Abstract
The nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), an important carcinogen found in tobacco products, causes lung cancer in genetically susceptible animals. In addition to mutations of the K-Ras gene, NNK has non-mutagenic effects that include alterations in gene expression and immunomodulation in the lung. Here we report the identification of two gene sets associated with NNK-induced pulmonary tumourigenesis. First, to identify genes involved in the susceptibility to NNK, we compared the lung transcriptomes of NNK-resistant C3H mice with that of the NNK-susceptible A/J mice, identifying differential expression of genes related to innate immunity and inflammation. Second, to identify gene expression induced by NNK, we compared the lung transcriptomes of C3H and A/J mice post-treatment. The Resistin-like alpha (Retnla) gene was highly upregulated in response to NNK only in susceptible mice. This gene product is known to recruit immune cells to the lung, and accumulation of CD45 positive cells in A/J lungs correlated with increased Retnla expression. Genetic susceptibility to NNK-induced lung tumourigenesis may relate in part to gene expression changes and alterations in the immune response to create a protumourigenic environment, acting in concert with NNK’s mutagenic effects.
Keywords: 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, Nitrosamine, Susceptibility, Lung cancer, Resistin-like alpha, Inflammation, Retnla
1. Introduction
Lung cancer is one of the most common cancers worldwide, with over one million new cases and over one million deaths annually in the US.1 Cigarette smoke, a factor in nearly nine out of 10 cases of lung cancer, contains many carcinogenic compounds, including the nitrosamine 1-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK), believed to be a cause of lung cancer in smokers.2,3 Nitrosamines are known to induce cancer through direct damage to DNA (mutagenesis), affecting oncogenes and/or tumour suppressor genes including the oncogene K-ras.4,5 In addition, nitrosamines have non-mutagenic effects that likely promote tumourigenesis, including the direct activation of nicotinic acetylcholine receptors.6 Previous studies have only identified a handful of gene expression changes related to NNK treatment in lungs and their tumours, including increased levels of Cox27 and contactin-1 via transduction of the alpha7 nicotinic acetylcholine receptor; nitrosamines likely act to promote lung cancer through direct mutagenesis as well as through nicotinic receptor-mediated effects.8 One of the goals of this study was to use transcript profiling to capture additional non-mutational effects that could provide insights into the lung tumourigenic process.
The other concept studied in this work is that differential sensitivity to nitrosamine plays a role in lung tumourigenesis. Despite the strong carcinogenic effects of cigarette smoke, many smokers do not develop lung cancer. This suggests that genetic susceptibility plays an important role in lung carcinogenesis.9 One approach to gain insights into the susceptibility to cigarette smoke-induced lung cancer is through the use of animal models with differential susceptibility to NNK-induced carcinogenesis. The advantages of these mouse models are that tumour formation occurs in a biological context (stroma, angiogenesis, immune-system and hormones) and that in vivo responses to NNK can be directly measured. A/J mice readily develop lung tumours when exposed to NNK while more resistant mouse strains, including C3H and BL6, do not.10,11 Since NNK induces nearly identical numbers of mutation and comparable levels of mutagenic DNA adducts in both susceptible and resistant lungs,12 insights gained from this and other studies can identify non-mutational molecules that confer sensitivity/resistance to nitrosamine-induced tumourigenesis. We hypothesise that by studying the lung transcriptomes of both sensitive and resistant mouse strains exposed to NNK we can gain insights into nitrosamine-induced tumourigenesis.
It is generally believed that a multistep process of genetic alterations is responsible for pulmonary carcinogenesis. 13–15 Initiation and progression of tumourigenesis, however, is complex and involves inactivation of tumour suppressor genes, activation of oncogenes, inflammatory processes, as well as alterations in the tissue microenvironment.16–18 In this study, we demonstrate that there are global gene expression changes during the pre-tumour stages of NNK-induced lung carcinogenesis and that susceptible (A/J) and resistant (C3H) mice show differential gene activation in response to NNK.
2. Materials and methods
2.1. Animals
A/J, C3H, Bl6, DBA and 129 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed in filter-top cages and maintained in standard 12 h light/dark conditions with food ad libitum. Mice were treated in accordance to National Institutes of Health guidelines and as approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
2.2. Reagents and antibodies
NNK from ChemSyn Labs (San Diego, CA); Retnla antibody from Abcam (Cambridge, MA); CD45 antibody from BD Biosciences (San Jose, CA) and Krt8 antibody from Epitomics (Burlingame, CA).
2.3. NNK treatment
Mice were treated with NNK by subcutaneous injection of 100 μl in the upper back at a dose of 100 mg/kg once a week for 4 weeks (the week 3 time-point was taken 7-d after the second injection). NNK was dissolved in DMSO in 10× concentration and diluted in corn oil to final concentration. Control mice were injected with the same solution without NNK.
2.4. Preparation tissues
Tissues were collected prior to treatment (time 0) and 3, 6, 9 and 12 weeks after NKK treatment. Animals were sacrificed by CO2 asphyxiation, and lungs removed. The right lobe was preserved in 10% formalin for embedding, and the left lobe preserved with RNAlater (Ambion, Austin, TX) for RNA extraction.
2.5. RNA extraction and microarray processing
Microarray results have been uploaded to the Gene Expression Omnibus GSE38952. Lungs were homogenised in Trizol (Invitrogen, Carlsbad, CA). Total RNA was isolated using chloroform phase separation with column purification (PureLink RNA Mini Kit, Invitrogen). RNA was eluted in RNase-free water, quantitated by Nanodrop spectrophotometry (Thermo Scientific), then analysed for degradation on the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). A minimum RNA Integrity Number of 7 was used for microarray experiments. RNA was processed and hybridised to Gene ST1.0 arrays (Affymetrix, Santa Clara, CA) by the University of California Irvine High Throughput Genomics Core as previously described.19
2.6. Statistical analysis of microarrays
Processing and normalisation (PLIER Algorithm) were performed using Gene expression console software (Affymetrix). Genes were called absent if their normalised expression value was below 200 at all time-points. Differential gene expression analysis was performed using the CyberT algorithm (http://cybert.microarray.ics.uci.edu/). Time-course gene expression was analysed with the BETR algorithm using Multi Experiment Viewer software (http://www.tm4.org/mev). Significant genes were called with a p-value less than 0.001 (CyberT and BETR) and a fold change of ±1.5 (CyberT). Gene ontology analysis was performed using DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/). K-means clustering was performed as previously described.20
2.7. Quantitative Real-Time PCR
The total RNA (500 ng) was converted to cDNA using iScript (Bio-Rad, Hercules, CA) and qRT-PCR run on the CFX-384 Real-Time PCR System using SSO-Fast SYBR reagents (Bio-Rad). Results were derived from the linear amplification curve and normalised to β-actin. The ΔΔCT method was used to calculate the fold change in gene expression.19,21
2.8. Immunohistochemistry
Briefly, formalin-fixed and paraffin-embedded lung sections were deparaffinised, re-hydrated, and the antigens were retrieved in Sodium Citrate (100 °C). Blocking was performed with dual-endogenous enzyme block and serum free protein block (Dako North America Inc., Carpinteria, CA), and primary antibody was incubated at 4 °C overnight. Slides were washed with PBS, then treated with species-specific biotinylated secondary antibodies, washed with PBS, then treated with Vectastain ABC-Reagent (Vector Laboratories, Burlingame, CA), washed with PBS, and then developed with the DAB Quanto UltraVision detection system (Thermo Scientific, Kalamazoo, MI).
3. Results
In order to quantify and characterise pre-NNK gene expression differences between the lungs of A/J and C3H mice, we compared the gene expression profiles of the control mice (vehicle treated) at all five time-points, ages 8–20 weeks. We identified 392 differentially expressed genes between these two strains of mice (Fig. 1A and Supplementary Table 1). Two-thirds, 248, of these genes were under-expressed in A/J mice while one-third, 144, were expressed at a higher level in A/J than C3H mice (Fig. 1A and B). Gene ontology (GO) analysis of the differentially expressed genes revealed that genes underexpressed in the lungs of A/J mice were significantly enriched in chemotaxis, response to wounding, immune response, inflammatory response, regulation of cytokine production and innate immunity, while those overexpressed in A/J mice had only weak enrichment in immune response, cation transport, inflammatory response and oxidative phosphorylation (Fig. 1C and D). When the immune related genes under-expressed in A/J mice were examined in further detail they fell into both innate and adaptive immunity categories including leucocyte activity and complement activity. It thus appears that the C3H mice may have an immune activation advantage over the A/J strain, perhaps in part explaining their resistance to NNK. The expression of a subset of significantly differentially expressed genes was validated by qPCR. Interestingly, the differential expression patterns of these genes were conserved in at least two of three additional NNK-resistant mouse strains, Bl6, DBA and 129 (Fig. 1E). Together, these experiments show that differential gene expression related to immunity and inflammation, key processes involved in tumourigenesis, define the transcriptome difference between the lungs of susceptible A/J and resistant C3H mouse strains.
Fig. 1.
A/J and C3H lung transcriptomes show immune related gene expression differences. (A) A flow chart depicting the microarray analysis methodology and results. (B) A heatmap of the 392 genes differentially expressed between the indicated strains of mice. (C) Gene ontology of genes under-expressed in A/J compared to C3H mice. (D) Gene ontology of genes expressed at a higher level in A/J compared to C3H mice. (E) Quantitative real time PCR of genes differentially expressed in A/J and C3H mice and three additional NNK-resistant strains (B6, DBA and 129). AJ, A/J mice; C3H, C3H mice; B6, C57Bl6 mice; DBA, DBA mice; 129, 129 mice.
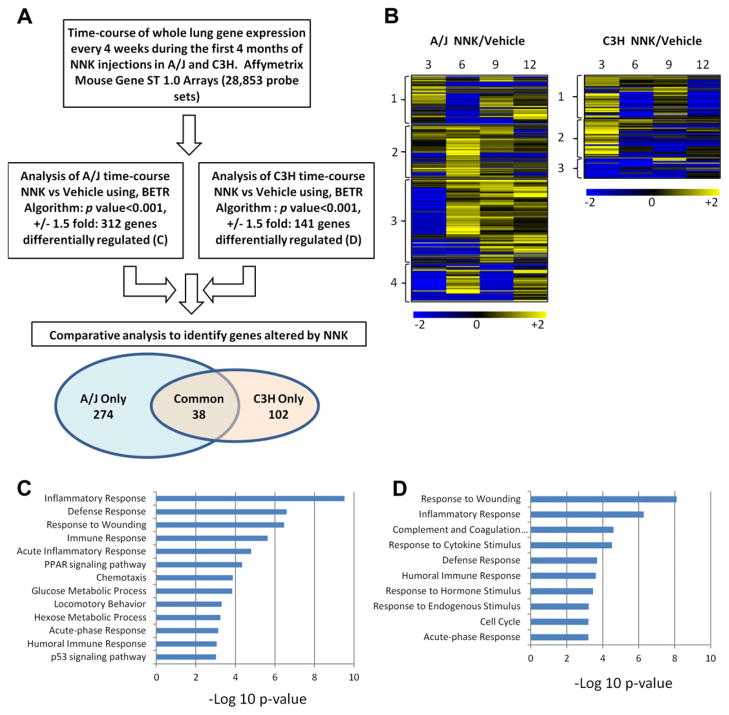
In order to define the early gene expression changes induced by NNK in lungs, we performed gene expression analysis at 3, 6, 9 and 12 weeks after NNK or vehicle treatment in both A/J and C3H mice (Supplementary Figs. 1A and 2A). Since small Krt8-positive tumours (Supplementary Fig. 1B and C) were first detected in the A/J mice by histology at 12 weeks post NNK injection, our results primarily reflect pre-tumour changes. During the time-course in A/J mice, 314 genes were differentially regulated, while 143 genes were altered in C3H mice (Supplementary Tables 2 and 3, and Fig 2A and B). NNK-induced gene expression changes are highly time-dependent, forming 4 and 3 major clusters, respectively, in A/J and C3H lungs (Fig. 2B). About one-third of NNK-induced genes in A/J lungs were upregulated at 6 weeks and maintain upregulation throughout the time-course (A/J cluster 3). In contrast, a large fraction of the NNK-induced genes in the C3H lungs was primarily upregulated 3 weeks after treatment (C3H clusters 1 and 2). GO analysis of the genes affected by NNK in A/J mice showed over-representation of responses to inflammation, wounding, immune stimuli, glucose metabolism, PPAR signalling and p53 signalling (Fig. 2C). GO analysis of the genes affected by NNK in C3H mice revealed over-representation of responses to wounding, inflammation and cytokine stimuli, complement and coagulation cascades and cell cycle (Fig. 2D). Thus, NNK-induced significant gene expression changes in both A/J and C3H lungs after NNK exposure even before tumours were histologically detected in A/J mice.
Fig. 2.
Differential gene expression changes in A/J and C3H mouse lungs in response to NNK. (A) A flow chart depicting the microarray analysis methodology and results. (B) Heatmaps depicting the genes differentially regulated by NNK in AJ (left panel) and C3H (right panel) mice. The numbers to the left of the heatmaps indicate groups of genes clustered by K-means. (C) Gene Ontology analysis of genes differentially regulated by NNK in A/J mice. (D) Gene Ontology analysis of genes differentially regulated by NNK in C3H mice. For GO analysis the x-axis refers to significance (negative log of the p-value).
We next determined the degree of overlap in lung gene expression triggered by NNK in A/J and C3H mice. NNK affected the expression of 38 genes in the same direction in both strains, 276 genes were only affected in A/J mice, and 105 genes were only affected in C3H mice (Figs. 2A, and 3A–C). The common genes affected in both susceptible and resistant strains are involved in the inflammatory and wound response, likely representing a common injury response to NNK (Supplementary Fig. 2C). Genes selectively regulated in the A/J mice included inflammation-related genes as well as genes involved in immune response and PPAR signalling, which could play pro-tumourigenic roles (Supplementary Fig. 2A). Lastly, the genes selectively altered in C3H by NNK revealed enrichment in wound response and cell division, but neither inflammation nor immunity (Supplementary Fig. 2B). Thus, while NNK caused gene expression changes in both susceptible and resistant mouse strains, there was little overlap in this response. The A/J-specific response included genes involved in processes with known links to tumour induction and progression.
Fig. 3.
Differential lung gene expression after NNK injections in A/J and C3H Mice. (A) Examples of transcripts altered only in A/J mice. A/J NNK, NNK treated A/J; A/J Ctrl, vehicle treated A/J; C3H NNK, NNK treated C3H; and C3H Ctrl, vehicle treated C3H. (B) Examples of transcripts altered only in C3H mice. (C) Examples of transcripts altered in both strains of mice. (D) Lung Retnla gene expression after NNK treatment by qPCR in A/J and C3H mice, as well as in 3 additional resistant mouse strains.
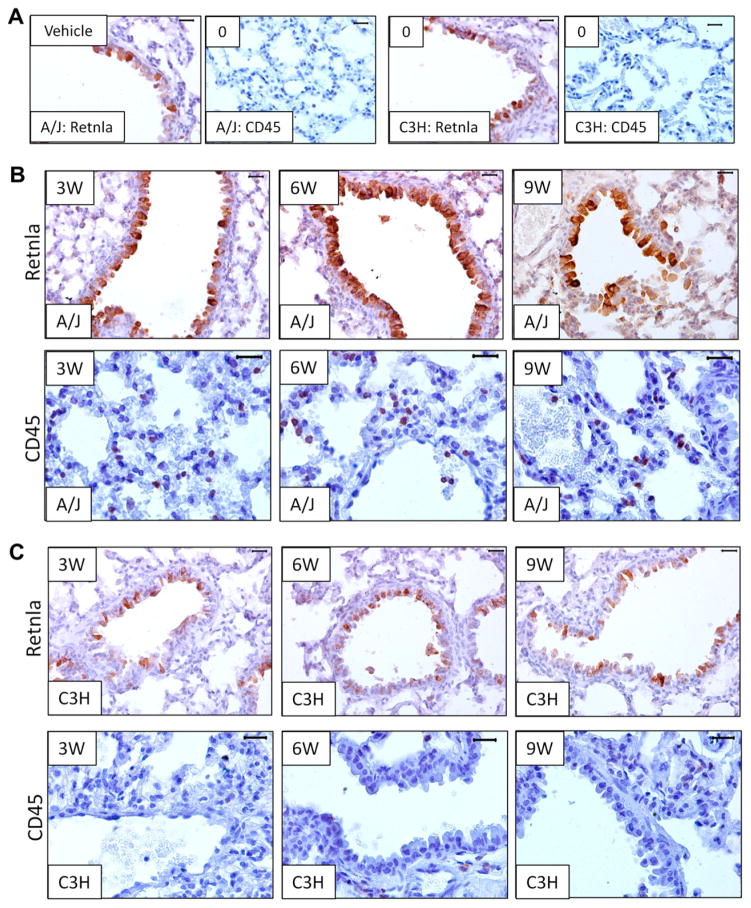
The gene most highly upregulated across the first 9 weeks of NNK treatment in A/J mice was Resistin-like alpha (Retnla), a known chemotactic modulator of immune infiltration in lungs and intestine.22–24 This gene, whose basal expression was identical in A/J and C3H as well as three additional resistant strains Bl6, DBA and 129 (Fig. 3D), was upregulated greater than 5-fold at both 3 and 6 weeks post-treatment in the A/J mice and continued to be over-expressed at 9 and 12 weeks (Fig. 3A). This expression pattern was confirmed by qPCR in A/J mouse lungs (Fig. 3D). The lungs from C3H and three other NNK-resistant mouse strains showed no Retnla up-regulation (Fig. 3D). Immunohistochemistry confirmed that Retnla protein expression after NNK treatment was highest at 6 weeks in A/J lungs (Fig. 4A and B). Consistent with the transcript data, there was no change in Retnla protein expression in C3H lungs (Fig. 4A and C). Retnla is also heterogeneously expressed within the A/J lung tumours (Supplementary Fig. 3). Furthermore, immunohistochemical staining of human lung tissue sections revealed epithelial expression in multiple tumour types including small cell carcinoma, adenocarcinoma and bronchialveolar carcinoma, as well as in inflammatory pseudotumours and histologically normal tissue adjacent to cancer (Supplementary Fig. 4).
Fig. 4.
Retnla protein is upregulated and CD45 positive cells are recruited in response to NNK treatment only in A/J lungs. (A) Immunohistochemical staining with the indicated antibodies before NNK treatment in A/J (left panels) and C3H lungs (right panels). (B) Immunohistochemical staining at 3, 6 and 9 weeks post NNK for Retnla (upper) and CD45 (lower) in A/J lungs. (C) Immunohistochemical staining at 3, 6 and 9 weeks post NNK for Retnla (upper) and CD45 (lower) in C3H lungs. All images are from 40× magnification and have a reference bar of 100 μM in the upper right corner.
Since many gene expression changes found only in A/J mice related to inflammatory and immune responses and Retnla have a known role in immune cell recruitment and activation,22,23 we examined immune cell infiltration into A/J and C3H lungs after NNK treatment. Utilising an antibody detecting CD45, an antigen present on all nucleated haematopoietic cells, we found a little to no CD45 staining in the lungs of vehicle treated mice (A/J and C3H) or NNK treated C3H mice (Fig. 4A–C). In contrast, CD45 positive cells were already present at 3 weeks in NNK treated A/J lungs (Fig. 4B lower panel), with the greatest number of positive cells at 6 weeks. No CD45 positive cells were detected within NNK-induced tumours (Supplementary Fig. 5). Thus, infiltration by CD45 positive cells prior to tumour formation correlated with increased Retnla expression. Since immune cells and chronic inflammation facilitate tumour formation and progression,25–27 we propose that NNK-induced gene expression changes alter the pulmonary microenvironment to facilitate tumourigenesis, acting in conjunction with NNK’s known mutagenic activities.
4. Discussion
We utilised the lung cancer-susceptible A/J and non-susceptible C3H strains of mice to identify differentially expressed transcripts in their lungs as well as those transcripts that are altered by treatment with NNK. The A/J mice provide a sensitive and specific test-system for assessing the carcinogenic potential of tobacco products and chemopreventive agents.28–35
In this study we identified gene expression changes reflective of innate immunity and the inflammatory response as key differences between A/J and C3H lungs prior to NNK treatment. Since NNK is known to induce immunomodulatory effects only in susceptible mice,36 together these findings provide evidence that key immune and inflammatory changes characterise NNK-induced lung tumour development. We have also demonstrated that the Retnla gene is highly upregulated in response to NNK specifically in the susceptible A/J mice. Interestingly, previous gene expression studies found upregulation of Retnla in response to other pulmonary stresses, including hypoxia, infection, non-nitrosamine carcinogens and cigarette smoke.37–40 These findings suggest that increased Retnla is a component of the lung damage/stress response. Since Retnla, is also a known chemotactic molecule for immune cells in vitro and in vivo,22,24 it is likely that prolonged high levels of Retnla in A/J lungs following NNK exposure could contribute to an overactive stress/inflammatory response, helping to create a tumour promoting environment. Since the Retnla gene product is secreted,41 it could also be explored as a plasma/serum biomarker in lung cancer.
A previous study10 reported that the nicotinic acetylcholine receptor α-nAChR7 was expressed at a higher level in A/J compared to C3H lungs and that it was induced by treatment with NNK. We, however, found no measurable difference in the expression of α-nAChR7 between A/J and C3H lungs, either by microarray or by qPCR, although we did find lower α-nAChR7 expression in the BL6, DBA and 129 mouse strains (Supplementary Fig. 5A and B). Also, we did not detect increased α-nAChR7 expression after treatment with NNK at any time-point examined in the 5 mouse strains (Supplementary Fig. 5B and C). These differences may relate to variations in experimental procedures and time-points investigated. In addition we did not study the levels of α-nAChR7 protein; we cannot rule out that α-nAChR7 is post-translationally affected by treatment with NNK. Stability of nicotinic acetylcholine receptors, including α-nAChR7, is affected by tyrosine phosphorylation and the ubiquitin proteasome system.42–44
In conclusion, while it is well known that NNK can induce mutations leading to cancer,45 our results demonstrate that it also induces global changes in gene expression that may act to increase susceptibility of respiratory cells to neoplastic transformation by eliciting chronic inflammation. These results lay the groundwork for future studies on the role of Retnla in lung tumourigenesis and biomarkers of NNK toxicity related to lung cancer.
Supplementary Material
Acknowledgments
Role of the funding source
The study sponsor, the UCI Cancer Center, only provided funding. They did not help in planning the study or in its analysis.
This study was supported by NIH Grant ES017009 (to S.G.); NIH Grant AR44882 and the Irving Weinstein Foundation (to B.A.); NIH training program T32-HD60555 (to W.G.); and UCI Cancer Center Seed Grant (to B.A. and S.G.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ejca.2012.08.027.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. Epub 2011/02/08. [DOI] [PubMed] [Google Scholar]
- 2.Burke L. Tobacco-specific carcinogen NNK has an important role in lung cancer. Thorax. 2011 Epub 2010/10/15. [Google Scholar]
- 3.Derby KS, Cuthrell K, Caberto C, et al. Exposure to the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers from 3 populations with different risks of lung cancer. Int J Cancer. 2009;125(10):2418–24. doi: 10.1002/ijc.24585. Epub 2009/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matzinger SA, Crist KA, Stoner GD, et al. K-ras mutations in lung tumors from A/J and A/J × TSG-p53 F1 mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and phenethyl isothiocyanate. Carcinogenesis. 1995;16(10):2487–92. doi: 10.1093/carcin/16.10.2487. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 5.Herzog CR, Desai D, Amin S. Array CGH analysis reveals chromosomal aberrations in mouse lung adenocarcinomas induced by the human lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Biochem Biophys Res Commun. 2006;341(3):856–63. doi: 10.1016/j.bbrc.2006.01.043. Epub 2006/02/04. [DOI] [PubMed] [Google Scholar]
- 6.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9(3):195–205. doi: 10.1038/nrc2590. Epub 2009/02/06. [DOI] [PubMed] [Google Scholar]
- 7.El-Bayoumy K, Iatropoulos M, Amin S, Hoffmann D, Wynder EL. Increased expression of cyclooxygenase-2 in rat lung tumors induced by the tobacco-specific nitrosamine 4-(methylnitrosamino)- 4-(3-pyridyl)-1-butanone: the impact of a high-fat diet. Cancer Res. 1999;59(7):1400–3. Epub 1999/04/10. [PubMed] [Google Scholar]
- 8.Hung YH, Hung WC. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) enhances invasiveness of lung cancer cells by up-regulating contactin-1 via the alpha7 nicotinic acetylcholine receptor/ERK signaling pathway. Chem Biol Interact. 2009;179(2–3):154–9. doi: 10.1016/j.cbi.2008.10.042. Epub 2008/11/26. [DOI] [PubMed] [Google Scholar]
- 9.Sugimura H, Tao H, Suzuki M, et al. Genetic susceptibility to lung cancer. Front Biosci (Schol Ed) 2011;3:1463–77. doi: 10.2741/237. Epub 2011/05/31. [DOI] [PubMed] [Google Scholar]
- 10.Razani-Boroujerdi S, Sopori ML. Early manifestations of NNK-induced lung cancer: role of lung immunity in tumor susceptibility. Am J Respir Cell Mol Biol. 2007;36(1):13–9. doi: 10.1165/rcmb.2005-0330OC. Epub 2006/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander MC, Balogh AR, Liwanag J, et al. Strain-specific spontaneous and NNK-mediated tumorigenesis in Pten mice. Neoplasia. 2008;10(8):866–72. doi: 10.1593/neo.08406. Epub 2008/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux TR, Belinsky SA, Maronpot RR, et al. Comparison of pulmonary O6-methylguanine DNA adduct levels and Ki-ras activation in lung tumors from resistant and susceptible mouse strains. Mol Carcinog. 1993;8(3):177–85. doi: 10.1002/mc.2940080308. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JC, Wiseman RW. Cellular and molecular mechanisms of multistep carcinogenesis: relevance to carcinogen risk assessment. Environ Health Perspect. 1987;76:65–70. doi: 10.1289/ehp.877665. Epub 1987/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco D, Vicent S, Fraga MF, et al. Molecular analysis of a multistep lung cancer model induced by chronic inflammation reveals epigenetic regulation of p16 and activation of the DNA damage response pathway. Neoplasia. 2007;9(10):840–52. doi: 10.1593/neo.07517. Epub 2007/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantuejoul S, Salameire D, Salon C, Brambilla E. Pulmonary preneoplasia – sequential molecular carcinogenetic events. Histopathology. 2009;54(1):43–54. doi: 10.1111/j.1365-2559.2008.03182.x. Epub 2009/02/04. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. Epub 2010/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sautes-Fridman C, Cherfils-Vicini J, Damotte D, et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30(1):13–25. doi: 10.1007/s10555-011-9279-y. Epub 2011/01/29. [DOI] [PubMed] [Google Scholar]
- 19.Yu Z, Mannik J, Soto A, Lin KK, Andersen B. The epidermal differentiation-associated Grainyhead gene Get1/Grhl3 also regulates urothelial differentiation. EMBO J. 2009;28(13):1890–903. doi: 10.1038/emboj.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin KK, Kumar V, Geyfman M, et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009;5(7):e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkin AS, Gordon W, Klein RH, et al. GRHL3/GET1 and trithorax group members collaborate to activate the epidermal progenitor differentiation program. PLoS Genet. 2012;8(7):e10002829. doi: 10.1371/journal.pgen.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelini DJ, Su Q, Kolosova IA, et al. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One. 2010;5(6):e11251. doi: 10.1371/journal.pone.0011251. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munitz A, Waddell A, Seidu L, et al. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122(6):1200–7. e1. doi: 10.1016/j.jaci.2008.10.017. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair MG, Du Y, Perrigoue JG, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206(4):937–52. doi: 10.1084/jem.20082048. Epub 2009/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougan M, Li D, Neuberg D, et al. A dual role for the immune response in a mouse model of inflammation-associated lung cancer. J Clin Invest. 2011;121(6):2436–46. doi: 10.1172/JCI44796. Epub 2011/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Brodt P, Sun H, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70(1):57–67. doi: 10.1158/0008-5472.CAN-09-2472. Epub 2010/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colby JK, Klein RD, McArthur MJ, et al. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia. 2008;10(8):782–96. doi: 10.1593/neo.08330. Epub 2008/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castonguay A, Pepin P, Stoner GD. Lung tumorigenicity of NNK given orally to A/J mice: its application to chemopreventive efficacy studies. Exp Lung Res. 1991;17(2):485–99. doi: 10.3109/01902149109064434. [DOI] [PubMed] [Google Scholar]
- 29.Estensen RD, Wattenberg LW. Studies of chemopreventive effects of myo-inositol on benzo[a]pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14(9):1975–7. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 30.Wattenberg LW, Estensen RD. Chemopreventive effects of myo-inositol and dexamethasone on benzo[a]pyrene and 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone-induced pulmonary carcinogenesis in female A/J mice. Cancer Res. 1996;56(22):5132–5. [PubMed] [Google Scholar]
- 31.Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18(3):575–86. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- 32.Witschi H, Espiritu I, Uyeminami D. Chemoprevention of tobacco smoke-induced lung tumors in A/J strain mice with dietary myo-inositol and dexamethasone. Carcinogenesis. 1999;20(7):1375–8. doi: 10.1093/carcin/20.7.1375. [DOI] [PubMed] [Google Scholar]
- 33.Obermueller-Jevic UC, Espiritu I, Corbacho AM, Cross CE, Witschi H. Lung tumor development in mice exposed to tobacco smoke and fed beta-carotene diets. Toxicol Sci. 2002;69(1):23–9. doi: 10.1093/toxsci/69.1.23. [DOI] [PubMed] [Google Scholar]
- 34.Witschi H, Espiritu I, Dance ST, Miller MS. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol Sci. 2002;68(2):322–30. doi: 10.1093/toxsci/68.2.322. [DOI] [PubMed] [Google Scholar]
- 35.Castonguay A, Pepin P, Stoner GD. Lung tumorigenicity of NNK given orally to A/J mice: its application to chemopreventive efficacy studies. Exp Lung Res. 1991;17(2):485–99. doi: 10.3109/01902149109064434. [DOI] [PubMed] [Google Scholar]
- 36.Proulx LI, Paré G, Bissonnette EY. Alveolar macrophage cytotoxic activity is inhibited by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a carcinogenic component of cigarette smoke. Cancer Immunol Immunother. 2007;56(6):831–8. doi: 10.1007/s00262-006-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebel S, Diehl S, Pype J, et al. The transcriptome of Nrf2−/− mice provides evidence for impaired cell cycle progression in the development of cigarette smoke-induced emphysematous changes. Toxicol Sci. 2010;115(1):238–52. doi: 10.1093/toxsci/kfq039. Epub 2010/02/06. [DOI] [PubMed] [Google Scholar]
- 38.Gebel S, Gerstmayer B, Kuhl P, Borlak J, Meurrens K, Muller T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicol Sci. 2006;93(2):422–31. doi: 10.1093/toxsci/kfl071. Epub 2006/07/28. [DOI] [PubMed] [Google Scholar]
- 39.Thomas RS, O’Connell TM, Pluta L, Wolfinger RD, Yang L, Page TJ. A comparison of transcriptomic and metabonomic technologies for identifying biomarkers predictive of two-year rodent cancer bioassays. Toxicol Sci. 2007;96(1):40–6. doi: 10.1093/toxsci/kfl171. [DOI] [PubMed] [Google Scholar]
- 40.Sciuto AM, Phillips CS, Orzolek LD, Hege AI, Moran TS, Dillman JF. Genomic analysis of murine pulmonary tissue following carbonyl chloride inhalation. Chem Res Toxicol. 2005;18(11):1654–60. doi: 10.1021/tx050126f. [DOI] [PubMed] [Google Scholar]
- 41.Asano T, Sakosda H, Fujishiro M, et al. Physiological significance of resistin and resistin-like molecules in the inflammatory process and insulin resistance. Curr Diabetes Rev. 2006;2(4):449–54. doi: 10.2174/1573399810602040449. Epub 2008/01/29. [DOI] [PubMed] [Google Scholar]
- 42.Cho CH, Song W, Leitzell K, et al. Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J Neurosci. 2005;25(14):3712–23. doi: 10.1523/JNEUROSCI.5389-03.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanamaker CP, Christianson JC, Green WN. Regulation of nicotinic acetylcholine receptor assembly. Ann N Y Acad Sci. 2003;998:66–80. doi: 10.1196/annals.1254.009. [DOI] [PubMed] [Google Scholar]
- 44.Rezvani K, Teng Y, De Biasi M. The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40(1–2):177–84. doi: 10.1007/s12031-009-9272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng HC, Takano Y. NNK-induced lung tumors: a review of animal model. J Oncol. 2011;2011:635379. doi: 10.1155/2011/635379. Epub 2011/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.