Abstract
At the blood–brain and blood–spinal cord barriers, P-glycoprotein, an ATP-driven drug efflux pump, is a major obstacle to central nervous system (CNS) pharmacotherapy. Recently, we showed that signaling through tumor necrosis factor-α (TNF-α), sphingolipids, and sphingosine-1-phosphate receptor 1 (S1PR1) rapidly and reversibly reduced basal P-glycoprotein transport activity in the rat blood–brain barrier. The present study extends those findings to the mouse blood–brain and blood–spinal cord barriers and, importantly, identifies multidrug resistance-associated protein 1 (Mrp1, Abcc1) as the transporter that mediates S1P efflux from brain and spinal cord endothelial cells. In brain and spinal cord capillaries isolated from wild-type mice, TNF-α, sphingosine, S1P, the S1PR agonist fingolimod (FTY720), and its active, phosphorylated metabolite, FTY720P, reduced P-glycoprotein transport activity; these effects were abolished by a specific S1PR1 antagonist. In brain and spinal cord capillaries isolated from Mrp1-null mice, neither TNF-α nor sphingosine nor FTY720 reduced P-glycoprotein transport activity. However, S1P and FTY720P had the same S1PR1-dependent effects on transport activity as in capillaries from wild-type mice. Thus, deletion of Mrp1 alone terminated endogenous signaling to S1PR1. These results identify Mrp1 as the transporter essential for S1P efflux from the endothelial cells and thus for inside-out S1P signaling to P-glycoprotein at the blood–brain and blood–spinal cord barriers.
Keywords: blood–brain barrier, confocal microscopy, endothelium, pharmacology, physiology
Introduction
The bioactive sphingolipid metabolite sphingosine 1-phosphate (S1P) regulates a wide range of biologic processes, including cell growth, survival, and immune system function. Within cells, S1P is synthesized by phosphorylation of sphingosine through two closely related sphingosine kinase (SphK) enzymes: SphK1 and SphK2. SphK1 and SphK2 have distinct kinetic properties, subcellular localizations, and cellular functions.1 S1P can act as an intracellular second messenger to induce calcium release,2 and regulate histone acetylation in the nucleus.3 S1P also signals through a family of five membrane-bound G protein-coupled receptors (S1PRs), each of which targets multiple intracellular cascades.4 Because S1P is generated within cells and is sufficiently polar to limit membrane permeability, access to external S1PR-binding sites requires carrier-mediated efflux. Several studies have implicated ATP-binding cassette (ABC) transporters, which function as ATP-powered efflux pumps, in S1P efflux from cells.5, 6 ABCA1 and ABCC1 (multidrug resistance-associated protein 1 (Mrp1)) appear to be leading candidates. Recently, a novel S1P transporter (Spns2), not a member of the ABC transporter family, was discovered in zebrafish.7, 8 An ortholog of this transporter is expressed in mammals.9 It transports both S1P and the phosphorylated, active metabolite of the drug fingolimod, which is a S1P analog and nonselective S1PR agonist. In mice, it is expressed in endothelial cells and appears to be involved in the regulation of lymphocyte trafficking. Currently, there is no consensus as to which transporter provides the primary efflux pathway, but one could envision the identity of the primary transporter being cell specific.
The present study is concerned with one aspect of the mechanism by which sphingolipids signal changes in brain barrier function. The brain and spinal cord capillary endothelia that comprise these barriers are major elements in maintaining central nervous system (CNS) homeostasis, determining to a large extent which substances enter and leave the CNS.10 Relative to other capillary beds, they possesses a unique phenotype, with permeability being determined by highly restrictive tight junctional complexes between cells, by a low rate of transcytosis and by expression of specific plasma membrane transporters that facilitate entry of nutrients, metabolites and ions, and the efflux of potentially toxic waste products of brain metabolism. In this regard, brain and spinal cord capillary endothelial cells express ATP-driven xenobiotic efflux pumps, e.g., P-glycoprotein (ABCB1) and breast cancer-related protein (BCRP, ABCG2), on the luminal, blood-facing, plasma membrane. For small-molecule drugs, especially lipophillic drugs, P-glycoprotein presents a formidable obstacle to entry into the CNS.11
We recently identified S1PR1-based signaling as a key element of a pathway that rapidly reduces basal P-glycoprotein activity at the blood–brain barrier in isolated rat brain capillaries and in intact rats (Figure 1).12 Activation of signaling upstream of S1P, e.g., by tumor necrosis factor-α (TNF-α) and sphingosine, is blocked by inhibitors of sphK1, implying that S1P is generated from sphingosine within the capillary endothelial cells. Consistent with that finding and with the need for mediated S1P efflux, MK571, a nonselective inhibitor of Mrps, blocks signaling upstream of S1P, e.g., by added TNF-α or sphingosine.12 Multiple Mrps are expressed at the blood–brain barrier, i.e., Mrp1, Mrp2, Mrp4, and Mrp5,13, 14 and there are no specific inhibitors available that can be used to sort out the contributions of individual Mrps to S1P signaling. In the present study, we used FVB/N wild-type and isogenic Mrp1-null mice to show that: (1) a similar signaling pathway regulating basal P-glycoprotein activity is present at the mouse blood–brain and blood–spinal cord barriers, and (2) Mrp1 mediates S1P efflux from brain and spinal cord capillary endothelial cells, providing access to extracellular S1PR1.
Figure 1.

Signaling pathway that regulates basal P-glycoprotein activity in brain capillaries.12 Agents that activate signaling at various points in the pathway are shown above each affected step. Identifying the molecular basis for sphingosine-1-phosphate (S1P) efflux from brain and spinal cord capillary endothelial cells (curved arrow) is the focus of the current study.
Materials and Methods
Materials
Mouse anti-P-glycoprotein (C219) was purchased from Covance (Princeton, NJ, USA) whereas rabbit anti-Mrp1 was acquired from Acris Antibodies (San Diego, CA, USA). Mouse anti-β-actin, Ficoll, pyruvic acid, glucose, and bovine serum albumin (BSA) were all obtained from Sigma (St Louis, MO, USA). NBD-CSA ((N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A) was custom synthesized as described previously.15 Recombinant rat TNF-α and S1P were purchased from Sigma. The pan Mrp inhibitor, MK571, FTY720, FTY720P, and the specific S1P1 receptor antagonist, W123, were obtained from Cayman Chemical (Ann Arbor, MI, USA). Sphingosine was from Avanti Polar Lipids (Alabaster, AL, USA). The P-glycoprotein inhibitor, PSC833 (valsprodar), was a generous gift from Novartis (Basel, Switzerland). All other chemicals were of highest analytical grade and were obtained from commercial sources.
Animals
All experiments were carried out in compliance with the NIH animal care and use guidelines (Guide for the Care and Use of Laboratory Animals, National Research Council) and approved by the NIEHS Animal Care and Use Committee (ARRIVE Guidelines). Male 8- to 9-week-old FVB/N (mrp1+/+), C3H (mrp1+/+), and Mrp1-deficient (mrp1−/−; FVB/N background) mice were obtained from Taconic Farms (Germantown, NY, USA). Adult male Sprague-Dawley rats (6 months of age) were obtained from Charles River (Portage, MI, USA). Animals were housed in temperature-controlled rooms under a 12-hour light/12-hour dark cycle and given ad libitum access to food and water.
Brain and Spinal Cord Capillary Isolation
Brain and spinal cord capillaries were isolated as described previously.16, 17 Briefly, mice and rats were killed by CO2 inhalation and decapitated. Brains were removed immediately and placed in ice-cold phosphate-buffered saline (PBS; 2.7 mmol/L KCl, 1.5 mmol/L KH2PO4, 136.9 mmol/L NaCl, 8.1 mmol/L Na2HPO4, 1 mmol/L CaCl2, 0.5 mmol/L MgC12, 5 mmol/L 𝒟-glucose, and 1 mmol/L sodium pyruvate, pH 7.4). After removal of the meninges, choroid plexuses, mid-brain, white matter, blood vessels, and olfactory lobes, the remaining brain tissue was homogenized. The brain homogenate was then centrifuged in an equal volume of 30% Ficoll for 20 minutes at 5,800 g and 4°C. Next, brain capillary-enriched pellets were resuspended in PBS with 1% BSA and passed over a glass bead column supported by a 30-μm nylon mesh. Brain capillaries adhering to the glass beads were then collected by gentle agitation in PBS with 1% BSA. Finally, brain capillaries washed in BSA-free PBS were either used immediately for transport experiments or snap frozen in liquid nitrogen and stored at −80°C. The procedure for spinal cord capillary isolation (5 to 10 animals per preparation) was identical except that the final Ficoll concentration was 20%.
P-Glycoprotein Transport Assay
Confocal microscopy-based transport assays with isolated brain capillaries have been described previously;17 an identical procedure was used for spinal cord capillaries. All experiments were carried out at room temperature in coverslip-bottomed imaging chambers filled with PBS. In most experiments, capillaries were exposed for 30 minutes to inhibitors of signaling. NBD-CSA was then added to each chamber and luminal substrate accumulation was assessed 1 hour later. In one experiment, capillaries were first loaded to steady state with NBD-CSA, and then modulators were added. To acquire images, the chamber containing the capillaries was mounted on the stage of a Zeiss Model 510 inverted confocal laser scanning microscope and imaged through a × 40 water-immersion objective (numeric aperture=1.2) using a 488-nm laser line for NBD-CSA. Images (8-bits) were saved to disk and luminal fluorescence was subsequently quantitated by ImageJ software (NIH, Bethesda, MD, USA). Each experiment was performed 2 to 3 times to provide independent verification. Data are presented as arbitrary fluorescence units.
Capillary Membrane Isolation
Liquid nitrogen frozen mouse and rat brain capillary pellets were resuspended in ice-cold PBS containing protease and phosphatase inhibitors (Mini Complete Protease Inhibitor tablet (Roche, Indianapolis, IN, USA) and 2 mmol/L EGTA, 5 mmol/L EDTA, 30 mmol/L NaF, 20 mmol/L pyrophosphate, 10 mmol/L orthovanadate, and 8.6 mg/ml β-glycerophosphate), followed by centrifugation at 16,200 g for 2 minutes at 4°C. The resulting supernatant (cytosolic fraction) was collected for western blot analysis of aqueous proteins, whereas the remaining pellet was gently triturated in ice-cold CelLytic MT Mammalian Tissue Lysis/Extraction Reagent (Sigma Aldrich, St Louis, MO, USA) containing 10% PBS with protease and phosphatase inhibitors. This protein suspension was then sonicated for 30 seconds, cooled on ice for 10 minutes, and centrifuged at 16,200 g for 12 minutes at 4°C. Membrane protein concentrations were then determined from the resulting supernatant using the Bradford protein assay (Bio-Rad, Hercules, CA, USA). Samples were either stored at −80°C or used immediately for western blot analysis.
Western Blot Analysis
Equal amounts of membrane protein (3.5 μg for P-glycoprotein and 20 μg for Mrp1) were loaded on a 4 to 12% Bis-Tris NuPAGE gel (Invitrogen, Carlsbad, CA, USA), and subjected to electrophoresis. After separation, proteins were transferred onto methanol-soaked Immobilon-FL polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA) via the XCell II blot module (Invitrogen). Membranes were then blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA) for 1 hour at room temperature followed by overnight incubation at 4°C with primary antibodies: anti-P-glycoprotein (C219; 170 kDa; 1:200), anti-Mrp1 (184 kDa; 1:50), and anti-β actin (42 kDa; 1:2,000). After three washing with PBS plus 0.1% Tween-20, membranes were then incubated with the corresponding infrared-fluorescence IRDye 800CW conjugated goat anti-mouse (1:1,500; Li-Cor Biosciences) or IRDye 800CW conjugated goat anti-rabbit (1:7,500; Li-Cor Biosciences) secondary antibodies for 45 minutes at room temperature in the dark. In some cases, the Millipore Snap i.d. blotting system (Millipore) was used according to the manufacturer's specifications for performing blocking and antibody incubations. Washed membranes were scanned to visualize the signal at 680 nm by the Odyssey detection system (Li-Cor Biosciences). All western blots depicted in the figures are representative of two to three independent experiments.
Statistical Analysis
Data are expressed as mean±s.e.m. Mean values were compared using one-way analysis of variance (Newman–Keuls multiple-comparison test). Differences between mean values were deemed statistically significantly when P<0.05.
Results
Blood–Brain Barrier
We previously developed an assay to measure efflux transporter activity in freshly isolated brain and spinal cord capillaries from rat and mouse.17 The assay is based on the use of confocal imaging and digital image analysis to measure concentrative and specific accumulation of fluorescent substrates within capillary lumens. To measure P-glycoprotein transport activity, we use a fluorescent cyclosporine A derivative (NBD-CSA) as substrate and PSC833 as specific inhibitor. Figure 2A shows representative confocal images of mouse (FVB/N strain) brain capillaries incubated to steady state (60 minutes) in medium with 2 μmol/L NBD-CSA without (control) and with 5 μmol/L PSC833. In brain and spinal cord capillaries, this concentration of PSC833 maximally reduces luminal NBD-CSA fluorescence, but does not alter luminal fluorescence of substrates for other ABC transporters, i.e., Mrp2 or Bcrp.16, 17 These micrographs clearly show NBD-CSA accumulation in lumens of control capillaries and substantially reduced luminal accumulation in capillaries exposed to PSC833. In the experiments that follow, we report specific P-glycoprotein transport activity, measured as the difference between luminal fluorescence intensities in control and PSC833-exposed capillaries.
Figure 2.
Tumor necrosis factor-α (TNF-α) signaling through sphingolipids and the S1P1 receptor reduce P-glycoprotein transport activity in wild-type mouse brain capillaries. (A) Representative confocal images of brain capillaries after a 60-minute incubation with 2 μmol/L NBD-CSA ((N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A) and 100 ng/ml TNF-α; note the high luminal fluorescence in the control capillary and decreased luminal fluorescence in capillaries exposed to PSC833 and TNF-α. The scale bar indicates 20 μm. (B) TNF-α acts through sphingosine-1-phosphate receptor 1 (S1PR1) to reduce P-glycoprotein activity. (C) Sphingosine acts through S1PR1 to reduce P-glycoprotein activity. (D) S1P acts through S1PR1 to reduce P-glycoprotein activity. (E) FTY720 and FTY720P act through S1PR1 to reduce P-glycoprotein activity. Each bar represents the mean±s.e.m. for 8 to 15 capillaries from a single preparation (pooled tissue from 5 to 7 mice). Statistical comparisons: *** significantly lower than controls, P<0.001.
Rat brain capillaries possess a proinflammatory/sphingolipid signaling pathway that, when activated, rapidly reduces basal P-glycoprotein transport activity.12 Signaling is activated upstream through TNF-α binding to TNFR1 and downstream by S1P binding to S1PR1 (Figure 1). Activating this pathway does not alter transport activity of other ABC transporters, nor does it alter tight junction permeability in vitro or in vivo.12 Figure 2A shows that exposing mouse brain capillaries to 100 ng/ml TNF-α for 1 hour reduced luminal NBD-CSA fluorescence. Quantitation of confocal images indicated that P-glycoprotein transport activity was reduced by ∼80% (Figure 2B). As in rat brain capillaries, TNF-α did not reduce transport activity in mouse capillaries exposed to the S1PR1 receptor antagonist, W123 (Figure 2B). Similar reductions in P-glycoprotein transport activity were seen for the downstream effecters of TNF-α, sphingosine (Figure 2C) and S1P (Figure 2D), as well as the nonselective S1PR agonist fingolimod (FTY720) and its active metabolite, FTY720P (Figure 2E). The effects of sphingosine, S1P, FTY720, and FTY720P were abolished by the S1PR1 receptor antagonist, W123 (Figure 2).
To determine the time course of S1P action, we incubated brain capillaries to steady state with NBD-CSA, added 1 μmol/L S1P, and followed changes in luminal fluorescence with time. S1P rapidly reduced P-glycoprotein-mediated accumulation of NBD-CSA into the capillary lumen (Figure 3). Within 30 minutes, specific luminal fluorescence was reduced by 80%, an effect similar to that seen in our 60-minute experiments (Figure 2). On removal of S1P from the medium, luminal fluorescence rapidly returned to the control steady-state level (Figure 3). Taken together, these data show that mouse brain capillaries, like rat brain capillaries, rapidly reduce basal P-glycoprotein transport activity through a S1PR1-dependent signaling pathway activated by TNF-α, sphingosine, S1P, FTY720, and FTY720P.
Figure 3.
Sphingosine-1-phosphate (S1P) rapidly reduces P-glycoprotein transport activity. Brain capillaries from wild-type mice were incubated to steady state (60 minutes) in medium with 2 μmol/L NBD-CSA ((N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A). Then, 1 μmol/L S1P was added to the medium (time 0 on graph); 30 minutes later, capillaries were washed and S1P-free medium was added. Each point represents the mean±s.e.m. for 7 to 9 capillaries from a single preparation (pooled tissue from 5 mice).
Within endothelial cells, sphingosine kinase phosphorylates sphingosine to S1P.18 However, S1PR1 binds ligands from the exterior of the cells. To access the receptor, S1P generated intracellularly must be transported out of the cells. We previously showed that sphingosine's ability to reduce P-glycoprotein activity in rat brain capillaries was abolished by the nonselective Mrp inhibitor, MK571. Figure 4A shows that was also the case for mouse brain capillaries. As expected, MK571 did not alter the ability of added S1P to reduce transport activity (Figure 4B).
Figure 4.
MK571, a nonselective multidrug resistance-associated protein 1 (Mrp) inhibitor, blocks the effects of (A) sphingosine, but not (B) sphingosine-1-phosphate (S1P), on P-glycoprotein transport activity in brain capillaries from wild-type mice. NBD-CSA, (N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A. Each point represents the mean±s.e.m. for 8 to 15 capillaries from a single preparation (pooled tissue from 5 mice). Statistical comparisons: *** significantly lower than controls, P<0.001.
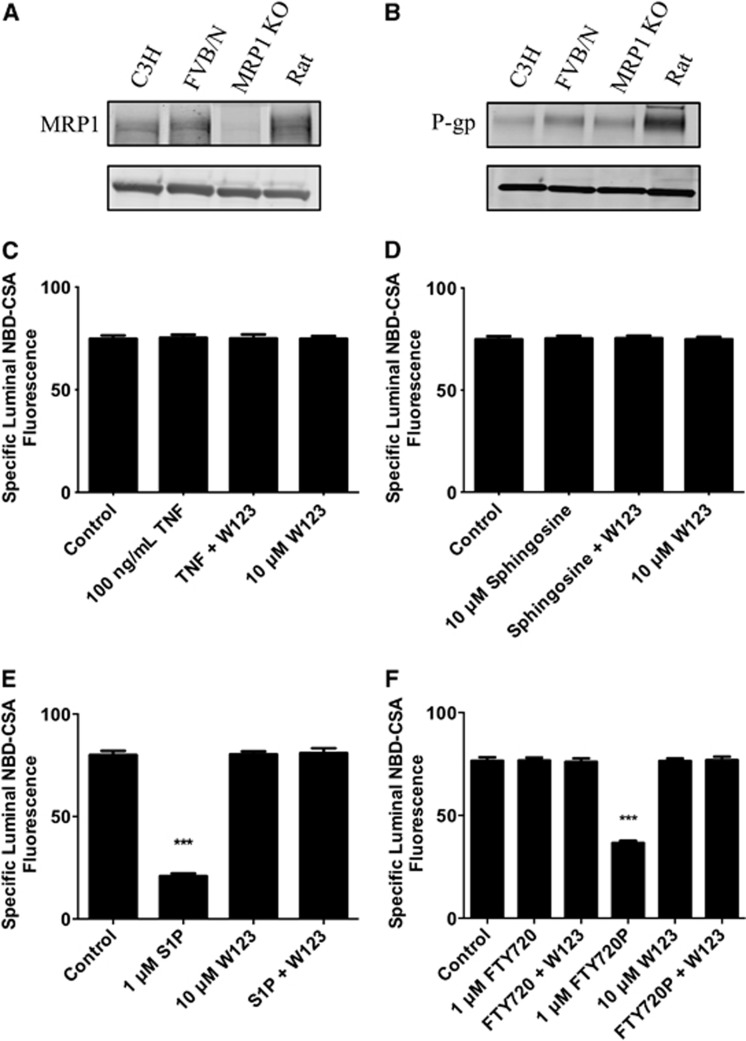
Multiple Mrps are expressed at the blood–brain barrier, including Mrp1, Mrp2, Mrp4, and Mrp5.13 We used brain capillaries from Mrp1-null mice to define the role of Mrp1 in sphingolipid signaling to P-glycoprotein. Although brain capillaries from these mice express no detectable Mrp1 protein (Figure 5A), they do express comparable amounts of P-glycoprotein protein as capillaries from wild-type mice (Figure 5B). In brain capillaries from Mrp1-null mice, TNF-α, sphingosine, and FTY720 had no effect on P-glycoprotein transport activity (Figures 5C, 5D, and 5F). In contrast, S1P and FTY720P were as effective in these capillaries as they were in capillaries from wild-type mice; S1P and FTY720P effects were blocked by the S1PR1 receptor antagonist, W123 (Figures 5E and 5F). These results indicate that in the absence of Mrp1, TNF-α, sphingosine and FTY720, agents that normally activate signaling upstream of sphingosine kinase, did not reduce P-glycoprotein transport activity. In contrast, phosphorylated effectors, S1P and FTY720P, still reduced transport activity, indicating that signaling downstream of S1PR1 is still intact. Thus, in these brain capillaries, Mrp1 functionally connects SK with S1PR1.
Figure 5.
Signaling in brain capillaries from multidrug resistance-associated protein 1 (Mrp1)-null mice. Western blots showing (A) Mrp1 and (B) P-glycoprotein (P-gp) expression in mouse brain capillaries from wild-type and Mrp1-null mice. Rat brain capillaries are shown for comparison. (C) Tumor necrosis factor-α (TNF-α), (D) sphingosine, (E) sphingosine-1-phosphate (S1P), and (F) FTY720 and FTY720P signaling to P-glycoprotein in brain capillaries from Mrp1-null mice. NBD-CSA, (N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A. Each point represents the mean±s.e.m. for 8 to 15 capillaries from a single preparation (pooled tissue from 5 mice). Statistical comparisons: *** significantly lower than controls, P<0.001.
Blood–Spinal Cord Barrier
Our recent publication showed that the confocal imaging-based assay described above provided a measure of specific P-glycoprotein transport activity in rat and mouse spinal cord capillaries.16 In the present study, experiments with spinal cord capillaries from wild-type (FVB/N) mice showed that P-glycoprotein transport activity was reduced by exposure to TNF-α (Figure 6A), sphingosine (Figure 6B), S1P (Figure 6C), and FTY720 and FTY720P (Figure 6D). For all of these chemicals, effects on transport activity in spinal cord capillaries were abolished by the S1PR1 receptor antagonist, W123 (Figure 6). As in brain capillaries, MK571 abolished the effects of sphingosine, but not S1P (Figures 6B and 6C). Thus, spinal cord capillaries from wild-type mice also possess a TNF-α- and sphingolipid-based signaling pathway that rapidly reduces basal P-glycoprotein transport activity.
Figure 6.
(A) Tumor necrosis factor-α (TNF-α), (B) sphingosine, (C) sphingosine-1-phosphate (S1P), and (D) FTY720 and FTY720P signaling to P-glycoprotein in spinal cord capillaries from wild-type mice. NBD-CSA, (N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A. Each point represents the mean±s.e.m. for 8 to 15 capillaries from a single preparation (pooled tissue from 5 mice). Statistical comparisons: *** significantly lower than controls, P<0.001.
In spinal cord capillaries from Mrp1-null mice, TNF-α (Figure 7A), sphingosine (Figure 6B), and FTY720 (Figure 7D) were without effect. However, S1P and FTY720P reduced P-glycoprotein transport activity to the same extent as in capillaries from wild-type mice (Figures 7C and 7D). Thus, in both the blood–brain and blood–spinal cord barriers, efflux mediated by Mrp1 is essential for phosphorylated metabolites that are generated intracellularly to access the S1PR1 receptor and signal reduced P-glycoprotein transport activity.
Figure 7.
(A) Tumor necrosis factor-α (TNF-α), (B) sphingosine, (C) sphingosine-1-phosphate (S1P), and (D) FTY720 and FTY720P signaling to P-glycoprotein in spinal cord capillaries from multidrug resistance-associated protein 1 (Mrp1)-null mice. NBD-CSA, (N-ɛ(4-nitrobenzofurazan-7-yl)-𝒟-Lys8)-cyclosporine A. Each point represents the mean±s.e.m. for 8 to 12 capillaries from a single preparation (pooled tissue from 5 mice). Statistical comparisons: *** significantly lower than controls, P<0.001.
Discussion
Wide specificity limits and the ability to generate large concentration gradients across the luminal plasma membrane makes blood–brain barrier P-glycoprotein a primary obstacle to the entry of small therapeutic drugs into the brain.14 Recent studies indicate that this is also the case for the blood–spinal cord barrier, a tissue that expresses high levels of the transporter on the luminal membrane of the spinal cord capillary endothelium.16 Certainly, for both of these CNS barrier tissues, an understanding of the mechanisms that regulate P-glycoprotein transport activity would be a critical step toward improving pharmacotherapy with many small-molecule drugs that are substrates for this transporter.
Over the past several years, work from this laboratory has defined a signaling pathway in rat brain capillaries that rapidly and reversibly reduces basal P-glycoprotein transport activity without changing transporter protein expression, transport activity of other ABC transporters, or tight junction permeability (Figure 1). Within this pathway, TNF-α signals through TNFR1, ETBR, iNOS, and PKCβ1.17, 19, 20 Recent experiments place sphingosine conversion to S1P and signaling through S1PR1 downstream of the TNFR1/PKCβ1 pathway.12 In situ brain perfusion in rats shows that activating the pathway through PKCβ1 or S1PR1 increases brain delivery of drugs that are P-glycoprotein substrates.12, 20 Importantly, we have identified a S1PR1 agonist, FTY720, that reduces blood–brain barrier P-glycoprotein transport activity in vitro and in vivo; this drug is a highly effective oral treatment for relapsing–remitting multiple sclerosis.21 As noted previously, these findings define a signaling-based strategy to improve drug delivery to the CNS in patients where brain barrier P-glycoprotein limits pharmacotherapy. They identify a suitable signaling pathway, a ‘drugable' target within the pathway, and a clinically practical means of hitting that target.
The present study extends previous work in two ways. First, it shows that essential elements of the signaling pathway found in rat blood–brain barrier are present in the blood–brain and blood spinal cord barriers of wild-type mice. In both barrier tissues, TNF-α, sphingosine, S1P, FTY720, and FTY720P reduced P-glycoprotein transport activity and these effects were abolished by the specific S1PR1 antagonist, W123. We previously showed that ABC transporter expression and upregulation by ligand-activated nuclear receptors in spinal cord capillaries was similar to that found in brain capillaries.16 Thus, accumulating evidence indicates that the same signals regulate ABC transporter expression and transport activity in the blood–brain and blood–spinal cord barriers.
Second, the present study identifies Mrp1 as the transporter essential for S1P efflux from brain and spinal cord endothelial cells, and thus defines a role for Mrp1 in inside-out signaling by sphingolipids in the those blood–CNS barrier tissues. Several transporters have been proposed as mediating S1P efflux from cells, including Mrp1, P-glycoprotein, Abca1, and Spns2.5, 6, 7 Based on disruption of signaling by MK571, previous work suggested that the transporter responsible for S1P efflux from rat brain capillary endothelial cells was a Mrp.12 We show here that neither TNF-α nor sphingosine nor FTY720 could reduce P-glycoprotein transport activity in brain and spinal cord capillaries from Mrp1-null mice. In contrast, both S1P and FTY720P had the same effects on transport activity as in capillaries from wild-type mice, indicating that signaling downstream of S1PR1 was intact in capillaries from Mrp1-null mice. Thus, deleting Mrp1 alone was sufficient to completely terminate the pathway following the step where SK generates intracellular S1P from sphingosine (Figure 1).
Note that the lack of any effect of sphingosine or FTY720 on P-glycoprotein transport activity in capillaries from Mrp1-null mice indicates that other candidate S1P transporters, e.g., other ABC transporters and Spns2, do not contribute to efflux of S1P or FTY720P in our system. Although there is still some controversy, most studies place Mrp1 at the abluminal (brain side) plasma membrane of the endothelial cells.22 We found S1PR1 to be present on both sides of the rat brain capillary endothelium.12 Thus, in isolated capillaries, the signaling pathway appears to used only the abluminal receptor. We suspect that is also the case in vivo when FTY720 is administered by carotid infusion, because its active, phosphorylated metabolite is generated within brain capillary endothelial cells and then must be pumped out of the cells to interact with an extracellular S1PR1-binding site. Of course, this would not be the case when FTY720P was administered by carotid infusion, because FTY720P can react directly with S1PR1 on the luminal membrane of the endothelium.
Note also that one could invoke an alternative sequence of events involving pericytes. These cells are integral to the neurovascular unit and are retained in our isolated capillary preparation along the abluminal surface of the vessels. They are essential for the development and maintenance of the blood–brain (and likely blood–spinal cord) barrier.23 Available data indicate that pericytes in culture express Mrp1 at the mRNA level,24 and that S1P secreted by pericytes can alter endothelial cell barrier function at least in a culture model of the blood–retinal barrier, a structure similar to the blood–brain barrier.25 At this time, we do not know whether pericytes secrete S1P in response to TNF-α. However, having an additional cell type contribute to the signaling pathway would add further to its complexity.
We also suspect that additional events must connect S1PR1 signaling to P-glycoprotein. Indeed, our unpublished experiments now identify an extended protein kinase cascade downstream of S1PR1; it involves phosphoinositol-3-kinase, phosphoinositide dependent kinase-1, and Akt (RE Cannon et al, unpublished data). However, how P-glycoprotein transport capacity decreases is not clear. Recent work indicates that transporter protein is not degraded.12 Experiments using an in vivo protease protection assay indicate that the protein is still exposed at the outer face of the luminal plasma membrane of the endothelial cells (lack of protection from proteolysis by luminal proteinase K).26 This implies that transporter turnover number is reduced through covalent modification or through altered interactions of the transporter protein with membrane lipids or other membrane proteins. In this regard, McCaffrey et al27 recently showed that the blood–brain barrier increases P-glycoprotein activity in response to localized, peripheral inflammatory pain, likely through altered protein–protein interactions. Membrane subfractionation experiments show that the increase in activity is because of redistribution of the transporter within the plasma membrane of the brain capillary endothelial cells. In control animals, P-glycoprotein localizes to membrane domains containing high-molecular-weight, disulfide-bonded, P-glycoprotein-containing structures; these are associated with membrane domains enriched in monomeric and high-molecular-weight, disulfide-bonded, caveolin-1-containing structures. Peripheral inflammatory pain causes an increase in blood–brain barrier P-glycoprotein ATPase activity and a redistribution of P-glycoprotein and caveolin-1, involving disassembly of high-molecular-weight, P-glycoprotein-containing structures.27 Further studies are needed to define the exact mechanism of regulation by S1PR1-mediated signaling.
The present study provides an additional example of how a member of the ABC transporter C-family plays an essential role in moving a signaling molecule out of cells to interact with a specific, outward-facing receptor. In this regard, MRPs are known to transport cyclic-AMP and cyclic-GMP (MRP4, MRP5, and MRP8), leukotriene C4 (MRP1, MRP2, MRP3, MRP4, MRP6, and MRP8), and certain eicosanoids, including prostaglandins (MRP1 and MRP4; reviewed inSlot et al28 and Deeley et al29). Consider the relationship between leukotriene C4 (LTC4, a glutathione-conjugated leukotriene) and the inflammatory response. LTC4 is produced in a number of cells that contribute to the immune response, including mast cells, eosinophils, neutrophils, and macrophages. It is a high-affinity substrate for MRP1. Once released from cells, it is converted to other proinflammatory, nonconjugated leukotrienes, which contribute to anaphalaxis and asthma pathologies. In Mrp1-null mice, leukotriene release from mast cells and eosinophils is reduced and the immune response to specific stimuli is impaired.30 Based on the present findings, we expect the contributions of S1P to the immune response to be similarly impaired by Mrp1 deficiency, not just in circulating cells but also in the brain endothelium.
Finally, in addition to endogenous signaling molecules and metabolites, MRP family members transport a large number of therapeutic drugs, some handled by P-glycoprotein.13 For those drugs that possess a high affinity for transport on one or more MRPs, one can envision competition at the transporter as a mechanism by which inside-out signaling or metabolite excretion is disrupted. Such interactions may underlie transporter-based mechanisms of toxicity or, conversely, may provide a tool to target specific signaling events.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
The authors declare no conflict of interest.
References
- Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, et al. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- Meyer ZU, Heringdorf D, Liliom K, Schaefer M, Danneberg K, Jaggar JH, et al. Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G-protein-coupled receptors. FEBS Lett. 2003;554:443–449. doi: 10.1016/s0014-5793(03)01219-5. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA. 2012;109:15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58:140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Madole EK, Miller DS, Bauer B. Estrogen receptor beta signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein. J Pharmacol Exp Ther. 2010;334:467–476. doi: 10.1124/jpet.110.168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm U, Fricker G, Wenger R, Miller DS. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol. 1995;268:F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Campos CR, Schroter C, Wang X, Miller DS. ABC transporter function and regulation at the blood-spinal cord barrier. J Cereb Blood Flow Metab. 2012;32:1559–1566. doi: 10.1038/jcbfm.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–1383. doi: 10.1038/jcbfm.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol. 2011;10:1026–1034. doi: 10.1016/S1474-4422(11)70228-9. [DOI] [PubMed] [Google Scholar]
- Kilic E, Spudich A, Kilic U, Rentsch KM, Vig R, Matter CM, et al. ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain. 2008;131:2679–2689. doi: 10.1093/brain/awn222. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- McGuire PG, Rangasamy S, Maestas J, Das A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol. 2011;31:e107–e115. doi: 10.1161/ATVBAHA.111.235408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30:1593–1597. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122:962–975. doi: 10.1111/j.1471-4159.2012.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs) Essays Biochem. 2011;50:179–207. doi: 10.1042/bse0500179. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]