Abstract
Publications relating esophageal radiation toxicity to clinical variables and to quantitative dose and dose–volume measures derived from three-dimensional conformal radiotherapy for non–small-cell lung cancer are reviewed. A variety of clinical and dosimetric parameters have been associated with acute and late toxicity. Suggestions for future studies are presented.
Keywords: Esophagitis, Lung cancer, Radiotherapy, Esophagus, Toxicity
1. Clinical Significance
Acute esophagitis (occurring ≤90 days after treatment initiation) is a common side effect of patients undergoing radiotherapy (RT) for thoracic tumors. Concurrent chemoradiotherapy (CCT) or hyperfractionation results in a 15–25% rate of severe (Radiation Therapy Oncology Group [RTOG] Grade 3 or greater) acute esophagitis (1–3) that can require hospitalization, invasive diagnostic tests (e.g., endoscopy), surgical intervention (e.g., percutaneous endoscopic gastrostomy tube) or RT breaks that could lower local tumor control.
Late injury is less commonly reported, perhaps because the patients might not live long enough to manifest toxicity (e.g., the disease-specific survival is relatively short for many thoracic cancers). Dose escalation of standard fractionated RT and hypofractionated RT regimens (4, 5) can increase the risk of late esophageal toxicity, especially if the survival rates improve. Esophageal stricture often requires periodic dilation, usually with good results (6). Death related to late esophageal injury (e.g., tracheoesophageal fistula or esophageal perforation) has been reported in only 0.4–1% of patients (7, 8).
2. Endpoints
The assigned toxicity grade varies with the scoring system used, making interstudy comparisons challenging. In general, Grade 1 toxicities cause minor changes in a patient's lifestyle, and Grade 2 or greater toxicities might require medical intervention. The currently accepted grading system is the Common Terminology Criteria for Adverse Events, version 3 (9); however, the studies cited in the present report mostly used the RTOG scoring system. In the present review, Grade 2 or greater acute esophagitis (because it constituted the end-point of many studies) and any late esophagitis (Grade 1 or greater), independent of the duration of the late symptoms, were considered clinically significant.
Acute esophagitis occurs during RT and often persists for several weeks after RT. The symptoms of severe esophagitis (Grade 3 or greater) typically peak 4–8 weeks from the beginning of RT (10). Late esophageal damage, typically stricture and associated dysphagia, develops ∼3–8 months (range, 5–40) after RT (11). Abnormal esophageal motility can be noted within 3–4 weeks from RT alone and as early as 1 week after starting concurrent chemoradiotherapy (12).
Some of the pitfalls in assigning the acute esophagitis grade are as follows:
Esophageal infection can mimic treatment (RT or concurrent chemoradiotherapy)-related esophagitis. Candidiasis (usually suggested by co-existing oral candidiasis) or, rarely, herpes simplex esophagitis are the main culprits.
Pre-existing gastroesophageal reflux can worsen the symptoms of esophagitis and should be treated. Constant burning, unrelated to the act of swallowing, and localized in the lower part of the esophagus is more likely related to the reflux than to the treatment-related esophagitis.
Incidental irradiation of the stomach, and associated gastritis symptoms, can occur when a lower lobe lung mass has been treated.
The assignment of Grade 2 (brief intravenous fluid for ≤24 hours) vs. Grade 3 (hospitalization) esophagitis might be physician-dependent.
3. Challenges Defining Volumes
The adult esophagus length is ≈ 25 cm and is defined by its external contour on axial computed tomography (CT) images. The esophagus remains closed when not involved in swallowing, and its lumen is often not easily identifiable throughout its entire length, particularly in the middle and caudal levels. Administration of a thick barium paste can help localize the esophagus, but the swallowing times are short (10 seconds), and the barium paste might not fully opacify the entire organ. In addition, high-contrast barium can affect the heterogeneity-corrected dose calculations. It is recommended that the entire length of the esophagus, from the cricoid cartilage to the gastroesophageal junction, be identified, requiring that a portion of the neck and upper abdomen be included in the planning CT scan. In some of the studies (8, 11, 13), the cephalad (“cervical”) esophagus was not included, causing the absolute esophageal volume to be ∼20% smaller than if its entirety had been contoured.
The esophagus is slightly mobile. In a study of 29 patients undergoing four-dimensional CT scans three times during RT, the cephalad, middle, and caudal esophagus can move ≤5,7, and 9 mm in the combined anteroposterior and craniocaudal directions, respectively (14). Thus, dose–volume analyses using the planning CT scan (as was done in the studies we reviewed), could have some inaccuracies, although no specific margin recommendations can be given at this time.
The esophageal circumference varies markedly on sequential axial CT images, a reflection of the swallowing act. This appearance does not reflect the anatomic reality of a relatively uniform circumference (15). Thus, conventional dose–volume histograms (DVHs) might not accurately reflect the partial volume doses. In the single study to consider this issue, the predictive value of metrics that were “corrected” for this anatomic reality were slightly better predictors of outcome than were the “traditional” DVH-based metrics (15). Nevertheless, the use of alternative three-dimensional dosimetric parameters (e.g., dose–surface-area, dose–circumference histograms, “anatomically corrected” DVHs) as improved predictors of outcome is of unclear utility (11, 15, 16).
4. Review of Dose–Volume Published Data
A total 12 studies published between 1999 and January 2009 that assessed the dose–volume outcome in ≥90 patients treated for non–small cell lung cancer were reviewed (7, 8, 11, 13, 16–19, 20–23) (Table 1). All but one study (17) used three-dimensional planning. The endpoint was usually RTOG Grade 2 or greater or Grade 3 or greater. Two studies (7, 8) combined acute and late toxicities in a single analysis. The others either analyzed only acute (13, 16, 17, 19, 20, 22, 23) or analyzed acute and late toxicity separately (11, 18). The studies found a correlation with these endpoints for a variety of dose–volume factors.
Table 1.
Summary of large published series investigating treatment-related esophagitis in patients with NSCLC
| Series/investigator | Patients (n) | Prescription dose (Gy) range [median]* (special fractionations) | CCT (%) | Endpoint† (rate) | Univariate significant factors | Multivariate significant factors |
|---|---|---|---|---|---|---|
| Duke/Maguire et al.(18), 1999 | 91 | 64–86 [79]‡ (64% twice daily, 1.25–1.6 Gy/fx) | 47 | Acute G 3≥(G3, 11%; G4-5, 0%) | None | None |
| Any late,§ 18% (G1, 9%; G2, 6%; G3, 3%) | V50, A50, length of 100% circumference >50 Gy | Gender, pre-RT dysphagia, V50, maximum percentage of circumference >80 Gy | ||||
| Thomas Jefferson/Werner-Wasik et al. (17), 2000¶ | 105 | 45–70 [60] (7% twice daily)║,# | 55 | Acute G ≥3 (G3, 12%; G4, 1%) | CCT, twice-daily treatment, female gender | CCT, twice-daily treatment |
| Washington University/Singh et al. (7), 2003 | 207 | 60–74 [70]** | 25.6 | Acute G ≥3 (G3, 4.3% G4, 0.5%) and/or†† late G ≥3 (G3, 4.8%; G4, 0.5%; G5, 0.5%)‡‡ | CCT, Dmax ≥58 Gy, mean dose >34 Gy, subcarinal nodes, race | CCT, Dmax ≥58 Gy |
| Washington University/Bradley et al. (16), 2004§§ | 166 | 60–74 [70]¶¶ | 24.7 | Acute G ≥2 (G2, 22.3%; G3, 4.2%; G4, 0.6%) | CCT, aA range (aA5–aA70), aA55║║, aV range (aV5–aV70), aV60║║ | CCT and aV60; CCT, aV60, and aV80; CCT and aA55; CCT, aA55, and aA80 “volume and area equally predictive” |
| Duke/Ahn et al. (11), 2005## | 254 | 30–86 [66]§§ (39% twice daily, 1.25–1.6 Gy/fx) | 12.6 | Acute G ≥3 (G3, 8.7%; G4, 0.4%) | Twice daily; nodal stage; pretreatment dysphagia; Dmax; mean dose; V50; length of 50%, 75%, or 100%; circumference ≥50 Gy; maximal percentage circumference ≥50, 60, 70 Gy | Twice daily RT, noda stage, pretreatment dysphagia |
| Any late§ (G2, 2%; G3, 2%; G4, 1%) | Length with 75% circumference ≥70 Gy, length with 100% circumference ≥50, 55 Gy; maximal percentage circumference ≥60–80 Gy | Previous acute toxicity dominated all dosimetric factors | ||||
| NKI/Belderbos et al. (19), 2005 | 156 | Group 1 (n = 88), 50–95 at 2.25/fx#,***,††† Group 2 (n = 68), 66 at 2.75/fx#,*** | 23.7‡‡‡ | Acute G ≥2 (G2, 20%; G3, 6%; G4, 0.6%) | Lyman NTCP§§§,V range (V20–V60); V35║║, percentage of length, 100%; circumference ≥40 Gy or ≥66 Gy; treatment group (column 3); CCT worse than sequential C/RT or RT only; sequential C/RT worse than RT alone; T stage and nodal stage; age¶¶¶ | V35, CCT |
| University of Michigan/Chapet et al. (13), 2005 | 101 | 65–103#,***,††† | 0 | Acute G ≥2 (G2, 13%; G3, 3%) | Nodal stage, V range (V40–V70), Dose-percentage volume range (D5–D60), D30║║, D1 cc, 2.5 cc, 5 cc | Lyman model NTCP with study-specific parameters |
| Goyang/Kim et al. (20), 2005 | 124 | 54–66 [60]#,*** | 60 | Acute G ≥3-4 (G3, 12%; G4, 0.8%) | CCT, V range (V58– V63), Dmax, Lyman model NTCP (Burman et al. [24] parameters) | CCT, V60 (in patients with CCT) |
| Harbin University/Qiao et al. (8), 2005 | 208 | 60–72 [70]** | 26 | Acute G ≥3 (G3, 5%; G4, 0.5%; G5, 1%) and/or late G ≥3 (G3, 5%; G4, 0.5%) | CCT, Dmax ≥60 Gy, mean dose ≥40 Gy, subcarinal lymph nodes | CCT, Dmax ≥60 Gy |
| MDACC/Wei et al. (22), 2006 | 215 | 60–70 [63]*** (16% twice daily, 1.2 Gy/fx) | 100 | Acute G ≥3║║║ (G3, 20%; G4, 0.5%) | aV range (aV15–V45); V range (V10–V45); mean dose ≥34.5 Gy | V20 |
| Barcelona/Rodriquez et al. (23), 2009 | 100 | 55–65 [62] | 100 | Acute G ≥1 (G2, 29#x00025;; G3, 4%) esophagitis index### | V50–V55 | NA |
Abbreviations: NSCLC = non–small-cell lung cancer; CCT = concurrent chemotherapy; fx = fraction; G = grade; Vdose (e.g., V20) = relative volume receiving specified dose or more (e.g., ≥ 20 Gy); RT = radiotherapy; Dmax = maximal dose; Adose = relative surface area receiving specified dose or greater; aVdose, aAdose = absolute volume (V) or area (A) receiving specified dose or greater; D# = dose encompassing hottest percentage of esophagus. D #cc =dose encompassing hottest cubic centimeters of esophagus; NTCP = normal tissue complication probability; RTOG = Radiation Therapy Oncology Group.
All doses at standard fractionation of 1.8–2.2 Gy/d, 5 d/wk, unless otherwise stated.
Unless otherwise specified, RTOG grading was used; RTOG Grade 2, moderate dysphagia or odynophagia, requiring narcotic agents or liquid diet; RTOG Grade 3, severe dysphagia or odynophagia with dehydration or weight loss, requiring nasogastric feeding.
Clinical calculations and prescriptions done without inhomogeneity correction; doses for study retrospectively corrected for inhomogeneity and tabulated above.
Late complications determined from fraction of patients assessable for late toxicity.
No three-dimensional conformal RT but correlation with irradiated esophagus length inferred from length of spine in field was investigated.
All twice-daily patients also underwent CCT.
Doses were fraction size-corrected using linear-quadratic model and α/β = 10 Gy.
Doses reported without tissue heterogeneity correction.
Acute and Late complications analyzed together.
Percentage of late complications from raw numbers (e.g., 4.8% = 10 patients of 207 patients).
Same patients analyzed by El Naqa et al. (21).
Various treatment techniques and fractionation schedules used; most common was standard fractionation for 45 Gy to clinical target volume with cone-down to 66 Gy total to gross target volume; dose range quoted was overall dose to isocenter, corrected for tissue heterogeneity.
Lowest p value.
Doses were heterogeneity corrected.
Esophagus constraint on treatment plan.
All CCT patients were in 66-Gy group, a randomized trial of concurrent vs. sequential chemotherapy; they constituted 54% of that group but only 23.7% of total.
Found Lyman NTCP model parameters that gave visually good fit to data; significance not stated.
Not specified whether toxicity was more likely at older age.
Grading by institutional modification of RTOG.
See Rodriguez et al. (23) for definition
The maximal esophagus dose had significant univariate correlation (p ≤ .05), with severe esophagitis in all the studies that included it as a variable (7, 8, 11, 13, 20). However, it only remained significant in multivariate analyses in some of them (7, 8, 11).
Ten studies (8, 13, 16, 18, 19–24) searched for correlations between severe acute esophagitis and either the absolute volume (aVdose), absolute area (aAdose), or percentage of a reference volume (Vdose), or reference area (Adose) receiving more than a specified dose. Eight of these studies (13, 16, 19–24) found significant univariate correlations with exposure over a wide dose range (10–80 Gy; Table 1 and Fig. 1). Multivariate analysis (16, 19, 20, 22, 24) identified fewer dose–volume combinations. Because of the diverse reporting metrics, we could not find a consensus for the dose–volume thresholds. For example, one study (19) found V35 was the only dosimetric predictor of RTOG Grade 2 or greater acute esophagitis on multivariate analysis, both with and without CCT, and another study (22) found V20 to be the only multivariate significant factor for 215 patients receiving CCT. However, a third study (16) found a much greater dose region (aA55 and aA80 or aV60 and aV80) to be significant.
Fig. 1.
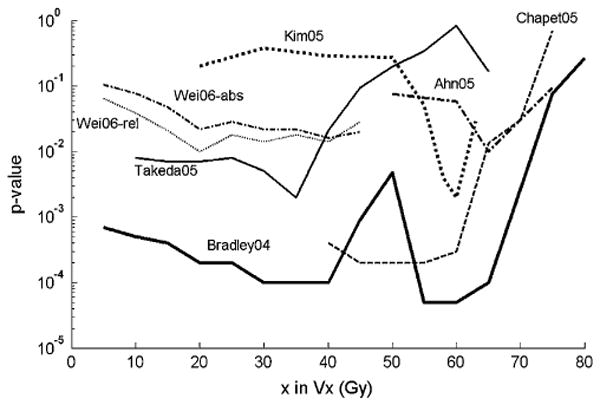
Correlations between acute esophagitis and Vx values (volume greater than x Gy). p Values correlated with relative or absolute volumes (in cubic centimeters); relative volumes used except as noted for 2006 data from Wei et al. (22). Lower values indicate better correlations with outcomes. As the wide variety of correlation shapes suggests, there does not appear to be any singular “threshold” dose above which a toxic effect is observed.
Some studies found circumferential metrics (e.g., esophageal length receiving full circumference dose >40–66 Gy [19] or 50–65 Gy [11]) to be significant, although not superior to simpler volume or area metrics.
Four studies (7, 8, 11, 22) found a univariate correlation with the mean dose greater than levels ranging from 34 Gy (7) to 40 Gy (8). A 34-Gy mean dose recommendation was adopted in the RTOG Phase III comparison of 60 Gy vs. 74 Gy with CCT in Grade III non–small-cell lung cancer (RTOG 0617).
Dose–volume histogram parameters describing cumulative dose >50 Gy have been identified as highly statistically significantly correlated with acute esophagitis in several studies. Some studies (Fig. 1), however, have shown the strongest statistically significant correlations with esophagitis at lower doses (as low as V30), perhaps owing to technique differences. V30 was also implicated in a multivariate modeling study by El Naqa (21). Overall, the data are consistent with some risk of acute esophagitis at intermediate doses (30–50 Gy) and an increasing effect for greater doses.
A main obstacle to obtaining definitive dosimetric recommendations from the published data is the variety of volumetric metrics—the absolute volume or area, relative volume or area, and circumferential measures—all have been analyzed. Reports describing relative metrics might have used different reference volumes (9, 13). Differences in the way other technical factors were handled have less effect. For example, adjusting DVHs for conventional fraction size and the type of tissue heterogeneity correction used are likely to have only minor effect, the latter because the esophagus is embedded in bulky soft tissue and anteroposterior/posteroanterior beams are the main component in many treatment plans. Several studies have provide enough information to estimate the incidence of esophagitis to dosimetric parameters (Fig. 2). There does appear to be a dose–response relationship, although the interstudy variations have been large. Nevertheless, the data are somewhat consistent, with rates of acute Grade 2 or greater esophagitis increasing to >30% as V70 exceeds 20%, V50 exceeds 40%, and V35 exceeds 50%.
Fig. 2.
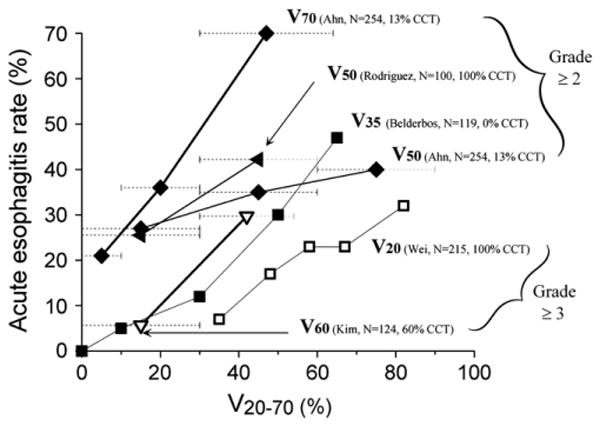
Incidence of acute esophagitis according to Vx (volume receiving more than x Gy). x-Axis values estimated according to range of doses reported. Each curve annotated as follows: Vdose (investigator, number of patients, percentage with concurrent chemotherapy [CCT]. Percentage of patients who received sequential chemotherapy in studies by Ahn et al. (11), Belderbos et al. (19), and Kim et al. (20) was 44%, 38%, and 15%, respectively. Data for V50 (Ahn et al. [11]) at 15, 45, and 75 Gy represent reported rates of Grade 2 or greater acute esophagitis plotted in dose bins at <30%, 30–60%, and >60%, respectively. Similarly, for V70 (Ahn et al. [11]), V50 (Rodriguez et al. [23]), and V60 (Kim et al. [20]), each symbol represents rates of acute esophagitis at <10% vs. 11–30% vs. 31–64%, and ≤30% vs. ≥30%, and ≤30 vs. >30%, respectively. Dashed horizontal lines reflect dose ranges ascribed to each data point. Upper x-axis range of greatest data point for V50 (Rodriguez et al. [23]), V50 (Ahn et al. [11]), and V60 (Kim et al. [20]), are indefinite according to data (light-gray dotted bars). Solid and open symbols represent reported rates of Grade 2 or greater acute esoph-agitis and Grade 3 or greater acute esophagitis, respectively. Thicker and thinner solid lines represent higher and lower doses of Vx, respectively (i.e., thicker line for V70 and thinner line for V20).
5. Factors Affecting Risk
Greater acute esophagitis rates are seen with increased RT aggressiveness (e.g., hyperfractionation, concurrent boost), the addition of CCT, and several clinical factors (e.g., pre-existing dysphagia and increasing nodal stage, with the latter likely a surrogate for larger tumors; Table 1). The incidence of Grade 3 or greater acute esophagitis is ≈ 1% for patients treated with once-daily RT alone. It is markedly increased with the addition of CCT (incidence, 6–24%) and is as great as 49% with concurrent gemcitabine. The Continuous Hyperfractionated Accelerated Radiation Therapy regimen (25) reported a 19% rate of severe (Grade 3 or greater) esophagitis. Older patients (>70 years of age) were more likely than younger patients to experience high-grade esophagitis in a secondary analysis of the RTOG 94-10 study (26).
Several studies have assessed the putative radioprotector amifostine. Three single-institution Phase III studies (27– 29) suggested a significant benefit (27, 28) or a trend (29) for amifostine in lowering Grade 2 or greater esophagitis. However, the findings are difficult to interpret because of the small patient numbers and low (28) or unknown (27) incidence of Grade 3 or greater esophagitis. These results were not confirmed in a large cooperative group Phase III randomized study of 243 patients (RTOG trial 98-01) (30).
6. Mathematical/Biologic Models
Statistical models
The statistical level of correlation between a complication and a set of variables is inadequate for treatment planning purposes. Statistical models aim to supply the missing link. They use the most significant dose–volume or dose–area variable and medical factors (e.g., CCT) as variables in a sigmoidal function. The typical functional form is
The summation (symbolized by Σi) represents a weighted combination of the patient-specific values of the significant dose–volume variables, Vdosei. CCT can be handled by an extra term or by having different sets of coefficients for patients with and without CCT. The model coefficients, ci, are chosen to best match the observed complication rates, and coefficient values are given in the cited studies. The simplest models (probably too simple) use a single dose–volume variable (e.g., V35 [19], V20, or mean dose [22]). Others use several DVH-based variables (e.g., a four-variable model [21] selected absolute area points with doses from 30 to 85 Gy). Such statistical models are more sensitive to the DVH shape than those based on a single Vdose point.
Lyman-Kutcher-Burman model
Two recent studies (13, 19) used the maximum likelihood method to find the Lyman-Kutcher-Burman model parameters that correlated well with the incidence of Grade 2 or greater acute esophagitis in their respective populations of patients without CCT. Both studies applied tissue inhomogeneity and linear-quadratic corrections to 2-Gy equivalent regimens but used different reference esophageal lengths. Chapet et al. (13) excluded the cervical esophagus; thus, their reference length was approximately 20% shorter than that of Belderbos et al. (19). Table 2 lists the parameters from these two studies and, for comparison, the 1991 parameters (31). Because the 1991 endpoint was a very severe and, in modern times very rare, toxicity of clinical stricture or perforation, it is not surprising that the 1991 Lyman-Kutcher-Burman parameters are different from those from the more recent studies for which the endpoint was RTOG Grade 2 or greater acute esophagitis. Both recent parameterizations (13, 19) yielded mid-size n values, consistent with the correlation with a wide range of significant dose–volume factors noted in the section, “Review of Dose–Volume Published Data.” The Lyman parameters of the two studies agreed within their broad 95% confidence intervals.
Table 2.
Three parameterizations of Lyman-Kutcher-Burman model for esophageal complications
| Investigator | TD50 (Gy) | n | m |
|---|---|---|---|
| Burman et al. (31), 1991 | 68 | 0.06 | 0.11 |
| Chapet et al. (13), 2005 | 51 (29–82) | 0.44 (0.11–1.41) | 0.32 (0.19–0.57) |
| Belderbos et al. (19), 2005 | 47 (41–60) | 0.69 (0.18–6.3) | 0.36 (0.25–0.55) |
Abbreviation: TD50= median toxic dose
Burman values derived from “Emami” estimates for more severe endpoint.
Relative Seriality Model
Parameters for relative seriality model were derived (32) from partial irradiation tabulation of Emami et al. (33). Recent planning study (34) found this model/parameter combination predicted a complication rate similar to Lyman model using Burman et al. (31) parameters. However, because both were parameterized to fit the Emami data, neither might be relevant to the studies and milder endpoints reviewed in section “Review of Dose–Volume Published Data.”
General comments
Because acute esophagitis events occur mainly during a course of therapy, the rapidity of dose accumulation might be more important than the final overall dose (much of which is delivered after the complication risk has peaked). No current models account for the course of a complication relative to the number of fractions delivered. It also follows that existing models and dose–volume parameters should not be applied to regimens in which the number of fractions is much different from 30–35 Gy without careful additional study.
7. Special Situations
Hypofractionation for central lesions can expose parts of the esophagus to relatively large doses per fraction. Predictions using conventional fractionation should not be applied to such treatments unless they have been validated by additional study. Although a few reports have been published of serious esophageal toxicity from hypofractionation (35), no comprehensive dose–volume-based analyses have been published. Similarly, no large body of data exists on long-term esophageal toxicity of other altered fractionation schemes (e.g., hyperfractionation; in-field boost).
8. Ecommended Dose–Volume Limits
At present, it is not possible to identify a single best threshold volumetric parameter for esophageal irradiation, because a wide range of Vdose parameters correlate significantly with severe acute esophagitis. In particular, the studies we analyzed illustrate a clear trend demonstrating that volumes receiving >40–50 Gy correlated significantly with acute esophagitis (Fig. 1) (24). In particular, for high-dose conventionally fractionated non–small-cell lung cancer treatments, it is prudent to ensure that the dose to even small volumes of the esophagus does not exceed the prescription dose. This is a particular risk of intensity-modulated RT if no esophagus constraints are imposed in the planning process and the radiation dose is “dumped” inadvertently in the region of the esophagus. The ongoing Phase III Intergroup trial (RTOG 0617) has recommended (but has not mandated) that the mean dose to the esophagus be kept to <34 Gy and that the esophageal V60 be calculated for each patient enrolled in the trial. These recommendations were based on the Washington University experience (7) (Table 2). An inability to provide specific “dose limits” for the esophagus in this large cooperative group trial illustrates the lack of evidence that any absolute limits can be imposed on the basis of current published data. However, from the clinical reports without detailed dosimetric esophageal dose correlates, it appears safe to give doses as great as 74 Gy to a segment of the esophagus with concurrent carboplatin and paclitaxel (36–38).
In the section “Mathematical/Biologic Models,” we described several mathematical models that correlate with the incidence of Grade 2 or greater acute esophagitis for specific study populations. Clinicians with appropriate treatment planning resources might find such models interesting and useful, particularly when making decisions between competing treatment plans. However, it is important to recognize that, at present, these models are tentative as best. A prudent approach to using any mathematical model is to first do a retrospective “test drive” to determine whether predictions are in qualitative agreement with the complications observed at one's own center, subject to local contouring protocols, treatment beam arrangements, and patient populations.
9. Future Toxicity Studies
New thoracic protocols that have acute esophagitis toxicity as an endpoint should specify one or more dose–volume models to test prospectively. Future analyses of esophagitis should ideally include the time of onset, because the complication occurs from the dose accumulated during the course of therapy, usually well before the total dose has been delivered. Complication models could potentially be constructed on the basis of the dose accumulated each week and the total dose. Thus, the data analysis would not be a continual cycle of hypothesis/model generation, such as is commonly the case today.
Peer-reviewed treatment planning and outcomes data should be pooled and made permanently available. This might enable a single analysis to confidently uncover the factors that lead to such an array of dose–volume correlations, such as seen in Fig. 1, to derive robust parameter sets for the Lyman or relative seriality models or to derive new semi-mechanistic models.
The exclusion of the entire esophageal length/volume from the high-dose radiation region is extremely difficult; however, reducing the radiation dose delivered to a part of esophageal circumference might be feasible. Intensity-modulated RT seems well suited for that purpose, with its ability to deliver concave-shaped RT dose distributions around organs at risk (39). Studies to better understand the importance of the spatial distribution of the dose (and hence the utility of partial circumferential sparing) would be useful.
Additional study is needed to understand the utility of radioprotectors.
A prospective assessment of the dose and volume and other factors relating to esophageal injury after hypofractionation is needed, given the growing interest in this approach.
The identification of biologic markers of radiation sensitivity will be important to explain individual variations in patients' reactions.
10. Toxicity Scoring
We recommend that the Common Terminology Criteria for Adverse Events, version 3, be used to score both acute and late injury. It is simple and consistent, and its use has been mandated by the National Cancer Institute in the cooperative group trials since October 2003 (40). Late injury might be scored under several endpoints, including necrosis, obstruction, perforation, or stricture, depending on the patient's symptoms.
Footnotes
Conflict of interest: none.
References
- 1.Curran W, Jr, Scott C, Langer C, et al. Phase III comparison of sequential vs. concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer: Initial report of RTOG 9410. Proc ASCO. 2000;19:484a. [Google Scholar]
- 2.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent vs. sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 3.Cox JD, Pajak TF, Asbell S, et al. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: Analysis of 1244 cases from 3 RTOG trials. Int J Radiat Oncol Biol Phys. 1993;27:493–498. doi: 10.1016/0360-3016(93)90371-2. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancers. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 5.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thoracic Oncol. 2007;2(7 Suppl 3):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 6.Choi GB, Shin JH, Song HY, et al. Fluoroscopically guided balloon dilation for patients with esophageal stricture after radiation treatment. J Vascular Intervent Radiol. 2005;16:1705–1710. doi: 10.1097/01.RVI.0000179813.93992.9E. [DOI] [PubMed] [Google Scholar]
- 7.Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:337–341. doi: 10.1016/s0360-3016(02)03937-8. [DOI] [PubMed] [Google Scholar]
- 8.Qiao W-B, Zhao Y-H, Zhao Y-B, et al. Clinical and dosimetric factors of radiation-induced esophageal injury: Radiation-induced esophageal toxicity. World J Gastroenterol. 2005;11:2626–2629. doi: 10.3748/wjg.v11.i17.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CTC/CTCAE Codes. [Last accessed December 15, 2009]; Available from: www.ctep.info.nih.gov/reporting/ctc.html.
- 10.Werner-Wasik M, Scott C, Curran WJ, Jr, et al. Correlation between acute esophagitis and late pneumonitis in patients (pts) with locally advanced non-small cell lung cancer (LA-NSCLC) receiving concurrent thoracic radiotherapy (RT) and chemotherapy: A multivariate analysis of the Radiation Therapy Oncology Group (RTOG) database [Abstract] Proc ASCO. 2002;21:299a. [Google Scholar]
- 11.Ahn S-J, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation- induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335–347. doi: 10.1016/j.ijrobp.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein HM, Rogers LF, Fletcher GH, et al. Radiological manifestations of radiation-induced injury to the normal upper gastrointestinal tract. Radiology. 1975;117:135–140. doi: 10.1148/117.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Chapet O, Kong F-M, Lee JS, et al. Normal tissue complication probability modeling for acute esophagitis in patients treated with conformal radiation therapy for non-small cell lung cancer. Radiother Oncol. 2005;77:176–181. doi: 10.1016/j.radonc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Dieleman EMT, Senan S, Vincent A, et al. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys. 2007;67:775–780. doi: 10.1016/j.ijrobp.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Kahn D, Zhou S, Ahn S-J, et al. Anatomically correct dosimetric parameters may be better predictors for esophageal toxicity than are traditional CT-based metrics. Int J Radiat Oncol Biol Phys. 2005;62:645–651. doi: 10.1016/j.ijrobp.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Bradley J, Deasy JO, Bentzen S, et al. Dosimetric correlates for acute esophagitis in patients treated with radiotherapy for lung carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:1108–1113. doi: 10.1016/j.ijrobp.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 17.Werner-Wasik M, Pequignot E, Leeper D, et al. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiated esophagus: A multivariate analysis of patients with lung cancer treated with non-operative therapy. Int J Radiat Oncol Biol Phys. 2000;48:689–696. doi: 10.1016/s0360-3016(00)00699-4. [DOI] [PubMed] [Google Scholar]
- 18.Maguire, Sibley GS, Zhou SM, et al. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97–103. doi: 10.1016/s0360-3016(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 19.Belderbos J, Heemsbergen W, Hoogeman M, et al. Acute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol. 2005;75:157–164. doi: 10.1016/j.radonc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Kim TH, Cho KH, Pyo HR, et al. Dose–volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:995–1002. doi: 10.1016/j.ijrobp.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 21.El Naqa I, Bradley J, Blanco AI, et al. Multivariable modeling of radiotherapy outcomes including dose–volume and clinical factors. Int J Radiat Oncol Biol Phys. 2006;64:1275–1286. doi: 10.1016/j.ijrobp.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Wei X, Liu HH, Tucker SL, et al. Risk factors for acute esophagitis in non–small-cell lung cancer patients treated with concurrent chemotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:100–107. doi: 10.1016/j.ijrobp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez N, Algara M, Foro P, et al. Predictors of acute esophagitis in lung cancer patients treated with concurrent three-dimensional conformal radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2009;73:810–817. doi: 10.1016/j.ijrobp.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Nemoto K, Saito H, et al. Dosimetric correlations of acute esophagitis in lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:626–629. doi: 10.1016/j.ijrobp.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Saunders MI, Dische S, Barrett A, et al. Randomized multi-centre trials of CHART vs. conventional radiotherapy in head and neck and non-small cell lung cancer: An interim report. Br J Cancer. 1996;73:1455–1462. doi: 10.1038/bjc.1996.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer C, Hsu C, Curran W, et al. Do elderly patients with locally advanced non-small cell lung cancer benefit from combined modality therapy? A secondary analysis of RTOG 94-10. Int J Radiat Oncol Biol Phys. 2001;51(Suppl 1):20–21. [Google Scholar]
- 27.Antonadou D, Coliarakis N, Synodinou M, et al. Randomized Phase III trial of radiation treatment plus/minus amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 28.Leong SS, Tan EH, Fong KW, et al. Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage III non-small cell lung cancer. J Clin Oncol. 2003;21:1767–1774. doi: 10.1200/JCO.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Komaki R, Lee JS, Milas L, et al. Effect of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small cell lung cancer: Report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369–1377. doi: 10.1016/j.ijrobp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Movsas B, Scott C, Langer C, et al. Phase III study of amifostine in patients with locally advanced non-small cell lung cancer receiving intensive chemoradiation: Radiation Therapy Oncology Group 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 31.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 32.Källman P, Agren A, Brahme A. Tumor and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol. 1992;62:249–262. doi: 10.1080/09553009214552071. [DOI] [PubMed] [Google Scholar]
- 33.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 34.Ragazzi G, Cattaneo GM, Fiorino C, et al. Use of dose–volume histograms and biophysical models to compare 2D and 3D irradiation techniques for non-small cell lung cancer. Br J Radiol. 1999;72:279–288. doi: 10.1259/bjr.72.855.10396219. [DOI] [PubMed] [Google Scholar]
- 35.Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–135. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y, Werner-Wasik M, Michalski D, et al. Comparison of three IMRT-based treatment techniques allowing partial esophagus sparing in patients receiving thoracic radiation therapy for lung cancer. Med Dosim. 2004;29:210–216. doi: 10.1016/j.meddos.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Stichcombe T, Lee C, Moore DT, et al. Long-term follow-up of a phase I/II trial of dose escalating three-dimensional conformal thoracic radiation therapy with induction and concurrent carbo-platin and paclitaxel in unresectable stage IIIA/B non-small cell lung cancer. J Thoracic Oncol. 2008;3:1279–1285. doi: 10.1097/JTO.0b013e31818b1971. [DOI] [PubMed] [Google Scholar]
- 38.Schild SE, McGinnis WL, Graham D, et al. Results of a phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–1111. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 39.Blackstock AW, Ho C, Butler J, et al. Phase IA/IB chemo-radiation trial of gemcitabine and dose-escalated thoracic radiation in patients with stage III A/B non-small cell lung cancer. J Thorac Oncol. 2006;1:434–440. [PubMed] [Google Scholar]
- 40.Colevas AD, Setser A. The NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new standard for oncology clinical trials. J Clin Oncol. 2004;22(14 Suppl):6098. [Google Scholar]


