Abstract
Whole-brain dynamic time-resolved computed tomography angiography (CTA) is a technique developed on the new 320-detector row CT scanner capable of generating time-resolved cerebral angiograms from skull base to vertex. Unlike a conventional cerebral angiogram, this technique visualizes pial arterial filling in all vascular territories, thereby providing additional hemodynamic information. Ours was a retrospective study of consecutive patients with ischemic stroke and M1 middle cerebral artery +/−intracranial internal carotid artery occlusions presenting to our center from June 2010 and undergoing dynamic time-resolved CTA and perfusion CT within 6 hours of symptom onset. Leptomeningeal collateral status was assessed by determining relative prominence of pial arteries in the ischemic region, rate and extent of retrograde flow, and various topographical patterns of pial arterial filling. Twenty-five patients were included in the study. We demonstrate the existence of the following novel properties of leptomeningeal collaterals in humans: (a) posterior (posterior cerebral artery (PCA)–MCA) dominant collateralization, (b) intra-territorial ‘within MCA region' leptomeningeal collaterals, and (c) significant variability in size, extent, and retrograde filling time in pial arteries. We also describe a simple and reliable collateral grading template that, for the first time on dynamic CTA, incorporates back-filling time as well as size and extent of collateral filling.
Keywords: acute ischemic stroke, dynamic CT angiography, leptomeningeal collaterals
Introduction
There is a growing body of evidence to suggest that leptomeningeal collaterals have an important role in maintaining blood flow to brain regions distal to an arterial occlusion.1, 2, 3, 4, 5, 6 Strategies aimed at augmenting blood flow through these collaterals in patients presenting with acute ischemic stroke have, however, met with limited success.7, 8 Questions regarding the in vivo anatomy of human leptomeningeal collaterals and the potential causes for interindividual variability in structure and function are, however, unanswered.9, 10, 11 Imaging assessment of leptomeningeal collaterals in humans does not visualize the small inter-arteriolar connections directly but instead relies on an indirect assessment of the extent and rate of backfilling of pial arteries receiving blood flow through these collateral vessels.1, 5, 12, 13 Conventional cerebral angiography is considered to be the reference standard for assessment of leptomeningeal collaterals using this principle. It is, however, an invasive test and is not performed at presentation in the majority of patients with acute ischemic stroke.5, 14 Even if undertaken, comprehensive assessment of collateral status using catheter angiography would require a detailed four-vessel study, a procedure often neither available, feasible, or appropriate in an acute stroke scenario where time is of essence. Imaging tools like computed tomography angiography (CTA) that are much quicker and simpler to obtain in an acute stroke setting have, until now, been limited by temporal resolution.1, 4, 13, 15
Whole-brain dynamic time-resolved CTA (dCTA) is a technique developed on the new generation 320-detector row 640-slice CT scanner. It is capable of generating time-resolved cerebral angiograms of the brain vasculature from skull base to vertex, similar to a conventional cerebral angiogram. Unlike a conventional cerebral angiogram, dCTA is able to visualize pial arterial filling in all vascular territories in a time-resolved manner, thereby potentially providing additional hemodynamic information.16, 17 In this study, we use this new imaging modality to assess leptomeningeal collaterals in patients presenting with acute M1 segment middle cerebral artery +/− intracranial internal carotid artery (ICA) occlusions and demonstrate several novel anatomical and functional properties of these vessels that could have significant research and clinical implications in this field.
Materials and methods
This study was performed in patients presenting with acute ischemic stroke and M1 MCA +/− intracranial ICA occlusions presenting to the John Hunter Hospital from June 2010 to March 2011. All patients had a baseline non-contrast CT scan, dCTA, and perfusion CT (CTP) on the Toshiba Aquilion One 320-detector row 640-slice CT scanner. Patient consent (informed or when applicable surrogate) was obtained. The Hunter New England Health Clinical Ethics Board approved the study.
Scanner
The Toshiba Aquilion One is a cone beam CT, with a detector width of 16 cm capable of generating a single-rotation whole-brain non-contrast CT, a cervico-cranial three-dimensional CTA using an incremental approach, and four-dimensional time-resolved whole-brain CTA (dCTA) and CTP acquired simultaneously.
Dynamic CTA and whole-brain CTP
All CT imaging was acquired on a Toshiba Aquilion One 320-detector row 640-slice cone beam CT. Whole-brain non-contrast CT was performed in one rotation (detector width 16 cm). Next, a four-dimensional dCTA and CTP was acquired simultaneously. For the CTA-CTP, 50 mL of contrast agent (Ultravist 370; Bayer HealthCare, Berlin, Germany) was injected at 6 mL/s chased by 50 mL of saline. Pulsed full-rotation scan beginning 7 seconds after contrast injection with 19 time points over 60 seconds with a total pulse image acquisition time of 9.5 seconds was used. Computed tomography angiogram of extra cranial vessels was acquired using bolus tracking with 50 mL of contrast injected at 6 mL per second chased by 50 mL of saline. Total radiation exposure was 5.5–6.0 mSev. Primary data were reconstructed on a modified Vitrea workstation upgraded for cone beam CT (Vitrea fX, Version 1.0, Vital Images, Minnetonka, MN, USA).
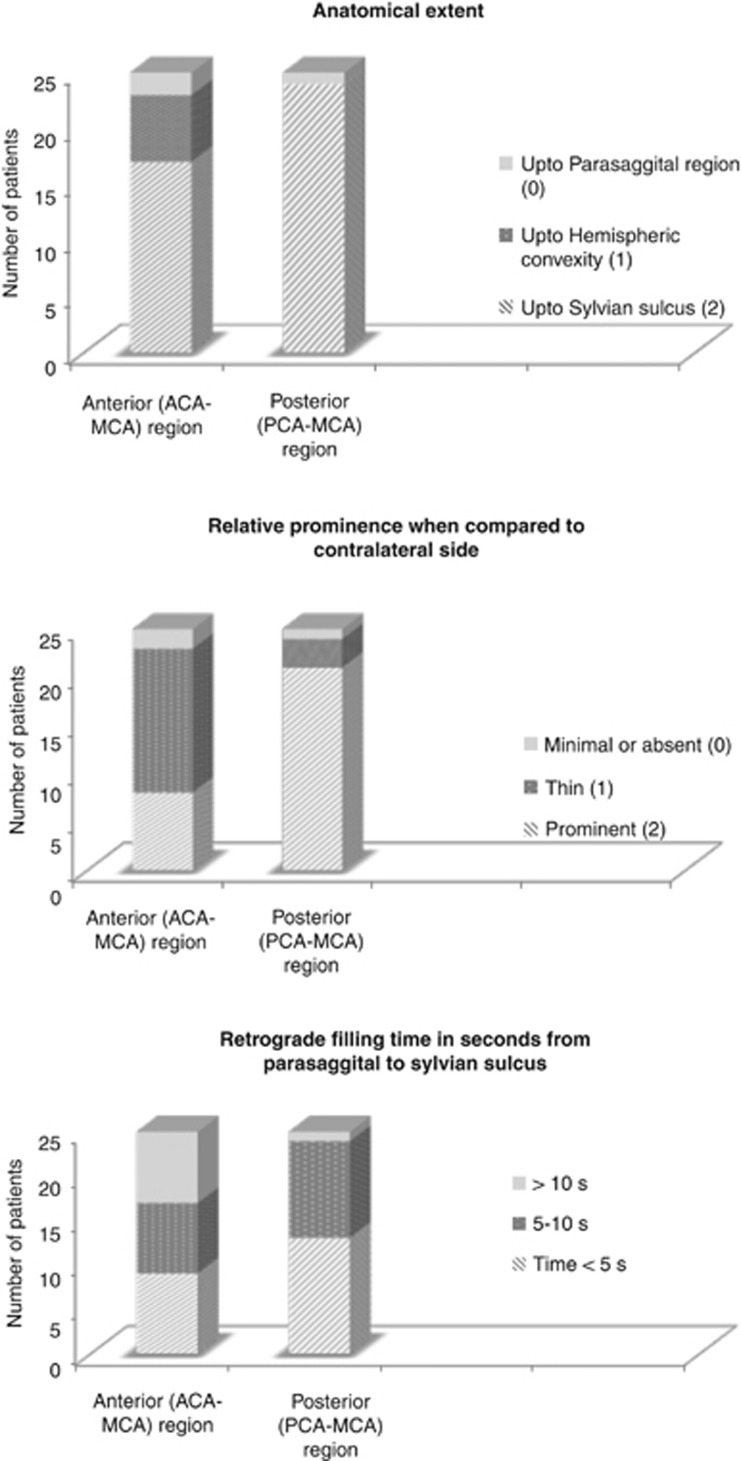
All images were read by consensus by two readers (BM and BB). Whole-brain dCTA identified retrograde filling pial arteries in the MCA territory distal to the occlusion. Pial arteries were distinguished from veins based on morphology, direction of filling, and whether visualized early or late. These retrograde filling pial arteries were divided into two groups based on origin from anterior or posterior circulation; namely anterior cerebral artery (ACA) to MCA and PCA to MCA and assessed for the following three properties using a simple grading system (Figure 1):
Anatomical extent (retrograde filling up to sylvian sulcus=2, up to cerebral convexity=1, and only up to parasaggital region=0).
Prominence of pial arteries when compared with similar vessels in the opposite MCA territory (same or more prominent=2, thin=1, and minimal or not visualized=0).
Time in seconds for retrograde filling from parasagittal region to the sylvian sulcus.
Figure 1.
Template for detailed assessment of leptomeningeal collaterals in patients with M1 middle cerebral artery +/− intracranial internal carotid artery occlusion using time-resolved dynamic computed tomography (CT) angiography on the 320-slice CT scanner.
We also looked for the presence of intra-territorial within MCA region collaterals as evidenced by filling up of either anterior from posterior MCA regions or vice versa. The circle of Willis was assessed for prominent, hypoplastic, or absent anterior communication artery (ACOM), posterior communication artery, and a fetal PCA. Data are described using standard descriptive and analytical statistics. Pearson's χ2 test was used to compare categorical variables. As data were not normally distributed and sample sizes were small, the nonparametric Spearman's rank correlation was used to assess correlation between extent, size, and rate of retrograde filling in pial arteries within and between ACA–MCA and PCA–MCA regions. Cox proportional hazards model was invoked to study the relative risk of retrograde backfilling reaching the sylvian sulcus in the two territories. As retrograde filling time from parasaggital to the sylvian sulcus was not noted in all patients within the acquisition time (60 seconds) and no patient who had filling to the sylvian sulcus had a filling time >10 seconds, an arbitrary value >10 seconds was assigned to such patients. Supplementary information is available at the Journal of Cerebral Blood Flow & Metabolism website—www.nature.com/jcbfm. Our choice of nonparametric statistics based on ranks ensures that this imputation does not affect results. Inter-rater reliability of each component of the assessment in the anterior and posterior regions was assessed in randomly chosen 20 patients by two independent raters (BM and BB) using a quadratic weighted kappa owing to the ordinal nature of the scale.18 We categorized time in seconds for retrograde filling from parasagittal region to the sylvian sulcus into three categories for this purpose (Figure 1). All P values are two-sided with P value <0.05 considered statistically significant. Software R, version 2.14.0, was used for analysis.
Results
Of the 41 patients with M1 MCA +/− intracranial ICA occlusions presenting to our hospital during the study period, 16 were excluded from the study because of poor image quality. Twelve of these 16 exclusions were during the first few months of installation of the machine when the imaging protocol was being refined. Four others excluded had substandard images owing to motion artifacts. Patients included ranged in age from 57 to 89 years, and consisted of 13 men and 12 women. Median stroke symptom onset to imaging time was 2.3 hours (range 1 to 6 hours) and median baseline stroke severity on the NIHSS was 15 (range 2 to 28). Seventeen patients were treated with IV t-PA. Of 25 patients with M1 MCA +/− intracranial ICA occlusions, 11 patients had M1 MCA+intracranial ICA occlusions and 14 patients had isolated M1 MCA occlusions.
Variability in extent and prominence of leptomeningeal collaterals in the anterior and posterior circulation of the brain
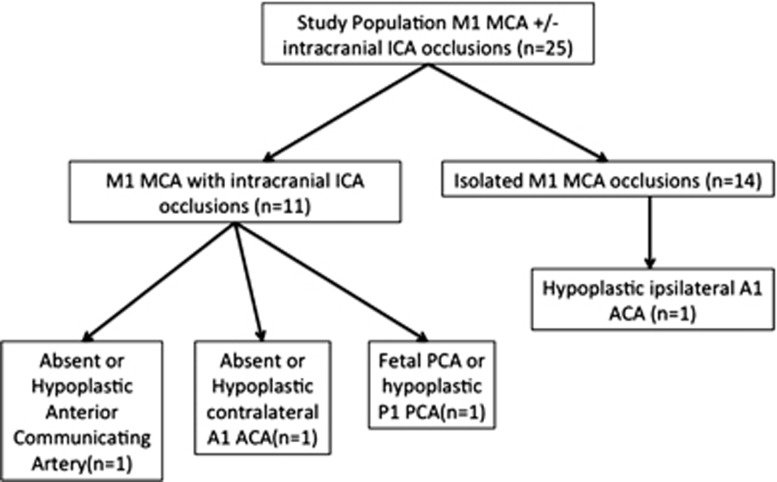
We noted significant variability in the presence of ACA–MCA and PCA–MCA leptomeningeal collaterals (Figure 2). Retrograde filling of pial arteries all the way to the sylvian sulcus was noted more often with PCA–MCA collaterals (24/25) than with ACA–MCA collaterals (17/25) (P=0.02) (Figures 3A and 3B). Twenty-one of twenty-five PCA–MCA pial arteries were of the same size or more prominent relative to contralateral vessel versus 8/25 ACA–MCA pial arteries (P=0.01). This pattern of posterior dominance of leptomeningeal collaterals was also noted in 14 patients with isolated M1 MCA occlusion (13/14 PCA–MCA pial arteries were of same size or more prominent relative to contralateral vessel versus 6/14 ACA–MCA pial arteries, P=0.01). Pattern of Willisian collateralization was assessed in detail to determine if a posterior or anterior dominant pattern is explained by a hypoplastic or absent circle of Willis (Figure 4). One patient with isolated M1 MCA occlusion and a dominant posterior pattern had an absent ACOM with hypoplastic ipsilateral A1 ACA segment. One patient with an intracranial ICA occlusion and a dominant posterior pattern had an absent ACOM and another had a hypoplastic contralateral A1 ACA segment (Figure 4). Posterior dominance of leptomeningeal collaterals persisted even when these patients were excluded from analysis (18/22 PCA–MCA pial arteries were of same size or more prominent relative to contralateral vessel versus 8/22 ACA–MCA pial arteries, P=0.01).
Figure 2.
Distribution of the three properties of leptomeningeal collateral status (prominence, extent, and retrograde filling time) assessed using dynamic computed tomography angiography in this study and stratified by anterior (anterior cerebral artery (ACA)–middle cerebral artery (MCA)) and posterior (posterior cerebral artery (PCA)–MCA) regions.
Figure 3.
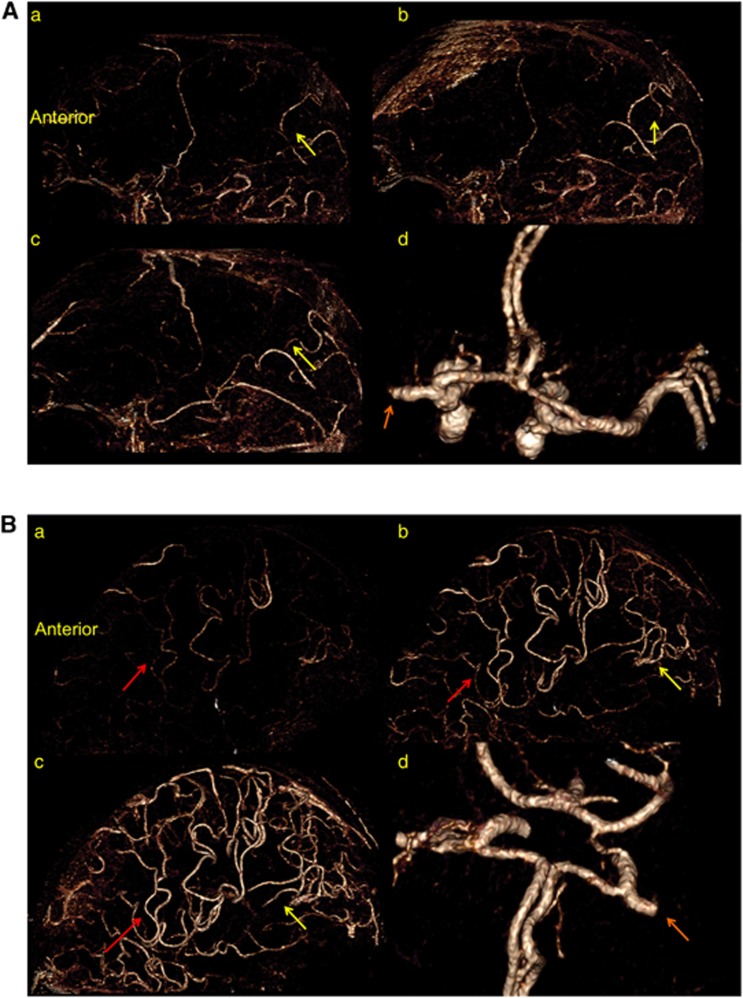
(A) Posterior dominant leptomeningeal collateral pattern. Dynamic time-resolved computed tomography (CT) angiography (saggital view) in a patient with an isolated M1 middle cerebral artery (MCA) occlusion (orange arrow) and patent circle of Willis (d). Note retrograde filling of pial arteries through posterior (posterior cerebral artery (PCA)–MCA) collaterals in sequential time-resolved images (a–c) (yellow arrows); there is no retrograde filling of pial arteries through anterior (ACA–MCA) collaterals. The sequential images (a–c) are 2 to 3 seconds apart. (B) Anterior dominant leptomeningeal collateral pattern. Dynamic time-resolved CT angiography (saggital view) in a patient with an isolated M1 MCA occlusion (orange arrow) and patent circle of Willis (d). Note retrograde filling of pial arteries through anterior (ACA–MCA, red arrows) and posterior (PCA–MCA, yellow arrows) collaterals in sequential time-resolved images (a–c); retrograde filling of pial arteries through posterior (PCA–MCA) collaterals are less prominent and slower than their anterior (ACA–MCA) counterpart. The sequential images (a–c) are 2 to 3 seconds apart.
Figure 4.
Distribution of incomplete/hypoplastic circle of Willis in the study population stratified by site of occlusion. In 11 patients with intracranial internal carotid artery (ICA) occlusions, only 2 patients had an incomplete anterior circle of Willis as a potential explanation for dominant posterior cerebral artery (PCA)–middle cerebral artery (MCA) leptomeningeal collateral pattern. In 14 patients with isolated M1 MCA occlusions, only 1 patient had an hypoplastic ipsilateral A1 anterior cerebral artery (ACA) as a potential explanation for dominant posterior PCA–MCA leptomeningeal collateral pattern.
Variability in retrograde filling time in pial arteries
The time in seconds for retrograde filling of pial arteries in the anterior and posterior MCA territory from the parasaggital region to the sylvian sulcus ranged from 2 to 10 seconds. In eight patients, however, retrograde filling failed to reach the sylvian sulcus in the anterior MCA territory during image acquisition when compared with one patient in the posterior MCA territory (P value=0.03). Using a Cox proportional hazards model, the relative risk of retrograde filling in the posterior MCA territory relative to the anterior MCA territory is 1.88 (P value=0.04), implying that retrograde filling time in the posterior MCA territory was consistently lesser than in the anterior MCA territory.
Correlation between extent, size, and retrograde filling time in pial arteries
We noted a positive correlation between extent and size in the ACA–MCA collaterals (r=0.61, P=0.001) and in the PCA–MCA collaterals (r=0.53, P<0.006). A negative correlation was observed between extent and retrograde filling time in the ACA–MCA collaterals (r=−0.82, P<0.001) implying that more extensive collaterals backfilled more quickly. This negative correlation was not observed in the PCA–MCA collaterals (r=−0.35, P=0.09). Similar negative correlation was observed between prominence and retrograde filling time in the ACA−MCA (r=−0.55, P=0.004) and in the PCA−MCA collaterals (r=−0.65, P<0.001). No correlation between ACA−MCA and PCA−MCA collateral extent (r=0.26, P=0.21) or between ACA−MCA and PCA−MCA collateral size (r=0.35, P=0.09) was noted. There was a positive correlation between retrograde filling time in the ACA−MCA and PCA−MCA pial arteries (r=0.67, P<0.001) (Table 1).
Table 1. Spearman's rank correlation matrix between the three properties of leptomeningeal collateral status (prominence, extent, and retrograde filling time) assessed using dynamic CTA in this study and stratified by anterior (ACA–MCA) and posterior (PCA–MCA) regions.
|
Anterior (ACA–MCA) region |
Posterior (PCA–MCA) region |
|||||
|---|---|---|---|---|---|---|
| Prominence | Extent | Time | Prominence | Extent | Time | |
| Prominence | 1 | 1 | ||||
| Extent | 0.61 (0.001) | 1 | 0.53 (0.006) | 1 | ||
| Time | −0.55 (0.004) | −0.82 (<0.001) | 1 | −0.65 (<0.001) | −0.35 (0.09) | 1 |
| |
Anterior—prominence |
|
Anterior—extent |
|
Anterior—time |
|
| Posterior—prominence | 0.35 (0.09) | Posterior—extent | 0.26 (0.21) | Posterior—time | 0.67 (<0.001) | |
Abbreviations: ACA–MCA, anterior cerebral artery–middle cerebral artery; CTA, computed tomography angiography; PCA–MCA, posterior cerebral artery–middle cerebral artery.
We noted good correlation between the three properties within anterior and posterior regions. (Rows 1 and 2, numbers in parentheses are P values.) We also noted a positive correlation between the rate of retrograde backfilling in the anterior and posterior regions (row 3). No correlation was, however, noted at the 5% significance level between anterior and posterior regions when assessing prominence and extent of back-filling pial arteries (row 3).
Inter-rater reliability for all components of leptomeningeal collateral assessment on dCTA are good except retrograde filling time in the anterior MCA territory, which can be described as fair19 (Table 2).
Table 2. Inter-rater reliability (quadratic-weighted kappa) of the three properties of leptomeningeal collateral status (prominence, extent, and retrograde filling time) assessed using dynamic CTA in this study and stratified by anterior (ACA–MCA) and posterior (PCA–MCA) regions18, 19.
|
Anterior (ACA–MCA) region |
Posterior (PCA–MCA) region |
|||||
|---|---|---|---|---|---|---|
| Extent | Prominence | Time | Extent | Prominence | Time | |
| Agreement (%) | 92.5 | 93.75 | 80 | 98.75 | 93.75 | 93.75 |
| Expected agreement (%) | 72.5 | 84.50 | 67.5 | 89.5 | 83.75 | 77.5 |
| Weighted kappa | 0.73 | 0.6 | 0.4 | 0.88 | 0.61 | 0.72 |
| P value | <0.01 | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 |
Abbreviations: ACA–MCA, anterior cerebral artery–middle cerebral artery; CTA, computed tomography angiography; PCA–MCA, posterior cerebral artery–middle cerebral artery.
Discussion
Heubner first noted the existence of leptomeningeal collaterals when he observed filling of the whole pial arterial tree with an injection of dye into one of the ACA, MCA, or PCA arteries.9 Fay confirmed these findings and suggested that leptomeningeal collaterals were ‘points of fusion' in the border zone of the three major cerebral arteries.9 Other studies suggested the existence of a continuous vascular network in the brain with anastomoses at the capillary, arteriolar, and pial artery level.10, 20 These micro-anatomical studies have increased our understanding of interindividual variability in the distribution, size, and number of leptomeningeal collaterals. Imaging modalities like conventional cerebral angiography, CTA, and magnetic resonance imaging have, in addition, increased our understanding of the role of leptomeningeal collaterals in preserving brain tissue until recanalization is achieved.1, 3, 5, 9, 21 Despite the gains made until now in understanding leptomeningeal collaterals, we do not fully understand the range or reasons for this variability.1, 22 In this study, we use a new imaging tool, dCTA, to study leptomeningeal collaterals in a uniform cohort of patients with M1 MCA +/− intracranial ICA occlusions. We present some properties of leptomeningeal collaterals not previously described in human imaging studies. In addition, we describe some other properties of collaterals that have been described using conventional cerebral angio or CTA, but never before using a single imaging modality.
Interterritorial leptomeningeal collaterals between the posterior circulation and the MCA territory are functionally better than those between the ACA and MCA territories
In a classic neuroanatomical study, van der Ecken and Adams demonstrated the existence of inter-arteriolar connections between the ACA, MCA, and PCA vascular territories. Their observation seemed to suggest that interterritorial collaterals are more prevalent anteriorly than posteriorly. Being a postmortem study was an obvious limitation.11 Other studies showed interindividual variability of the territories of the MCA, ACA, and PCA, possibly owing to variability in blood flow in the upstream arteries, or in the number and function of leptomeningeal collaterals.9, 20, 23, 24 Many of these studies, when in humans, were either postmortem or, when ante-mortem, were incomplete owing to lack of concurrent vertebral angiography.9 These studies were therefore unable to comment on interindividual variability in leptomeningeal collaterals between the PCA–MCA and ACA–MCA territories. Our study using dCTA is able to overcome this limitation. Our results suggest that interterritorial leptomeningeal collaterals between the PCA–MCA are functionally better than those between the ACA–MCA territories. We hypothesize that differences in sympathetic innervation and autoregulatory capacity between the two circulations could be a potential explanation for the observed differences.25, 26 The presence of the ACOM in all but one of our patients and of a hypoplastic contralateral A1 ACA segment in only two of our patients suggests that the posterior dominant pattern we observe is not due to the reduced pressure gradient in the second segment of the ipsilateral ACA owing to an incomplete anterior circle of Willis.
Variability in size and retrograde filling time of pial arteries probably related to blood flow regulation
Many previous CTA-based leptomeningeal collateral scores have used relative size of back-filling pial arteries as a marker of leptomeningeal collateral status.1, 3, 4, 13, 15, 27, 28 We note significant variability in size of these pial arteries when compared with anatomically similar vessels in the contralateral hemisphere. Relative size of these pial arteries could be a marker for increased or decreased functional capacity of leptomeningeal collaterals. Variability in size could also be genetic or owing to variability in autoregulatory mechanisms or myogenic tone of pial arteries.29, 30, 31, 32 Evidence suggests factors such as acute hyperglycemia might limit collateral status by actively promoting the constriction of leptomeningeal arteries.33, 34 Constriction could arise from the inhibitory effect of elevated glucose on voltage-gated K+ channel conductance in vascular smooth muscle34, 35 or owing to the production of endothelial-derived relaxing factors like nitric oxide.34, 36 Our study, along with other previous studies, points toward large variability in the size of pial arteries in the ischemic vascular bed.
Unlike in angiographic studies of the coronary vascular bed, rate of backfilling of brain arteries has rarely been used as a surrogate marker of collateral status.37 Our results show significant variability in the rate of backfilling of pial arteries. This temporal variability could be an indicator of the functional status of leptomeningeal collaterals and/or autoregulatory capacity of pial arteries. Further studies are needed to understand the relationship between leptomeningeal collateral status and the rate of backfilling in pial arteries. Our study also shows a positive correlation between retrograde filling time in the ACA–MCA versus PCA–MCA pial arteries in comparison with a lack of correlation for extent and size between these regions. We hypothesize that this unique difference may suggest that rate of backfilling is more likely a measure of upstream pressure gradients (similar in both ACA–MCA and PCA–MCA regions) when compared with extent and prominence that may indicate downstream leptomeningeal collateral functional capacity (and therefore different in ACA–MCA and PCA–MCA regions).
Our study has limitations. We are aware that the posterior dominant pattern of leptomeningeal collateralization observed could be influenced, to some extent, by variability in the functional status of the ACOM and its ability to support transhemispheric cross flow. We did, however, study the circle of Willis in great structural detail and found posterior dominance even after excluding cases where this pattern could be explained by structural variation. Other observations including the presence of intra-territorial leptomeningeal collaterals and differences between rate of backfilling of pial arteries and their size and extent will need verification in larger studies. Moreover, blood flow through leptomeningeal collaterals in patients with acute ischemic stroke is a dynamic process that could change over time. This is a potential limitation and needs further study. We also did not have conventional cerebral angiography in our patients for comparison; although conceptually we believe that dCTA may be inherently superior to the former technique owing to its ability to visualize blood flow in the anterior and posterior circulation simultaneously. Nonetheless, our use of dCTA to study functional status of leptomeningeal collaterals in patients with M1 MCA occlusions has helped us in identifying several novel properties of these vessels. Whole-brain coverage, resolution in time, and simultaneous visualization of vessels in all vascular beds had a significant role in helping us identify these phenomena when compared with previous imaging tools like conventional cerebral angiogram or non-time-resolved CTA.
Our study is hypothesis generating. We recognize the need for larger and more detailed studies using a combination of dCTA and techniques such as CTP and transcranial Doppler to better understand underlying physiological mechanisms and relationship to clinical outcomes. Our own future work focuses on this. The 320-detector row CT scanner with the ability to generate simultaneous dCTA and CTP images has the potential to greatly enhance our understanding of the haemodynamic influences on the survival of the ischemic penumbra.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011;32:1640–1645. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132 (Part 8:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Emery DJ, Schellinger PD, Selchen D, Douen AG, Chan R, Shuaib A, et al. Safety and feasibility of collateral blood flow augmentation after intravenous thrombolysis. Stroke. 2011;42:1135–1137. doi: 10.1161/STROKEAHA.110.607846. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, et al. Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of neuroflo in acute ischemic stroke trial. Stroke. 2011;42:1680–1690. doi: 10.1161/STROKEAHA.110.609933. [DOI] [PubMed] [Google Scholar]
- Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- Reina-De La Torre F, Rodriguez-Baeza A, Sahuquillo-Barris J. Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat Rec. 1998;251:87–96. doi: 10.1002/(SICI)1097-0185(199805)251:1<87::AID-AR14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vander Eecken HM, Adams RD. The anatomy and functional significance of the meningeal arterial anastomoses of the human brain. J Neuropathol Exp Neurol. 1953;12:132–157. doi: 10.1097/00005072-195304000-00002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke. 2004;35:1340–1344. doi: 10.1161/01.STR.0000126043.83777.3a. [DOI] [PubMed] [Google Scholar]
- Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- Qureshi AI.New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke Neurosurgery 2002501405–1414.discussion 1414-1415. [DOI] [PubMed] [Google Scholar]
- Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert E, Bohner G, Dewey M, Masuhr F, Hoffmann KT, Mews J, et al. 320-slice CT neuroimaging: initial clinical experience and image quality evaluation. Br J Radiol. 2009;82:561–570. doi: 10.1259/bjr/27721218. [DOI] [PubMed] [Google Scholar]
- Klingebiel R, Siebert E, Diekmann S, Wiener E, Masuhr F, Wagner M, et al. 4-D imaging in cerebrovascular disorders by using 320-slice CT: feasibility and preliminary clinical experience. Acad Radiol. 2009;16:123–129. doi: 10.1016/j.acra.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–268. [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Van Der Zwan A, Hillen B. Araldite F as injection material for quantitative morphology of cerebral vascularization. Anat Rec. 1990;228:230–236. doi: 10.1002/ar.1092280215. [DOI] [PubMed] [Google Scholar]
- Mohammad Y, Xavier AR, Christoforidis G, Bourekas E, Slivka A. Qureshi grading scheme for angiographic occlusions strongly correlates with the initial severity and in-hospital outcome of acute ischemic stroke. J Neuroimaging. 2004;14:235–241. doi: 10.1177/1051228404265716. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, et al. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–2131. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- Symon L, Ishikawa S, Meyer JS. Cerebral arterial pressure changes and development of leptomeningeal collateral circulation. Neurology. 1963;13:237–250. doi: 10.1212/wnl.13.3.237. [DOI] [PubMed] [Google Scholar]
- Symon L. Haemodynamic studies of the leptomeningeal circulation in primates. Proc R Soc Med. 1968;61:610–612. doi: 10.1177/003591576806100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Serrador JM, Larose SL, Moslehi F, Lipsitz LA, Sorond FA. Autoregulation in the posterior circulation is altered by the metabolic state of the visual cortex. Stroke. 2009;40:2062–2067. doi: 10.1161/STROKEAHA.108.545285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Owman C, Siesjo B. Physiological role of cerebrovascular sympathetic nerves in the autoregulation of cerebral blood flow. Brain Res. 1976;117:519–523. doi: 10.1016/0006-8993(76)90760-5. [DOI] [PubMed] [Google Scholar]
- Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Kulkens S, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin-Trescases N, Bevan JA. High levels of myogenic tone antagonize the dilator response to flow of small rabbit cerebral arteries. Stroke. 1998;29:1194–1200. doi: 10.1161/01.str.29.6.1194. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ Res. 1995;77:832–840. doi: 10.1161/01.res.77.4.832. [DOI] [PubMed] [Google Scholar]
- Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- Tsuruta R, Fujita M, Ono T, Koda Y, Koga Y, Yamamoto T, et al. Hyperglycemia enhances excessive superoxide anion radical generation, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Brain Res. 2010;1309:155–163. doi: 10.1016/j.brainres.2009.10.065. [DOI] [PubMed] [Google Scholar]
- Sercombe R, Vicaut E, Oudart N, Sercombe C, Girard P. Acetylcholine-induced relaxation of rabbit basilar artery in vitro is rapidly reduced by reactive oxygen species in acute hyperglycemia: role of NADPH oxidase. J Cardiovasc Pharmacol. 2004;44:507–516. doi: 10.1097/01.fjc.0000141477.59748.84. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Cooper EJ, Dowell FJ. Differential effects of glucose on agonist-induced relaxations in human mesenteric and subcutaneous arteries. Br J Pharmacol. 2008;153:480–487. doi: 10.1038/sj.bjp.0707592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Billinger M, Fleisch M, Meier B. Washout collaterometry: a new method of assessing collaterals using angiographic contrast clearance during coronary occlusion. Heart. 2001;86:540–546. doi: 10.1136/heart.86.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.