Abstract

This paper describes the catalytic asymmetric diamination of alkyl dienes using N,N’-di-tert-butylthiadiaziridine 1,1-dioxide in the presence of Pd(0) and a chiral phosphoramidite ligand to give cyclic sulfamides in high yield and high ee. The diamination is also amenable to gram scale.
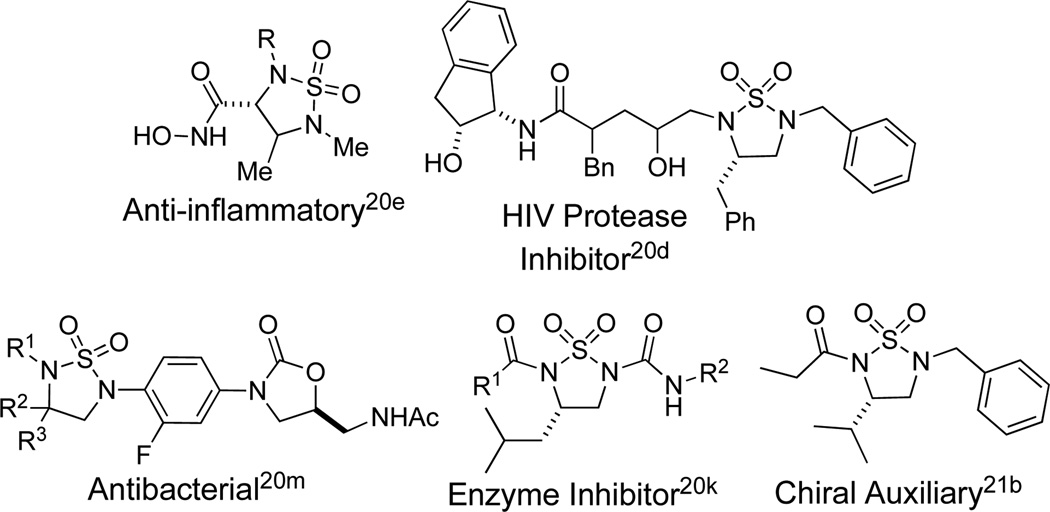
Vicinal diamines are found throughout biologically active molecules and are important chiral control agents in asymmetric synthesis.1 The synthesis of vicinal diamines through the metal-promoted diamination of olefins presents an attractive and efficient strategy to access these important functional motifs, and various methods have been reported.1–10 We have previously reported Pd(0)-11 and Cu(I)-catalyzed12 methods for the regioselective diamination of conjugated dienes using N,N’-di-tert-butyldiaziridinone (1) (Figure 1) as nitrogen source.13 Asymmetric variations of these reactions have also been disclosed.14,15 A related analogue, N,N’-di-tert-butylthiadiaziridine 1,1-dioxide (2),16 has also shown to be an effective nitrogen source in the synthesis of cyclic sulfamides using Pd(0),17 CuCl,18,19 or CuBr19 as catalyst. The resulting cyclic sulfamides are important functional motifs present in medicinal and biologically active molecules including HIV protease inhibitors, anti-inflammatory agents, antibacterials, blood pressure regulators, enzyme inhibitors, and treatments for Alzheimer’s disease (Figure 2).20 Cyclic sulfamides have also shown promise as chiral auxiliaries (Figure 2).21 Due to their biological and synthetic importance, an asymmetric synthesis of cyclic sulfamides is highly desirable. Commonly used methods employ multi-step syntheses from chiral amino acids,22 or more recently, the asymmetric hydrogenation of thiadiazole 1,1-dioxides, which are synthesized via reaction of α-hydroxy aryl ketones with sulfamide.23 Herein we wish to report the direct synthesis of optically active cyclic sulfamides via the catalytic, asymmetric diamination of conjugated dienes with N,N’-di-tert-butylthiadiaziridine 1,1-dioxide (2).
Figure 1.
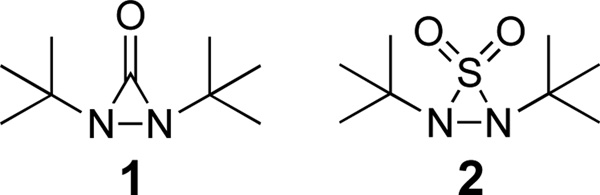
Nitrogen Sources for Diamination
Figure 2.
Cyclic Sulfamides as Biologically Active Molecules and Chiral Auxiliaries
Using trans-nona-1,3-diene as test substrate, thiadiaziridine 2 as nitrogen source, and catalysts generated from Pd2(dba)3 and a chiral ligand, the reactivity and selectivity of diamination was investigated (Scheme 1). No reaction was observed using bidentate ligand R-BINAP (L1).24 Of the ligands screened,25–28 BINOL-based phosphoramidite ligands displayed the most promising selectivity, and diamination was found to be highly regioselective for the internal double bond of the diene. Somewhat surprisingly, ligands L514a and L6,29 which were found to induce high selectivity for catalytic asymmetric diaminations using 1, exhibited lower selectivity in the current system. On the contrary, (R,R,R) ligand L7 was found to be optimal for both yield and selectivity providing the corresponding cyclic sulfamide in 76% yield and 90% ee, whereas ent-L7 had previously displayed poor reactivity using 1. As also shown in Scheme 1, (R,S,S) ligand L8 displayed decreased selectivity from that of L7, indicating that a matched relationship between the BINOL and amine portions of the ligand are important for high asymmetric induction. In both cases, the product configuration is determined by the BINOL skeleton and not the amine portion of the ligand. H8-BINOL-derived phosphoramidite ligand L9 also resulted in high ee, yielding the opposite enantiomer of the product sulfamide.
Scheme 1.
Initial Screening for Asymmetric Diamination
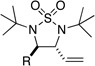
With optimal ligand in hand, the substrate scope was subsequently investigated. As shown in Table 1, a variety of alkyl-substituted conjugated (E)-dienes30 can be efficiently diaminated in 66–98% yield and 90–93% ee with 2.5 mol% Pd2(dba)3 and 10 mol% L7 in toluene at 65 °C for 3 hours. Alkyl chains can contain silyl (Table 1, entry 6), and aryl groups (Table 1, entries 7–9) as well as ethers (Table 1, entries 10–12). Double bonds in the alkyl groups remained unreacted in geometrically pure form (Table 1, entries 13 and 14). The catalytic asymmetric diamination is also amenable to gram scale and catalyst loading can be further reduced to 1.4 mol% Pd2(dba)3 (Table 1, entry 8). In all cases, the reaction occurs with high regioselectivity towards the internal double bond (Figure 3) and other regioisomers were barely detectable by 1H NMR analysis if there was any. The diamination most likely proceeds through a concerted reaction mechanism analogous to the previously reported catalytic cycle using 1 (Scheme 2).11a,c Insertion of the chiral Pd(0) complex into the N-N bond of 2 forms four-membered Pd(II) complex 5, which coordinates to diene 3 to give complex 6. The migratory insertion of 6 leads to π-allyl Pd complex 7. Upon reductive elimination, cyclic sulfamide 4 is formed with regeneration of the chiral Pd(0) catalyst.
Table 1.
Catalytic Asymmetric Diamination of Conjugated Dienes to Form Cyclic Sulfamidesa
| entry | diene (3) | product (4)b | yield (%)c | ee (%)d |
|---|---|---|---|---|
 |
||||
| 1 | 3a, R= Me | 4a | 97 | 90 |
| 2 | 3b, R= n-Pr | 4b | 79 | 90 |
| 3 | 3c, R= i-Bu | 4c | 84 | 90 |
| 4 | 3d, R= n-C5H11 | 4d | 91 | 91 |
| 5 | 3e, R= (CH2)2Cy | 4e | 89 | 91 |
| 6 | 3f, R= (CH2)2TMS | 4f | 98 | 91 |
 |
||||
| 7 | 3g | 4g | 90 | 93e |
 |
||||
| 8f | 3h, Ar= Ph | 4h | 88 | 92e |
| 9 | 3i, Ar= 4-MeOPh | 4i | 77 | 90e |
 |
||||
| 10 | 3j, R= n-C6H13 | 4j | 69 | 93 |
| 11g | 3k, R= Ph | 4k | 81 | 93h |
 |
||||
| 12 | 3l | 4l | 66 | 91 |
 |
||||
| 13 | 3m | 4m | 98 | 91 |
 |
||||
| 14 | 3n | 4n | 93 | 91 |
All reactions were carried out with diene 3 (0.20 mmol), 2 (0.30 mmol), Pd2(dba)3 (0.005 mmol), and L7 (0.02 mmol) in toluene (0.10 mL) under Ar at 65 °C for 3 h, unless otherwise stated.
For entries 4 and 7, the absolute configurations (R,R) were determined by comparison of the optical rotations with reported ones after complete deprotection to the free diamine (ref. 12b, 14a). For all other entries, the absolute configurations were not determined and the stereochemistry indicated represents relative stereochemistry.
Isolated yield.
The ee was determined by chiral HPLC (Chiralpak IC column) after removal of the t-Bu groups, unless otherwise stated.
The ee was determined without removal of the t-Bu groups.
The reaction was carried out with diene 3h (6.96 mmol), 2 (9.03 mmol), Pd2(dba)3 (0.10 mmol), and L7 (0.46 mmol) in toluene (3.5 mL) under Ar at 65 °C for 3 h.
Reaction time, 6 h.
The ee was determined by chiral HPLC (Chiralpak IA column).
Figure 3.
X-ray Structure of 4f
Scheme 2.
Proposed Catalytic Cycle for the Asymmetric Diamination of Dienes Using 2
As shown in Scheme 3, removal of the t-Bu groups is accomplished in a mixture of CF3CO2H-hexanes at room temperature, allowing possible further derivatization of the nitrogens. Complete removal of the t-Bu groups and the sulfone moiety in one step is also realized in aqueous HBr at elevated temperature to unmask the free diamine.31
Scheme 3.
Representative Deprotection of Cyclic Sulfamides
In summary, we have developed the direct and efficient synthesis of chiral cyclic sulfamides from conjugated dienes using Pd2(dba)3 and chiral phosphoramidite ligand L7 as catalyst, and N,N’-di-tert-butylthiadiaziridine 1,1-dioxide (2) as nitrogen source. Various alkyl-substituted conjugated dienes are smoothly diaminated in high yields and high selectivities, providing cyclic sulfamides with two adjacent chiral centers and the pendent vinyl group allows possible further functionalization. The reaction is amenable to gram scale and can produce the resulting cyclic sulfamides in multi-gram quantity. The resulting cyclic sulfamides can be deprotected via removal of the t-Bu groups and the corresponding free diamines can also be obtained without loss of ee. Cyclic sulfamides are valuable moieties in biologically relevant molecules and the described process provides a viable route to access a broad range of diverse analogues for future study.
Supplementary Material
Acknowledgment
We are grateful for generous financial support from the National Institutes of General Medical Sciences, National Institutes of Health (grant no. GM083944-05). R.G.C. thanks Eli Lilly for a graduate student fellowship.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and NMR spectra. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem. Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. Mortensen MS, O’Doherty GA. Chemtracts: Org. Chem. 2005;18:555. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x. Kizirian J-C. Chem. Rev. 2008;108:140. doi: 10.1021/cr040107v. Lin G-Q, Xu M-H, Zhong Y-W, Sun X-W. Acc. Chem. Res. 2008;41:831. doi: 10.1021/ar7002623. Jensen KH, Sigman MS. Org. Biomol. Chem. 2008;6:4038. doi: 10.1039/b813246a. de Figueiredo RM. Angew. Chem. Int. Ed. 2009;48:1190. doi: 10.1002/anie.200804362. Cardona F, Goti A. Nat. Chem. 2009;1:269. doi: 10.1038/nchem.256. Viso A, de la Pradilla RF, Tortosa M, García A, Flores A. Chem. Rev. 2011;111:PR1. doi: 10.1021/cr100127y. Chemler SR. J. Organomet. Chem. 2011;696:150. doi: 10.1016/j.jorganchem.2010.08.041. Jong SD, Nosal DG, Wardrop DJ. Tetrahedron. 2012;68:4067. doi: 10.1016/j.tet.2012.03.036.
- 2.For examples of metal-mediated diaminations, see, for Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420. Muñiz K, Iesato A, Nieger M. Chem.-Eur. J. 2003;9:5581. doi: 10.1002/chem.200305011. Muñiz K. Eur. J. Org. Chem. 2004:2243. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962. Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647.
- 3.For metal-catalyzed diamination with TsNCl2 or TsNBr2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem. Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I. Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769. Han J, Li T, Pan Y, Kattuboina A, Li G. Chem. Biol. Drug. Des. 2008;71:71. doi: 10.1111/j.1747-0285.2007.00604.x.
- 4.For Cu(II)-promoted intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v. Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713. Sequeira FC, Turnpenny BW, Chemler SR. Angew. Chem. Int. Ed. 2010;49:6365. doi: 10.1002/anie.201003499.
- 5.For Au(I)-catalyzed intramolecular diamination of allenes via dihydroamination, see: Li H, Widenhoefer RA. Org. Lett. 2009;11:2671. doi: 10.1021/ol900730w.
- 6.For Pd(II)-catalyzed intermolecular diamination of olefins, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d. Iglesias A, Pérez EG, Muñiz K. Angew. Chem. Int. Ed. 2010;49:8109. doi: 10.1002/anie.201003653. Muñiz K, Kirsch J, Chávez P. Adv. Synth. Catal. 2011;353:689. Martínez C, Muñiz K. Angew. Chem. Int. Ed. 2012;51:7031. doi: 10.1002/anie.201201719.
- 7.For Pd(II)-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a. Hövelmann CH, Streuff J, Brelot L, Muñiz K. Chem. Commun. 2008:2334. doi: 10.1039/b719479j. Muñiz K, Hövelmann CH, Campos-Gómez E, Barluenga J, González JM, Streuff J, Nieger M. Chem. Asian J. 2008;3:776. doi: 10.1002/asia.200700373. Muñiz K, Streuff J, Chávez P, Hövelmann CH. Chem. Asian J. 2008;3:1248. doi: 10.1002/asia.200800148. Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087. Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J. Am. Chem. Soc. 2009;131:15945. doi: 10.1021/ja906915w. Chávez P, Kirsch J, Streuff J, Muñiz K. J. Org. Chem. 2012;77:1922. doi: 10.1021/jo202507d.
- 8.For Ni(II) and Au(I)-catalyzed intramolecular diamination of olefins, see: Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem. Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160. Muñiz K, Hövelmann CH, Streuff J, Campos-Gómez E. Pure Appl. Chem. 2008;80:1089. Iglesias A, Muñiz K. Chem.-Eur. J. 2009;15:10563. doi: 10.1002/chem.200901199. de Haro T, Nevado C. Angew. Chem. Int. Ed. 2011;50:906. doi: 10.1002/anie.201005763.
- 9.For a recent Pd(II)-catalyzed intramolecular diamination of alkynes, see: Yao B, Wang Q, Zhu J. Angew. Chem. Int. Ed. 2012;51:5170. doi: 10.1002/anie.201201640.
- 10.For a recent Cu(II)/Fe(III)-co-catalyzed intermolecular diamination of alkynes, see: Zeng J, Tan YJ, Leow ML, Liu XW. Org. Lett. 2012;14:4386. doi: 10.1021/ol301858j.
- 11.(a) Du H, Zhao B, Shi Y. J. Am. Chem Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]; (b) Xu L, Du H, Shi Y. J. Org. Chem. 2007;72:7038. doi: 10.1021/jo0709394. [DOI] [PubMed] [Google Scholar]; (c) Zhao B, Du H, Cui S, Shi Y. J. Am. Chem. Soc. 2010;132:3523. doi: 10.1021/ja909459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a. [DOI] [PubMed] [Google Scholar]; (b) Zhao B, Peng X, Cui S, Shi Y. J. Am. Chem. Soc. 2010;132:11009. doi: 10.1021/ja103838d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao B, Peng X, Zhu Y, Ramirez TA, Cornwall RG, Shi Y. J. Am. Chem. Soc. 2011;133:20890. doi: 10.1021/ja207691a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For a leading review on diaziridinones, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. New York: John Wiley & Sons; 1983. p. 547.
- 14.For Pd(0)-catalyzed asymmetric diamination of conjugated dienes, see: Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t. Xu L, Shi Y. J. Org. Chem. 2008;73:749. doi: 10.1021/jo702167u.
- 15.For Cu(I)-catalyzed asymmetric diamination of conjugated dienes, see: Du H, Zhao B, Yuan W, Shi Y. Org. Lett. 2008;10:4231. doi: 10.1021/ol801605w. Zhao B, Du H, Shi Y. J. Org. Chem. 2009;74:8392. doi: 10.1021/jo901685c.
- 16.For preparation of N,N’-di-tert-butylthiadiaziridine 1,1-dioxide (2), see: Timberlake JW, Alender J, Garner AW, Hodges ML, Özmeral C, Szilagyi S, Jacobus JO. J. Org. Chem. 1981;46:2082. Ramirez TA, Zhao B, Shi Y. Tetrahedron Lett. 2010;51:1822. doi: 10.1016/j.tetlet.2010.01.114.
- 17.For Pd(0)-catalyzed dehydrogenative diamination of terminal olefins using 2, see: Wang B, Du H, Shi Y. Angew. Chem. Int. Ed. 2008;47:8224. doi: 10.1002/anie.200803184.
- 18.For CuCl-catalyzed diamination of activated terminal double bonds using 2, see: Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943. doi: 10.1021/ol702061s.
- 19.For regioselective diamination of conjugated dienes using Cu(I) and 2, see: Cornwall RG, Zhao B, Shi Y. Org. Lett. 2011;13:434. doi: 10.1021/ol102767j.
- 20.(a) Rosenberg SH, Dellaria JF, Kempf DJ, Hutchins CW, Woods KW, Maki RG, de Lara E, Spina KP, Stein HH, Cohen J, Baker WR, Plattner JJ, Kleinert HD, Perun TJ. J. Med. Chem. 1990;33:1582. doi: 10.1021/jm00168a009. [DOI] [PubMed] [Google Scholar]; (b) Castro JL, Baker R, Guiblin AR, Hobbs SC, Jenkins MR, Russell MGN, Beer MS, Stanton JA, Scholey K, Hargreaves RJ, Graham MI, Matassa VG. J. Med. Chem. 1994;37:3023. doi: 10.1021/jm00045a006. [DOI] [PubMed] [Google Scholar]; (c) McDonald IM, Dunstone DJ, Tozer MJ. WO-9905141. 1999 [Chem. Abst. 1999, 130, 168372].; (d) Tung RD, Salituro FG, Deininger DD, Bhisetti GR, Baker CT, Spaltenstein A. US-5945413. 1999 [Chem. Abst. 1999, 131, 185247].; (e) Cherney RJ, King BW. WO-0228846. 2002 [Chem. Abst.2002, 136, 309923].; (f) Zhong J, Gan X, Alliston KR, Lai Z, Yu H, Groutas CS, Wong T, Groutas WC. J. Comb. Chem. 2004;6:556. doi: 10.1021/cc030047r. [DOI] [PubMed] [Google Scholar]; (g) Bendjeddou A, Djeribi R, Regainia Z, Aouf N-E. Molecules. 2005;10:1387. doi: 10.3390/10111387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Sparey T, Beher D, Best J, Biba M, Castro JL, Clarke E, Hannam J, Harrison T, Lewis H, Madin A, Shearman M, Sohal B, Tsou N, Welch C, Wrigley J. Bioorg. Med. Chem. Lett. 2005;15:4212. doi: 10.1016/j.bmcl.2005.06.084. [DOI] [PubMed] [Google Scholar]; (i) Winum J-Y, Scozzafava A, Montero J-L, Supuran CT. Med. Res. Rev. 2006;26:767. doi: 10.1002/med.20068. [DOI] [PubMed] [Google Scholar]; (j) Winum J-Y, Scozzafava A, Montero J-L, Supuran CT. Expert Opin. Ther. Patents. 2006;16:27. doi: 10.1517/13543776.16.1.27. [DOI] [PubMed] [Google Scholar]; (k) Yang Q, Li Y, Dou D, Gan X, Mohan S, Groutas CS, Stevenson LE, Lai Z, Alliston KR, Zhong J, Williams TD, Groutas WC. Arch. Biochem. Biophys. 2008;475:115. doi: 10.1016/j.abb.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Benltifa M, García Moreno MI, Mellet CO, García Fernández JM, Wadouachi A. Bioorg. Med. Chem. Lett. 2008;18:2805. doi: 10.1016/j.bmcl.2008.04.004. [DOI] [PubMed] [Google Scholar]; (m) Kim SJ, Jung M-H, Yoo KH, Cho J-H, Oh C-H. Bioorg. Med. Chem. Lett. 2008;18:5815. doi: 10.1016/j.bmcl.2008.09.034. [DOI] [PubMed] [Google Scholar]; (n) Dou D, Tiew K-C, He G, Mandadapu SR, Aravapalli S, Alliston KR, Kim Y, Chang K-O, Groutas WC. Bioorg. Med. Chem. 2011;19:5975. doi: 10.1016/j.bmc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Eur. J. Med. Chem. 2012;47:59. doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Brodney MA, Barreiro G, Ogilvie K, Hajos-Korcsok E, Murray J, Vajdos F, Ambroise C, Christofferson C, Fisher K, Lanyon L, Liu J, Nolan CE, Withka JW, Borzilleri KA, Efremov I, Oborski CE, Varghese A, O’Neill BT. J. Med Chem. 2012;55:9224. doi: 10.1021/jm3009426. [DOI] [PubMed] [Google Scholar]
- 21.(a) Ahn KH, Yoo DJ, Kim JS. Tetrahedron Lett. 1992;33:6661. [Google Scholar]; (b) Fécourt F, Lopez G, Lee AVD, Martinez J, Dewynter G. Tetrahedron: Asymmetry. 2010;21:2361. [Google Scholar]
- 22.(a) Regaïnia Z, Abdaoui M, Aouf N-E, Dewynter G, Montero J-L. Tetrahedron. 2000;56:381. [Google Scholar]; (b) Kim I-W, Jung S-H. Arch. Pharm. Res. 2002;25:421. doi: 10.1007/BF02976594. [DOI] [PubMed] [Google Scholar]; (c) Berredjem M, Djebbar H, Regainia Z, Aouf N-E, Dewynter G, Winum J-Y, Montero J-L. Phosphorus,Sulfur Silicon Relat. Elem. 2003;178:693. [Google Scholar]; (d) Zhong J, Gan X, Alliston KR, Groutas WC. Bioorg. Med. Chem. 2004;12:589. doi: 10.1016/j.bmc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 23.Lee SA, Kwak SH, Lee K-I. Chem. Commun. 2011;47:2372. doi: 10.1039/c0cc04166a. [DOI] [PubMed] [Google Scholar]
- 24.Noyori R, Takaya H. Acc. Chem. Res. 1990;23:345. [Google Scholar]
- 25.For leading reviews on phosphoramidite ligands, see: Feringa BL. Acc. Chem. Res. 2000;33:346. doi: 10.1021/ar990084k. Minnaard AJ, Feringa BL, Lefort L, De Vries JG. Acc. Chem. Res. 2007;40:1267. doi: 10.1021/ar7001107. Teichert JF, Feringa BL. Angew. Chem. Int. Ed. 2010;49:2486. doi: 10.1002/anie.200904948.
- 26.For leading references on L2 and L3, see: Boele MDK, Kamer PCJ, Lutz M, Spek AL, de Vries JG, van Leeuwen PWNM, van Strijdonck GPF. Chem. Eur. J. 2004;10:6232. doi: 10.1002/chem.200400154. Burks HE, Liu S, Morken JP. J. Am. Chem. Soc. 2007;129:8766. doi: 10.1021/ja070572k.
- 27.For a leading reference on L4, see: Alexakis A, Rosset S, Allamand J, March S, Guillen F, Benhaim C. Synlett. 2001:1375.
- 28.For leading references on phosphoramidite ligands L7–L9, see: de Vries AHM, Meetsma A, Feringa BL. Angew. Chem. Int. Ed. 1996;35:2374. Sewald N, Wendisch V. Tetrahedron: Asymmetry. 1998;9:1341. Arnold LA, Imbos R, Mandoli A, de Vries AHM, Naasz R, Feringa BL. Tetrahedron. 2000;56:2865. Shintani R, Park S, Duan W-L, Hayashi T. Angew. Chem. Int. Ed. 2007;46:5901. doi: 10.1002/anie.200701529.
- 29.Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.No diamination product was observed when cis-1,3-pentadiene was subjected to the reaction conditions
- 31.Pansare SV, Rai AN, Kate SN. Synlett. 1998:623. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.