Abstract
Activation of interferon (IFN) signaling in the central nervous system (CNS) is usually associated with inflammation. However, a robust activation of type I IFN-stimulated genes (ISGs) at pre-symptomatic stages occurs in the spinal cord of SOD1(G93A) mice, an amyotrophic lateral sclerosis (ALS) animal model, without obvious signs of inflammation. To determine if the same signaling pathway is elevated in other types of neuronal injuries, we examined the protein expression levels of an IFN-stimulated gene, ISG15, in mouse models of acute and chronic neuronal injuries. We found that ISG15 protein was dramatically increased in the brains of mice subjected to global ischemia and traumatic brain injury, and in transgenic mice overexpressing HIV gp120 protein. These results suggest that activation of ISGs is a shared feature of neuronal injuries and that ISG15 may be a suitable biomarker for detecting neuronal injuries in the CNS.
Keywords: interferon-stimulated gene 15, neuronal injury, biomarker
Interferons (IFNs) are cytokines produced by a variety of cells primarily in response to viral infections as a mechanism of host defense and activated through both Toll-like receptor (TLR) dependent and independent mechanisms [1–3]. They exert antiviral and immunomodulatory responses, as well as growth regulation [4]. On the basis of their structure and receptor recognition they are classified as either type I (IFN-α and IFN-β) or type II (IFN-γ) [2, 3]. Type I and II IFNs initiate two similar but distinct signaling pathways. Type I and II IFNs bind to their heterodimeric receptor and trigger phosphorylation of JAK1 and TYK2 tyrosine kinases, which in turn phosphorylate STAT1 and STAT2 transcription factors. Phospho-STAT1 and phospho-STAT2 move into the nucleus and form a complex with ISGF3G, which activates the transcription of many interferon-stimulated genes (ISGs). In contrast, type II IFN acts on a tetrameric receptor to activate JAK1 and JAK2 kinases, which subsequently phosphorylate STAT1. The phospho-STAT1 forms a homodimer, translocates to the nucleus, and induces the expression of IFNγ-stimulated genes.
Similarly, activation of IFN signaling in the central nervous system (CNS) is usually associated with inflammation triggered by viral infection or autoimmunization [5–7]. However, a robust activation of IFN signaling at pre-symptomatic stages occurs in the spinal cord of mice with G93A mutations in the superoxide dismutase 1 (SOD1) gene, an amyotrophic lateral sclerosis (ALS) animal model, without obvious signs of inflammation [8]. Transcripts of a number of ISGs, including GBP2, IFI27L2A, IFI44, IFIT1, IFIT3, ISG15, and USP18, are up-regulated in the lumbar spinal cord of SOD1(G93A) mice. In particular, ISG15 protein levels are dramatically elevated in astrocytes in specific areas where spinal motor neurons are degenerating. Therefore, ISGs are reliable biomarkers for detecting early pathological changes in the ALS spinal cord [8].
In the present study, we examined the protein expression levels of ISG15 in mouse models of other acute and chronic neuronal injuries. We found that ISG15 protein was dramatically increased in the brains of mice subjected to global ischemia and traumatic brain injury (TBI), as well as in transgenic mice overexpressing HIV gp120 protein. These results suggest that ISG15 may be a suitable biomarker for detecting neuronal injuries in the CNS.
1 MATERIALS AND METHODS
1.1 Animals
C57black/6 mice and B6SJL GFAP-HIV gp120 transgenic mice [9] were used in the present study. B6SJL gp120 transgenic mice were maintained as hemizygotes by breeding transgenic mice with wild-type B6SJL mice. Non-transgenic littermates were used as controls. The mice were housed in a virus-free barrier facility under a 12-h light/dark cycle, with ad libitum access to food and water. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Society for Neuroscience “Guidelines for the Use of Animals in Neuroscience Research” using protocols approved by the Institutional Animal Care and Use Committee of the Sanford-Burnham Medical Research.
1.2 Global ischemia model
Global ischemia was induced by occluding both common carotid arteries (BCCA) for 30 min as described previously [10]. Briefly, six-month-old C57Black/6 mice were anesthetized with 2%–5% isoflurane in oxygen for induction and 0.25%–4% isoflurane in oxygen for maintenance, and kept in a supine position. The duration of anesthesia was approximately 5 min (4 min to occlude BCCA and 1 min to close the suture). After a ventral midline cervical incision, the common carotid arteries were exposed and ligated with 6-0 silk sutures. Immediately after the ligation, anesthesia was turned off. To reperfuse, animals were briefly anesthetized with 1% halothane and the ligatures removed. After observing blood flow return, the incision was closed.
1.3 Controlled cortical impact TBI
The controlled cortical impact TBI method used for this study has been described [11, 12]. Briefly, six-month-old C57Black/6 mice were anesthetized with isoflurane and placed in a stereotaxic frame. After a midline incision was made, the scalp was reflected to expose the skull. A 5 mm left lateral craniotomy was performed using a motorized drill. A controlled cortical impact injury was produced with an electromagnetic impact device using a 3-mm-diameter metal tip. The impact was centered at 2.7 mm lateral from midline and 3.0 mm anterior from lambda. The impact depth was set at 2.5 mm using the stereotaxic device. The impactor was then driven at a velocity of 5 m/s with a dwell time of 100 ms. This produces a moderately severe contusion in the left sensorimotor cortex and underlying hippocampus with pronounced behavioral deficits but virtually no mortality. After injury, a plastic skull cap was secured over the impact site, the skin incision was sutured closed, and the animals were allowed to fully recover. Sham mice underwent the same surgical procedure including anesthesia and craniotomy but were not injured.
1.4 Immunohistochemistry
Mice were anesthetized with isoflurane and perfused transcardially with chilled PBS followed by phosphate-buffered 4% paraformaldehyde (PFA) solution. The brain was cryoprotected in 25% sucrose. Cryostat sections (12 µm) were processed for immunohistochemistry. In brief, sections were incubated with primary anti-Isg15 (1:1 000, from Dr. D. E. Zhang), anti-GFAP (1:1 000, Sigma), or anti-Iba-1 (1:1 000, Wako) antibodies, followed by incubation with a biotinylated secondary antibody (Vector Laboratories). The detection of immunosignals was performed using a Vectastain Elite ABC kit (Vector Laboratories) and visualized with a DAB substrate solution (Roche Applied Science). For immunofluorescence, brain sections were incubated with rabbit anti-Isg15 (1:1 000), mouse anti-GFAP (1:1 000), or mouse anti-Iba-1 (1:1 000), followed by incubation with secondary antibodies conjugated with 488Alexafluor or 555Alexafluor (Invitrogen). After washing in PBS, the samples were mounted by adding Vectashield (Vector Laboratories) to the cover slips.
1.5 Western blot analysis
The cortex, hippocampus, striatum, and cerebellum of one-year-old B6SJL GFAP-HIV gp120 transgenic mice and their wild-type littermates were homogenized in SDS-sample buffer containing 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate (SDS), and 1% protease inhibitor cocktail (Sigma). Protein samples (30 µg) were fractionated by 4%–12% SDS-polyacrylamide gels and transferred to Hybond ECL nitrocellulose membranes (Amersham Pharmacia Biotech). The blots were probed with primary rabbit anti-Isg15 (1:3 000, from Dr. D. E. Zhang), and were subsequently probed with anti-rabbit IgG peroxidase conjugate (Amersham). The signals were detected using ECL reagents (Amersham).
2 RESULTS
2.1 ISG15 protein is increased in the hippocampus of mice subjected to global ischemia
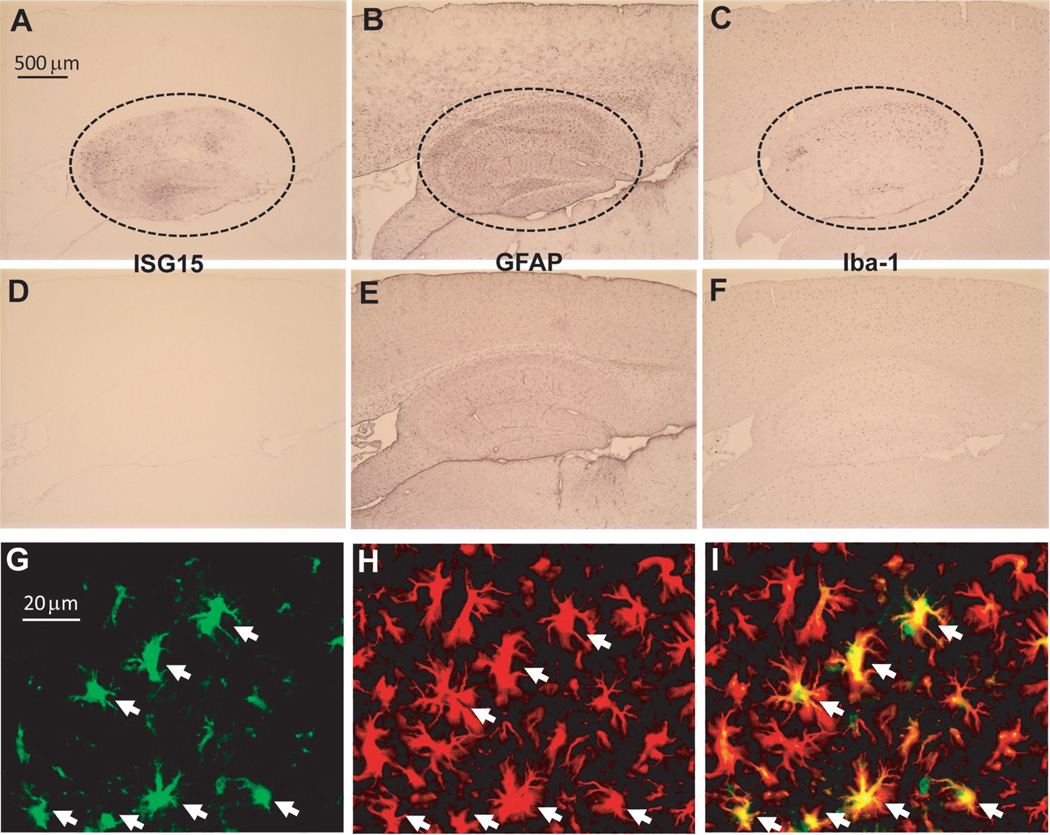
To test if acute neuronal injuries affected the expression of ISG15 in the brain, we first tested a mouse model of global ischemia by subjecting C57BL/6 mice to transient (30 min) BCCA occlusion. The mice were killed after reperfusion for 72 h, and their brains were then processed and examined with immunohistochemistry. As neuronal damage is mainly observed in the hippocampus in this model [10, 13], we examined the expression of ISG15 in the same area. Strong ISG15 immunosignals appeared consistently in the hippocampus of all mice subjected to ischemia (Fig. 1A). However, ISG15 immunoreactivity was very weak in the brain of sham-operated control mice (Fig. 1D). In contrast, GFAP-positive astrocytes (Fig. 1B and E) and Iba-1-positive microglia (Fig. 1C and F) were observed in all brain sections of all mice, although they were more in the hippocampus of ischemia (Fig. 1B and C) than sham-operated control mice (Fig. 1E and F). Furthermore, ISG15 was only co-localized with GFAP, an astrocyte marker (Fig. 1G–I), consistent with our findings in ALS mouse spinal cord [8].
Fig. 1.
Both common carotid arteries (BCCA) occlusion-induced increase of ISG15 expression in the hippocampus. Immunohistochemistry showing strong expression of ISG15 (A), GFAP (B), and Iba-1 (C) in the hippocampus of mice subjected to BCCA occlusion but not sham controls (D, E, F). Double-staining showing the co-localization of ISG15 (G) with GFAP-positive cells (H) in the hippocampus. (I): overlap of (G) and (H).
2.2 ISG15 protein is increased in the brains of mice subjected to controlled cortical impact TBI
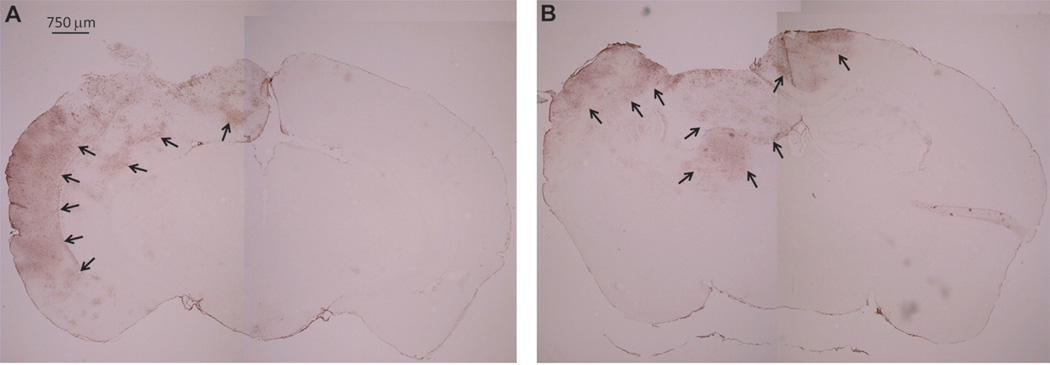
We next tested if another acute bran injury model, TBI, also affected the expression of ISG15 in the brain. C57BL/6 mice subjected to controlled cortical impact TBI incur significant hippocampal damage and behavioral deficits but low mortality [12]. This TBI model also produces a central contusion and pericontusional axonal injury [11]. We examined the brain of these mice at 48 or 96 h after TBI by immunohistochemistry. We found strong ISG15 protein expression in pericontusional areas at both 48 and 96 h after TBI (Fig. 2). The ipsilateral cortex and hippocampus appeared to have the most prominent ISG15 expression, with little ISG15 expression on the contralateral side.
Fig. 2.
Traumatic brain injury (TBI)-induced increase of ISG15 expression in the brain. Immunohistochemistry showing strong expression of ISG15 in the pericontusional areas at both 48 (A) and 96 h (B) after TBI.
2.3 ISG15 protein is increased in the brains of HIV gp120 transgenic mice
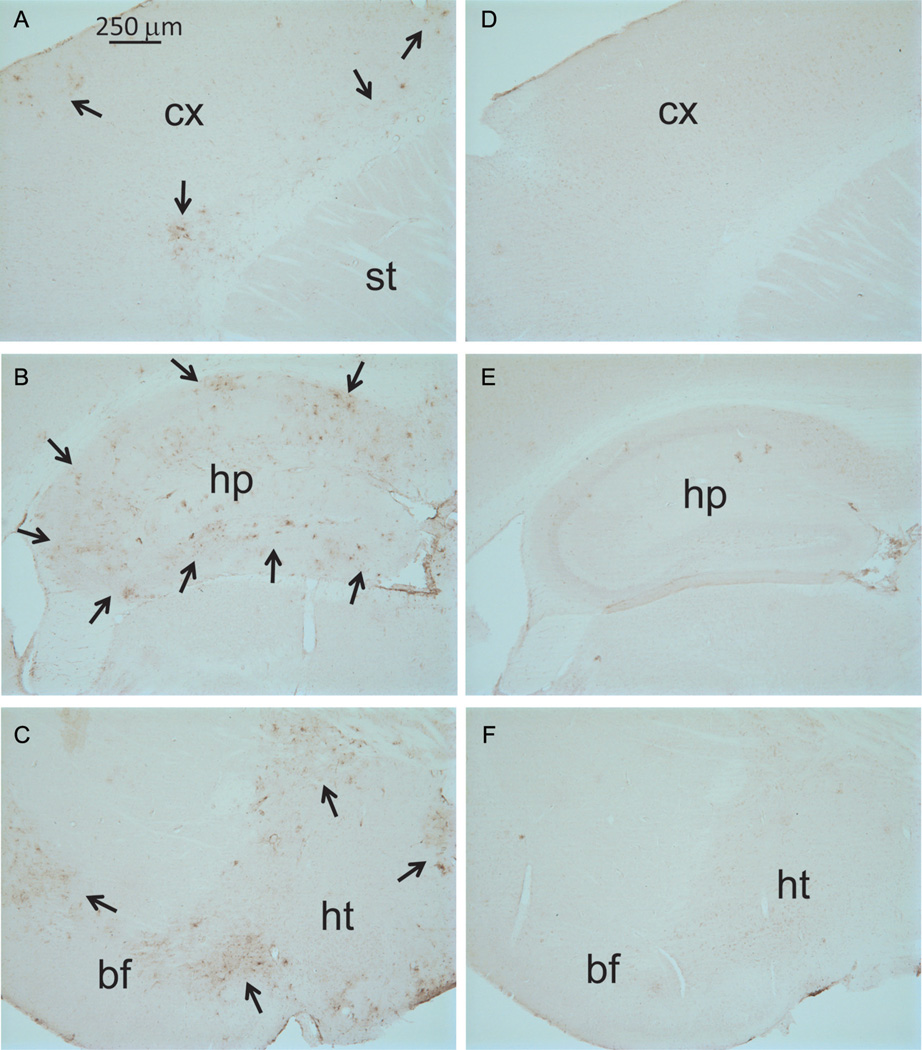
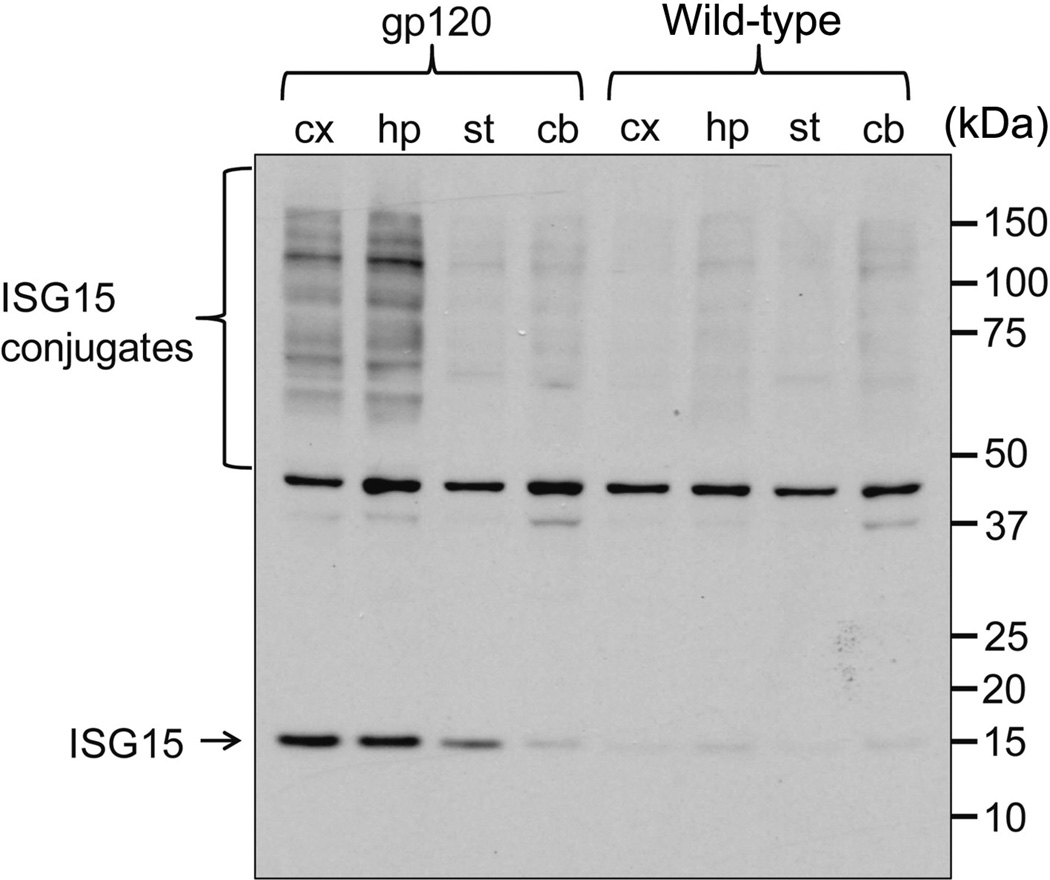
We further examined if a toxic viral protein, HIV gp120, affects the expression of ISG15 in the brain of a chronic neuronal injury mouse model. The B6SJL GFAP-HIV gp120 transgenic mice constitutively overexpressing HIV gp120 in the brain display a spectrum of neuronal and glial changes resembling abnormalities in the brains of HIV-infected humans [9]. The severity of damage in these mice correlated positively with brain levels of HIV gp120, which were highest in neocortex and hippocampus. We examined the brain sections from six-month-old gp120 mice using immunohistochemistry. Strong ISG15 immunoreactivity appeared in the cortex, hippocampus, basal forebrain, and hypothalamus of gp120 mice (Fig. 3A, B, and C). However, ISG15 immunosignals were very weak in the brain of wild-type littermate controls (Fig. 3D, E, and F). We further checked ISG15 levels in the whole nervous tissue lysates of one-year-old gp120 mice and wild-type littermates (Fig. 4). ISG15 is an ubiquitin-like modifier and is able to covalently modify target proteins to form ISG15 conjugates [14, 15]. The levels of ISG15 and its conjugates were similarly low in the cortex, hippocampus, striatum, and cerebellum of wild-type littermates, and in the striatum and cerebellum of gp120 mice. In contrast, ISG15 and its conjugates were extremely high in the cortex and hippocampus of gp120 mice (Fig. 4), correlating positively with the severity of neuronal damage in this mouse model [9].
Fig. 3.
Expression of ISG15 in the brain of HIV gp120 transgenic mice. Immunohistochemistry showing strong expression of ISG15 in the cortex (A), hippocampus (B), and basal forebrain and hypothalamus (C) of HIV gp120 mice but not of littermate controls (D, E, F). The positive cells were indicated by the arrows. cx, cortex; st, striatum; hp, hypothalamus; bf, basal forebrain; ht, hypothalamus.
Fig. 4.
Expression of both free ISG15 and ISG15 conjugates in the brain of HIV gp120 transgenic mice. Western blot showing increased levels of both free ISG15 and ISG15 conjugates in the cortex and hippocampus of HIV gp120 mice. cx, cortex; hp, hypothalamus; st, striatum; cb, cerebellum.
3 DISCUSSION
The major aim of the present study investigating the expression of ISG15 in several neuronal injury models is to demonstrate that the upregulation of ISGs is a shared feature in neuronal injuries involving inflammation rather than an isolated molecular event occurred only in motor neuron diseases [8]. We showed that ISG15 protein was strongly expressed in specific brain areas of acute and chronic neuronal injury models, including the hippocampus affected by global ischemia, pericontusional areas affected by TBI, and cortex and hippocampus affected by overexpression of HIV gp120 protein. Furthermore, ISG15 expression was selectively co-localized with GFAP, an astrocyte marker, similar to the expression pattern observed in the ALS model [8]. Our studies suggest that the increased expression of ISG15 induced by a broad spectrum of neuronal injuries occurs mainly in astrocytes, although a recent study in a transient focal ischemia model suggests that ISG15 is also induced in neurons in the infarct area [16]. Whether the activation of ISG15 occurs via ER stress-induced damage in neurons, as suggested in our ALS study, warrants further investigation.
ISG15 is an ubiquitin-like modifier and modifies proteins in a similar manner to ubiquitylation via a process termed ISGylation [14, 15]. Relative to ubiquitin, the biological function of ISG15 is still poorly understood, but ISG15 appears to play important roles in various biological and cellular functions, including innate immunity, anti-viral/bacterial infections, protein turnover, and tumorigenesis [17]. The role of ISG15 in normal and pathologic brains is largely unknown, although alterations in ISGylation (and De-ISGylation) are clearly associated with neuronal injuries, as shown here and elsewhere[8, 16]. Compared with other commonly used neuroinflammation markers[18, 19], such as cytokines TNF-α and IL-1 and gliosis markers GFAP and Iba-1, the expression of ISG15 in neuronal injuries showed much higher signal-to-noise ratio and better spatial resolution. Up-regulation of ISG15, as well as other ISGs, may affect astrocyte function. Equally important, as ISG15 levels were low in unaffected areas, the robust expression in response to neuronal injuries suggests that ISG15 may be a reliable biomarker for pathological changes in the CNS, especially for those that share disease mechanisms.
ACKNOWLEDGEMENTS
We thank Dr. Dong-Er Zhang, University of California, San Diego, CA, USA for the antibody against mouse ISG15.
REFERENCES
- 1.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 4.Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282(28):20047–20051. doi: 10.1074/jbc.R700004200. [DOI] [PubMed] [Google Scholar]
- 5.Dafny N, Yang PB. Interferon and the central nervous system. Eur J Pharmacol. 2005;523(1–3):1–15. doi: 10.1016/j.ejphar.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Reyes-Vazquez C, Prieto-Gomez B, Dafny N. Interferon modulates central nervous system function. Brain Res. 2012;1442:76–89. doi: 10.1016/j.brainres.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 7.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158(3):1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Yang B, Zhang D. Activation of interferon signaling pathways in spinal cord astrocytes from an ALS mouse model. Glia. 2011;59(6):946–958. doi: 10.1002/glia.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 10.Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28(9):1805–1810. doi: 10.1161/01.str.28.9.1805. discussion 1811. [DOI] [PubMed] [Google Scholar]
- 11.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31(26):9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, Kim E, Schwetye KE, Holtzman DM, Bayly PV. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24(4):657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagihara T, Matsumoto M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res. 1997;752(1–2):209–218. doi: 10.1016/s0006-8993(96)01453-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim KI, Zhang DE. ISG15, not just another ubiquitin-like protein. Biochem Biophys Res Commun. 2003;307(3):431–434. doi: 10.1016/s0006-291x(03)01216-6. [DOI] [PubMed] [Google Scholar]
- 15.Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17(4):455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakka VP, Lang BT, Lenschow DJ, Zhang DE, Dempsey RJ, Vemuganti R. Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab. 2011;31(12):2375–2384. doi: 10.1038/jcbfm.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Zhang DE. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res. 2011;31(1):119–130. doi: 10.1089/jir.2010.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol. 2011;238(1–2):1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]