MD), PG01037 was synthesized in the Medicinal Chemistry Section (NIDA-IRP, Baltimore, MD), using methods described in Grundt et al., (2005). Cocaine was dissolved in 0.9% saline and PG01037 in sterile water. Drug doses refer to the weights of the respective salts.
Data Analysis
The number of mice that met acquisition (food or cocaine) criteria within seven sessions was compared for each dose between and WT mice, and between and WT mice, respectively, using Fisher’s exact test. The amount of behavior during the acquisition phase (food or cocaine) was compared using mixed-model analysis of variance (ANOVA) with genotype and sex as between-subjects variables, and session and nose-poke hole (reinforced/nonreinforced) as within-subjects (repeated measures) variables. For cocaine, there was an additional between-subjects factor of dose. In mice that met acquisition criteria, reinforcers earned at criteria for acquisition, extinction, and reacquisition were likewise compared, with condition (training phase) as the within-subjects (repeated measures) variable. Significant effects were followed by Tukey’s test. For food concentration-effect functions and drug dose-effect functions, comparisons were made using a mixed-model ANOVA with genotype and sex as between-subjects variables and food concentration or drug dose as within-subjects (repeated measures) variables. The dependent variable was the number of reinforcers earned per session (i.e., the breaking point for the PR schedules). Significant effects were followed where appropriate by Dunnett’s multiple comparisons test versus vehicle, or the Bonferroni posttest between genotypes. Significance level was set at p < .05. There were no significant effects or consistent trends for sex differences, therefore all data are reported as sexes combined and statistical analyses involving the sex factor are omitted for brevity.
Results
Acquisition of Operant Behaviors (Experiments 1 and 2)
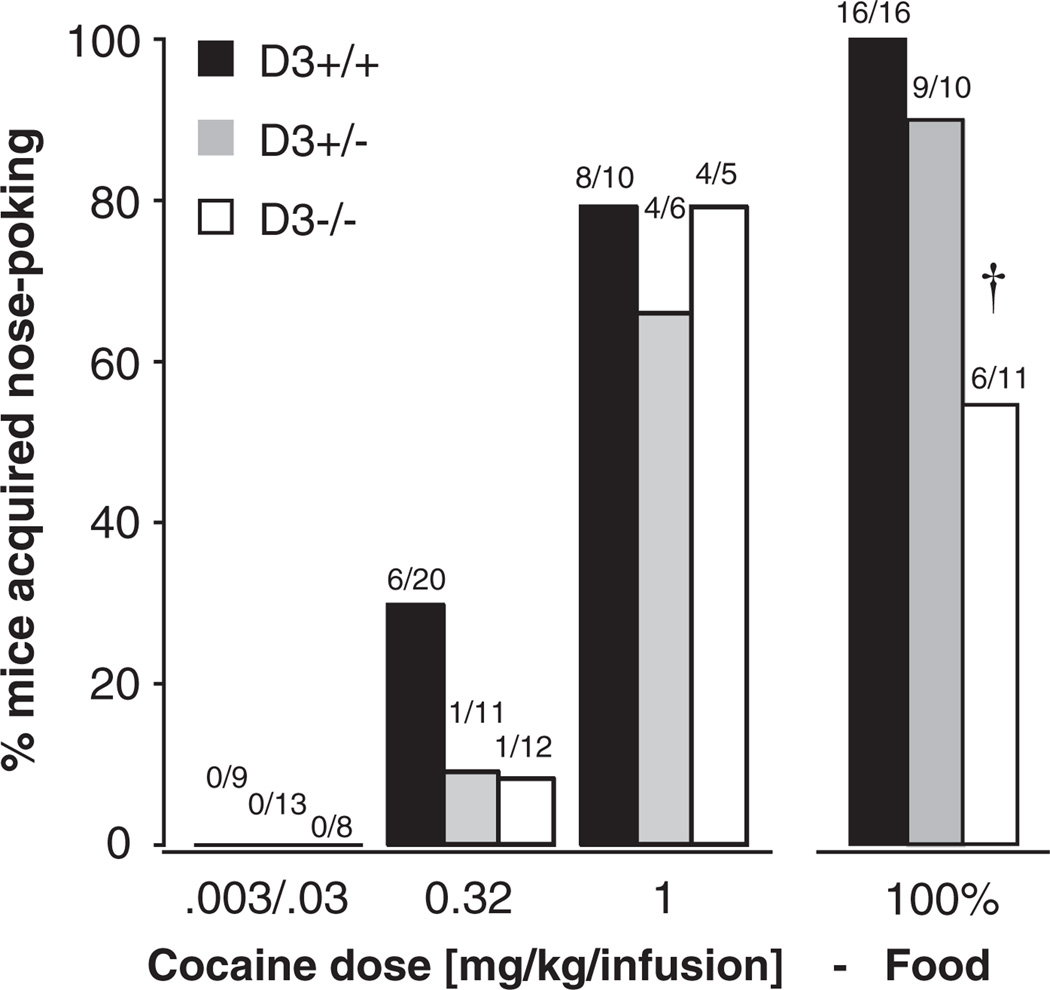
Acquisition of cocaine self-administration was evaluated in separate groups of experimentally naïve mice, having access to one of four doses of cocaine, 0.0032, 0.032, 0.32, or 1.0 mg/kg/infusion, under an FR 1 schedule of reinforcement. Because no mice acquired self-administration at the two lower doses, data from those two groups were pooled for presentation. For comparison, an additional group of mice were allowed to acquire nose poking for liquid food (undiluted Ensure, Experiment 2) under the same schedule of reinforcement. The number of mice meeting acquisition criteria within 7–10 sessions was compared at each dose using a Fisher’s exact test for versus WT and for versus WT, respectively (Figure 2). No comparison was significant for acquisition of cocaine self-administration, but for food, significantly fewer mice met criteria within seven sessions relative to WT (p < .05). mice did not differ significantly from WT mice for any comparison. Mice also showed no genotype effect in their ability to meet criteria for extinction and reacquisition of self-administration, as all mice that acquired initially met criteria for those phases as well. Males and females acquired in comparable proportions: at 0.32 mg/kg/infusion cocaine, 18% versus 20%, at 1.0 mg/kg/infusion cocaine, 81% versus 60%, for food, 85% versus 82% (all nonsignificant).
Figure 2.
Proportion of , and WT mice meeting acquisition criteria within 7 sessions for each cocaine dose and for food. Because no mice met criteria at 0.0032 and 0.032 mg/kg/infusion cocaine, these groups were pooled into one low-dose group. Abscissa: reinforcer; ordinate: % mice acquired. Numbers above columns indicate the number of mice meeting criteria/group size (see Methods for females/male distribution). † p < 0.05 vs. WT, Fisher’s exact test.
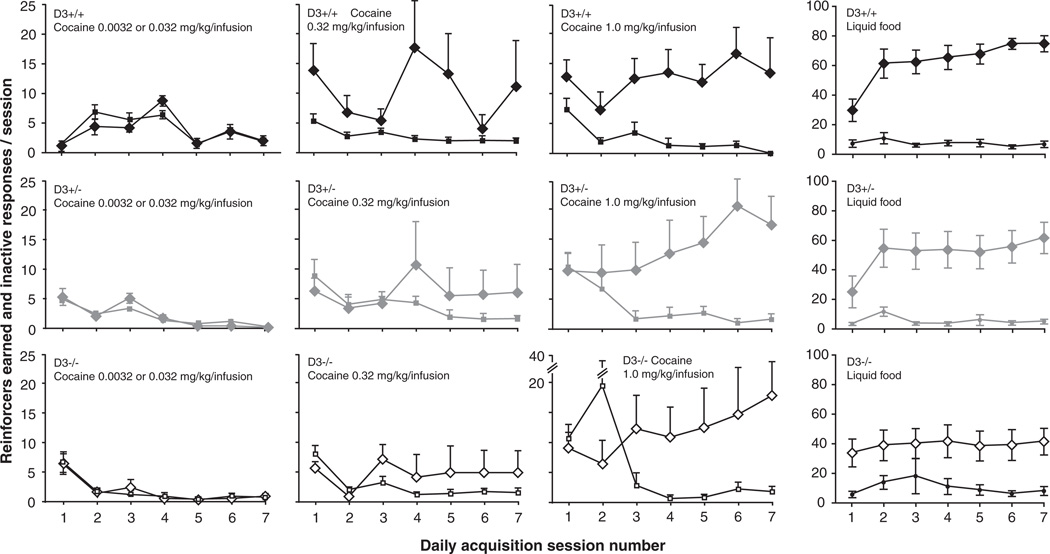
Figure 3 shows the reinforcers earned and inactive responses as a function of acquisition sessions for cocaine and food. For cocaine, ANOVA revealed no significant effect of genotype, or any significant interaction of genotype and other factors (see Figure 3). Responses were related to cocaine dose [F(2, 85) = 7.07, p < .01] and session [F(4, 340) = 2.97, p < .05]. Some interactions were significant, supporting the interpretation of dose-dependent acquisition of self-administration over the sessions: hole by session [F(4, 430) = 3.94, p < .01], and dose by hole by session [F(8, 340) = 3.09, p < .01]. Similarly for food, there was no significant effect of genotype or any significant interaction of genotype and other factors (see Figure 3). Responding was related to nose-poke side [F(1, 21) = 78.9, p < .0001], session [F(6, 162) = 6.40, p < .0001], with a side by session interaction [F(6, 162) = 8.72, p < .0001].
Figure 3.
Reinforcers earned and inactive responses as a function of session under acquisition of cocaine self-administration and food-maintained behavior in experimentally naïve WT mice (top panels), mice (center panels) and mice (lower panels). There was no significant effect of genotype for either reinforcer group. Abscissae: session number; ordinates: number of reinforcers earned and number of inactive responses per session. Diamonds represent reinforcers earned, small squares represent responses in the inactive manipulandum. Data are group means ± standard error of the mean (SEM). Group sizes as indicated in Figure 1.
In mice that met acquisition criteria, the D3 genotypes did not differ significantly from each other in the numbers of reinforcers earned at criteria for acquisition, extinction, or reacquisition, for all reinforcers (cocaine 0.32 or 1.0 mg/kg, food; see Table 1). For all reinforcers, the number of reinforcers earned was significantly affected by training phase (i.e., reinforcer availability; p < .0001). Specifically, mice earned significantly more reinforcers in the reinforced phases (acquisition and reacquisition) than in the vehicle extinction phase (Dunnett’s test after ANOVA, see Table 1 for details). Reacquisition levels were comparable to acquisition levels in all groups.
Table 1.
Reinforcers Earned at Criteria for Acquisition, Extinction, and Reacquisition
| Reinforcer/phase | WT | D3+/− | D3−/− |
|---|---|---|---|
| Cocaine 0.32 mg/kg/infusion | (N = 6) |
(N = 1) |
(N = 1) |
| Acquisition | 56.5 ± 9.8* | 58.0n/a | 47.8n/a |
| Extinction | 13.0 ± 2.6 | 24 | 22 |
| Reacquisition | 58.4 ± 16.2* | 50n/a | 54n/a |
| Cocaine 1.0 mg/kg/infusion | (N = 8) | (N = 4) |
(N = 4) |
| Acquisition | 23.9 ± 1.2*** | 23.7 ± 1.8*** | 23.1 ± 1.4** |
| Extinction | 8.3 ± 2.5 | 6.3 ± 0.9 | 7.3 ± 2.7 |
| Reacquisition | 22.8 ± 1.8*** | 25.7 ± 2.1*** | 24.8 ± 3.0** |
| Liquid food | (N = 14) |
(N = 10) |
(N = 11) |
| Acquisition | 69.9 ± 2.9*** | 70.5 ± 5.0*** | 61.3 ± 3.9*** |
| Extinction | 20.0 ± 1.9 | 21.4 ± 2.6 | 21.5 ± 2.8 |
| Reacquisition | 65.4 ± 4.7*** | 63.2 ± 7.3*** | 68.3 ± 4.6*** |
Note. In mice that met acquisition criteria, there was no significant effect of D3 genotype on the number of reinforcers earned at criteria for acquisition, extinction, or reacquisition.
p < .05.
p < .01.
p < .001 vs. extinction (Tukey's test).
Statistical analysis not applicable.
Dose-Effect Functions Under FR 1 and PR Schedules of Reinforcement (Experiment 3)
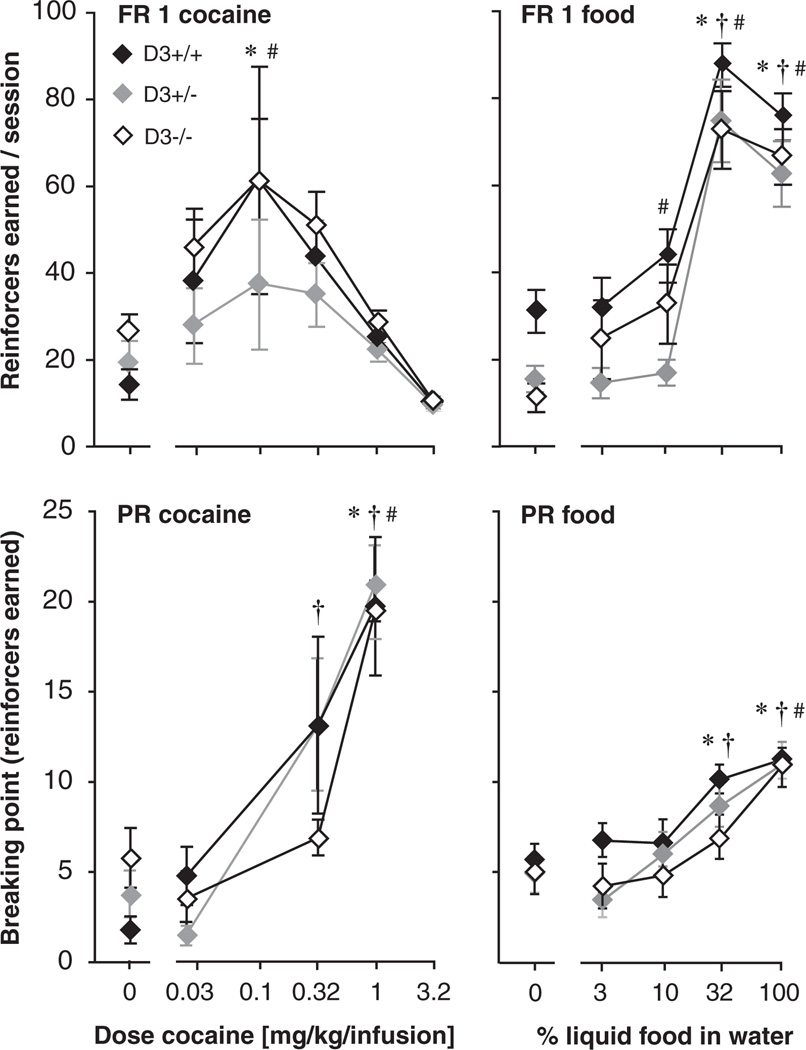
Cocaine self-administration dose-effect functions as well as food-maintained behavior were evaluated under the FR 1 and the PR schedules of reinforcement (Figure 4). Under the FR 1 schedule, reinforcers were related to cocaine dose [F(5, 105) = 7.49, p < .0001], with no significant effect of genotype or interaction. Further analysis revealed that 0.1 mg/kg/infusion cocaine maintained significant self-administration in both the mice and the WT mice (p < .05 vs. saline, Dunnett’s), although no dose reached significance post hoc in the mice. In contrast for food, numbers of reinforcers earned were related to both D3 genotype [F(2, 27) = 3.74, p < .05] and food concentration [F(4, 2108) = 85.7, p < .0001], with no significant interaction. Genotype effects were examined using the Bonferroni posttest, but no comparison reached significance, despite apparent lower levels in the mice when water and low food concentrations were available. Post hoc examination revealed that 10–100% food concentrations maintained higher responding than water in the mice, while only 32–100% reached significance in the and WT mice (all p < .01, Dunnett’s). Note that this difference reflects low levels of reinforcers earned in the mice when water was available, relative to WT mice, rather than high levels of responding for the 10% food concentration (see Figure 4 upper right panel).
Figure 4.
Cocaine dose-response functions (left panels) and food concentration-response functions (right panels) in , and WT mice under the FR 1 (top) and the PR (bottom) schedule of reinforcement. Abscissae: cocaine dose [mg/kg/infusion] or food concentration [% in water]; ordinates: reinforcers earned per session (i.e., breaking point for the PR schedule). Data are group means ± SEM. Group sizes: cocaine FR 1: WT, N = 7 (three females + four males), , N = 9 (two females + seven males), , N = 11 (four females + seven males); food FR 1: WT, N = 12 (two females + 10 males), and , N = 9 (both five females + four males); cocaine PR: WT, N = 4 (two females + two males), , N = 7 (two females + five males) and , N = 4 (all males); food PR: WT, N = 8 (all females), , N = 8 (three females + five males), and , N = 8, (four females + four males). * p < 0.05 vs. vehicle in WT mice, † p < 0.05 vs. vehicle in mice, # p < 0.05 vs. vehicle in mice (Dunnett’s multiple comparisons test).
Under the PR schedule of reinforcement, cocaine maintained dose-dependent responding [F(3, 36) = 29.9, p < .0001], with no significant effect of genotype or interaction. The highest dose, 1 mg/kg/infusion, maintained higher breaking points than saline in all three genotypes (p < .01, Dunnett’s), as did 0.32 mg/kg/infusion in the mice only (p < .05). In some cases, mice failed to reach a breaking point within the 6-hr session. This occurred at the highest dose (baseline and/or dose-effect determination) in all three strains (in about half the mice), and at the 0.32 mg/kg/infusion dose in three mice only. Thus the apparent difference between WT and mice at that intermediate dose was not associated with differences in reaching the cut-off. Similarly, the number of food reinforcers was related to concentration under the PR schedule [F(4, 84) = 56.2, p < .0001], with no significant effect of genotype or interaction. Food concentration of 32% and 100% maintained higher breaking points than water in all genotypes (p < .01, Dunnett’s). All food PR data were true breaking points, that is, no mice reached the maximum 6-hr session duration.
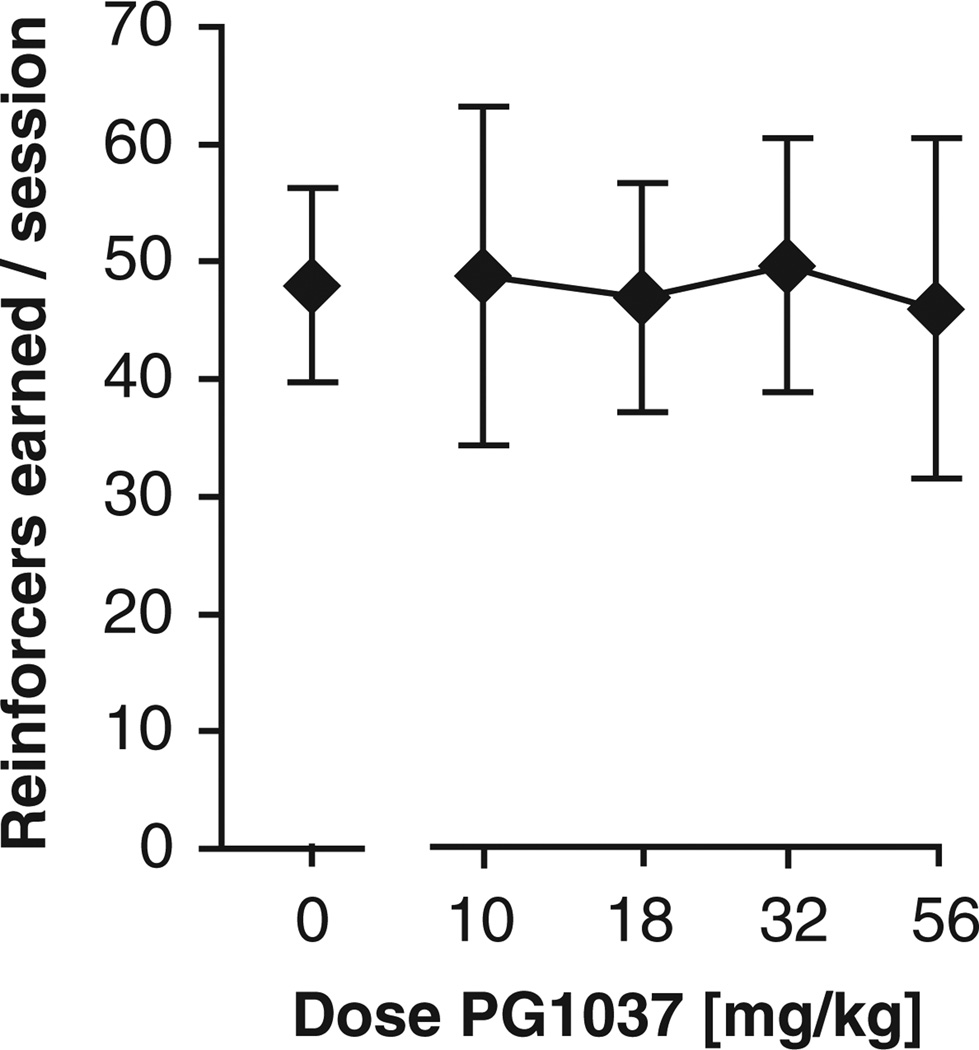
Effects of the D3 Antagonist PG01037 on Cocaine Self-Administration
We tested the D3 antagonist PG01037 as a pretreatment to cocaine self-administration (0.32 mg/kg/infusion) under the FR 1 schedule of reinforcement in WT mice (Figure 5). PG01037 failed to affect cocaine self-administration behavior when administered at doses up to 32 mg/kg (no significant effect of dose). Modest decreases were observed in some mice tested at 100 mg/kg (data not shown). However testing at this dose was discontinued because the administration of 56 mg/kg PG01037 produced side effects such as markedly reduced body temperature (i.e., readily noticeable by handling), in two of six mice, with lethality in one of the mice treated with 56.0 mg/kg, and another of the mice treated with 100 mg/kg. Note that no adverse effects of PG01037 were observed in a food assay at up to 100 mg/kg, so this likely represent an interaction with cocaine.
Figure 5.
Effect of PG01037 on self-administration of 0.32 mg/kg/infusion cocaine under the FR 1 schedule of reinforcement in WT mice. Abscissa: dose PG01037 [mg/kg]; ordinate: cocaine reinforcers earned per session. Data are group means ± SEM. N = 6 (two females, four males).
Discussion
mice did not differ significantly from WT controls in cocaine self-administration behaviors using a range of experimental conditions including acquisition, FR 1 and PR schedules of reinforcement. Small and nonsignificant decreases in self-administration relative to WT mice were apparent at a moderate dose of cocaine (0.32 mg/kg/infusion) under acquisition conditions and under the PR schedule, consistent with the idea that the D3 receptor may play a subtle role in the reinforcing effects of low cocaine doses under low fixed-ratio schedules. However, it should be noted that comparable or greater decreases were observed in food-maintained behavior. We tested both male and female mice, with no significant effect of sex, consistent with previous cocaine self-administration studies in mice from our laboratory (e.g., Thomsen, Han, Gu & Caine, 2009).Taken together, under a range of experimental conditions, we found no clear indication that the reinforcing effects of cocaine specifically were diminished in the mice relative to WT.
The present findings are consistent with earlier reports of unaltered cocaine-conditioned CPP and cocaine-induced increases in extracellular striatal dopamine in mice, suggesting that the D3 receptor does not play a predominant role in mediating abuse-related effects of cocaine in mice (Chen & Xu, 2010; Karasinska, George, Cheng, & O’Dowd, 2005; Zapata, Witkin, & Shippenberg, 2001). Studies using D3-preferring antagonists have suggested D3 manipulations are ineffective under self-administration conditions where the response requirement is low (FR 1), but can modulate cocaine self-administration under high FR, or PR schedules of reinforcement (Xi et al., 2005, 2006). These observations may extend to stimulant drugs more generally, as the D3 antagonist PG01037 was reported to decrease methamphetamine self-administration under a PR schedule of reinforcement in rats having extended access to self-administered methamphetamine, but did not affect behavior under an FR 1 schedule of reinforcement (Orio, Wee, Newman, Pulvirenti, & Koob, 2010). Similarly in knockout studies, we and others have found that PR schedules, as well as acquisition conditions in experimentally naïve mice, can uncover phenotypes not apparent under more permissive conditions (Rocha, Ator, Emmett-Oglesby, & Hen, 1997; Rocha et al., 1998; Thomsen et al., 2005; Ward & Walker, 2009). Here, we assessed both acquisition of cocaine self-administration over a range of doses and dose-effect functions under a PR schedule. While a trend for fewer relative to WT mice meeting acquisition criteria was apparent at the low and intermediate cocaine doses, this did not meet statistical significance and, importantly, was paralleled by a similar (and statistically significant) decrease in acquisition of food-maintained behavior in the mice. Similarly, under the PR schedule, an intermediate cocaine dose maintained significantly higher breaking points than saline in the WT mice, but not mice, however, the overall effect of the D3 genotype was not significant. Because relative small group sizes completed the PR dose-effect studies, it is possible that this apparent genotype effect would have reached significance if more mice had been included. However, a trend for lower breaking points in the mice was also apparent for food. Taken together, under a range of experimental conditions, cocaine self-administration was comparable across the three genotypes or only modestly decreased in the mice. The mice did however show some evidence of either mildly impaired operant performance, or decreased responsiveness to reinforcers more generally, including food.
In the present investigation, the D3 antagonist PG01037 did not decrease cocaine self-administration in WT mice under an FR 1 schedule of reinforcement, using a dose range and route of administration previously shown to be behaviorally active in mice (Collins et al., 2009). This finding is consistent with previous results in rats and squirrel monkeys using an FR 1, PR, or second order schedule of reinforcement in many studies (Achat-Mendes et al., 2010; Gál & Gyertyan, 2003; Orio et al., 2010), but not all (Xi et al., 2006). Other putative D3 receptor antagonists have been reported to decrease cocaine self-administration only under conditions of high response requirements and not under an FR 1 schedule of reinforcement, in rats and monkeys (Martelle et al., 2007; Xi et al., 2005). Beyond the straightforward interpretation that relatively moderate effects (of drugs or other manipulations) can be more easily detected under conditions of high response requirement, there is evidence that D3 receptors are involved in the conditioned reinforcing effects of cocaine, rather than its direct reinforcing effects (Collins & Woods, 2009). This interpretation is consistent with both second order schedules of reinforcement and cue-induced reinstatement of a behavior previously reinforced with cocaine being particularly sensitive to modulation by D3 antagonists (Cervo, Cocco, Petrella, & Heidbreder, 2007; Di Ciano, Underwood, Hagan, & Everitt, 2003). The failure of PG01037 to affect cocaine self-administration under a second order schedule in monkeys may reflect methodological or species differences, or may suggest confounding off target effects of PG01037 (Kumar et al., 2009). Another possibility is that the earlier findings were attributable to D2 rather than D3 receptor blockade by the D3 preferential antagonists. However, all of those possible explanations remain speculative without further empirical evidence.
In summary, our data add to the evidence failing to support a major role for D3 receptors in mediating the direct reinforcing effects of cocaine. However, it remains to be established how the D3 receptor contributes to reinstatement of operant behavior in laboratory animals or of relapse to drug use in humans, such as responses to cocaine-associated cues. In addition, the smaller density and restricted localization of D3 relative to D1 and D2 receptors may have allowed for especially robust developmental compensations to the constitutive D3 receptor knockout. In that regard, the observations in the present study of trends in the D3 receptor knockout mice toward decreased acquisition of self-administration under an FR 1 schedule and lower responding under a PR schedule are intriguing. All in all, the D3 receptor may not be necessary for cocaine’s reinforcing effects, but rather may be a target for subtle manipulations of brain systems related to reward generally and drug abuse in particular.
Acknowledgments
This work was supported by NIDA/NIH Grants DA12142 (to S. Barak Caine), DA02472 (to S. Barak Caine, Andrew C. Barrett, Gregory T. Collins), DA17323 (to Ming Xu), DA025088 (to Ming Xu) and the NIDA-Intramural Research Program (to Amy Hauck Newman). In addition, Morgane Thomsen was supported by NIH/NIDA Grant K99DA027825 while preparing this article. We thank Jennifer Dohrmann, Joon Y. Boon, Jill Berkowitz, Kate Woodard, and Melissa Gale for expert technical assistance.
Contributor Information
S. Barak Caine, Alcohol and Drug Abuse Research Center, McLean Hospital/Harvard Medical School, Belmont, Massachusetts.
Morgane Thomsen, Alcohol and Drug Abuse Research Center, McLean Hospital/Harvard Medical School, Belmont, Massachusetts.
Andrew C. Barrett, Alcohol and Drug Abuse Research Center, McLean Hospital/Harvard Medical School, Belmont, Massachusetts
Gregory T. Collins, Alcohol and Drug Abuse Research Center, McLean Hospital/Harvard Medical School, Belmont, Massachusetts
Paul Butler, Pfizer Worldwide R&D, Sandwich, Kent, United Kingdom.
Peter Grundt, Medicinal Chemistry Section, National Institutes on Drug Abuse-Intramural Research Program, National Institutes of Health, Baltimore, Maryland.
Amy Hauck Newman, Medicinal Chemistry Section, National Institutes on Drug Abuse-Intramural Research Program, National Institutes of Health, Baltimore, Maryland.
Ming Xu, Department of Anesthesia & Critical Care, The University of Chicago, Chicago, Illinois.
References
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: Studies with preferential antagonists in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology. 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikstrom H, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. European Journal of Pharmacology. 1995;281:R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Baker LE, Hood CA, Heidema AM. Assessment of D3 versus D2 receptor modulation of the discriminative stimulus effects of (+)-7-OH-DPAT in rats. Behavioural Pharmacology. 1999;10:717–722. doi: 10.1097/00008877-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Baker LE, Svensson KA, Garner KJ, Goodwin AK. The dopamine D3 receptor antagonist PNU-99194A fails to block (+)-7-OHDPAT substitution for D-amphetamine or cocaine. European Journal of Pharmacology. 1998;358:101–109. doi: 10.1016/s0014-2999(98)00582-2. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47 (1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Sokoloff P, Balster RL, Schwartz JC. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behavioural Pharmacology. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behavioural Pharmacology. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Risinger FO. Dopamine D3 receptor knockout mice and the motivational effects of ethanol. Pharmacology, Biochemistry and Behavior. 2003;75:373–379. doi: 10.1016/s0091-3057(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D1 and D2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. The Journal of Pharmacology and Experimental Therapeutics. 1994;270:209–218. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. Journal of Pharmacology and Experimental Therapeutics. 1999;291:353–360. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: Studies with D2 receptor mutant mice and novel D2 receptor antagonists. The Journal of Neuroscience. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology. 1999;147:22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Carta AR, Gerfen CR, Steiner H. Cocaine effects on gene regulation in the striatum and behavior: Increased sensitivity in D3 dopamine receptor-deficient mice. Neuroreport: For Rapid Communication of Neuroscience Research. 2000;11:2395–2399. doi: 10.1097/00001756-200008030-00012. [DOI] [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacology, Biochemistry and Behavior. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. International Journal of Neuropsychopharmacology. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. Journal of Neurochemistry. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian AJ, Goodwin AK, Baker LE. Antagonism of the discriminative stimulus effects of (+)-7-OH-DPAT by remoxipride but not PNU-99194A. Pharmacology, Biochemistry and Behavior. 2001;68:371–377. doi: 10.1016/s0091-3057(00)00470-6. [DOI] [PubMed] [Google Scholar]
- Collins GT, Truccone A, Haji-Abdi F, Newman AH, Grundt P, Rice KC, Woods JH. Pro-erectile effects of D2-like agonists are mediated by the D3 receptor in rats and mice. The Journal of Pharmacology and Experimental Therapeutics. 2009;329:210–217. doi: 10.1124/jpet.108.144048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behavioural Pharmacology. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyan I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Research Bulletin. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Audinot V, Cistarelli L, Spedding M, Vian J, Millan MJ. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: II. Both D2 and “silent” D3 autoreceptors control synthesis and release in mesolimbic, mesocortical and nigrostriatal pathways. Journal of Pharmacology and Experimental Therapeutics. 1995;275:899–913. [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal of Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: Differences compared to rats. Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Dijkstra D, Wikstrom H, Wise LD, Pugsley TA, Sedvall G. Autoradiographic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD 128907. Psychopharmacology. 1996;128:240–247. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. Journal of Psychopharmacology. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. Journal of Neurochemistry. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. European Journal of Neuroscience. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: Effects of selective antagonists and agonists. The Journal of Pharmacology and Experimental Therapeutics. 2000;294:680–687. [PubMed] [Google Scholar]
- Kong H, Kuang W, Li S, Xu M. Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neuroscience. 2011;176:152–161. doi: 10.1016/j.neuroscience.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss DS, Bergstrom DA, Gonzalez AM, Huang KX, Sibley DR, Walters JR. Dopamine receptor agonist potencies for inhibition of cell firing correlate with dopamine D3 receptor binding affinities. European Journal of Pharmacology. 1995;277:209–214. doi: 10.1016/0014-2999(95)00069-w. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Nader MA, Mello NK. Effects of the putative dopamine D3 receptor agonist 7-OH-DPAT in rhesus monkeys trained to discriminate cocaine from saline. Psychopharmacology. 1996;124:306–314. doi: 10.1007/BF02247435. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-npropyl-2-aminotetralin. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, Katz JL. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behavioural Pharmacology. 2010;21:171–181. doi: 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxami de] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzami de], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose-response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JA, Xu M, Woods SC, Seeley RJ, Benoit SC. Ethanol consumption in mice with a targeted disruption of the dopamine-3 receptor gene. Addiction Biology. 2003;8:295–303. doi: 10.1080/13556210310001602202. [DOI] [PubMed] [Google Scholar]
- Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Heidbreder C. 1,2,4-triazol-3-yl-thiopropyltetrahydrobenzazepines: A series of potent and selective dopamine D3 receptor antagonists. Journal of Medicinal Chemistry. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Narita M, Soma M, Tamaki H, Suzuki T. Intensification of the development of ethanol dependence in mice lacking dopamine D3 receptor. Neuroscience Letters. 2002;324:129–132. doi: 10.1016/s0304-3940(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addiction Biology. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Caine SB, Sokoloff P, Schwartz JC, Koob GF, Weiss F. Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. Journal of Neurochemistry. 1996;67:1078–1089. doi: 10.1046/j.1471-4159.1996.67031078.x. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Heffner TG. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1355–1366. [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: Comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. Journal of Pharmacology and Experimental Therapeutics. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Ator R, Emmett-Oglesby MW, Hen R. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacology, Biochemistry and Behavior. 1997;57:407–412. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. European Journal of Neuroscience. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaál J, Xi ZX, Gardner EL. YQA14: A novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addiction Biology. 2012;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacology, Biochemistry and Behavior. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Chapter 9, Unit 920. Current Protocols in Neuroscience. 2005 doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. The Journal of Neuroscience. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. The Journal of Pharmacology and Experimental Therapeutics. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Ralph RJ, Caine SB. Psychomotor stimulation by dopamine D1-like but not D2-like agonists in most mouse strains. Experimental and Clinical Psychopharmacology. 2011;19:342–360. doi: 10.1037/a0024053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. The Journal of Neuroscience. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. The Journal of Neuroscience. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Walker EA. Sex and cannabinoid CB1 genotype differentiate palatable food and cocaine self-administration behaviors in mice. Behavioural Pharmacology. 2009;20:605–613. doi: 10.1097/FBP.0b013e328331ba30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1984;230:678–683. [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. European Journal of Neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Zapata A, Witkin JM, Shippenberg TS. Selective D3 receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology. 2001;41:351–359. doi: 10.1016/s0028-3908(01)00069-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. The Journal of Neuroscience. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Adomako-Mensah J, Yuferov V, Ho A, Zhang J, Xu M, Kreek MJ. Effects of acute “binge” cocaine on mRNA levels of mu opioid receptor and neuropeptides in dopamine D1 or D3 receptor knockout mice. Synapse. 2007;61:50–59. doi: 10.1002/syn.20340. [DOI] [PubMed] [Google Scholar]