Abstract
Most forms of cerebral ischemia are characterized by damage to the entire neurovascular unit, which leads to an increase in the permeability of the blood–brain barrier (BBB). In response to permanent focal cerebral ischemia in mice, we detected an early concomitant increase in the expression of the vascular endothelial growth factor (VEGF), a key inducer of vascular leakage and pathological blood vessel growth, and of angiopoietin-2 (Ang2), which is closely associated with VEGF in vascular remodeling. Thus, the aim of this study was to evaluate the role of Ang2 alone, or in combination with VEGF, in the acute phase of cerebral ischemia. The effect of these angiogenic factors on the ischemic lesion volume was evaluated by magnetic resonance imaging. We observed that timely administration of VEGF exacerbates ischemic damage. In contrast, Ang2 decreases the ischemic volume and this beneficial effect is maintained in the presence of VEGF. This investigation reports, for the first time, a protective role of Ang2 following cerebral ischemia, an action associated with a reduced BBB permeability. We propose that Ang2 represents a pertinent molecular target for the treatment of cerebral ischemia since acute brain damage may be limited by a pharmacological protection of the vascular compartment.
Keywords: angiopoietins, blood–brain barrier, ischemia, MRI, vascular permeability, VEGF
Introduction
Stroke, a complex and devastating pathology, represents the second most common cause of death with someone succumbing to stroke every 4 minutes throughout the industrialized countries. It is also the leading cause of morbidity with up to 30% of patients permanently disabled.1 Although numerous studies have been conducted in the field of stroke, few therapeutic advances have been made over the last past decades. Cerebral ischemia, caused by either thrombosis or embolism, accounts for 87% of stroke.1 Stroke is characterized by damage to the entire neurovascular unit. Indeed, the deficit in energy supply to a specific brain region leads to neuronal death and a concomitant deleterious increase in blood–brain barrier (BBB) permeability.2 Accordingly, a therapeutic strategy for this pathology might be to limit acute brain damage by promoting not only protection of neuronal cells but also of the juxtaposed cells of the cerebrovascular bed.
Another characteristic of cerebral ischemia is active vascular remodeling, which is observed not only in the acute phase of the pathology, with an impairment of endothelial tight junctions forming the BBB, but also in the later poststroke recovery phase with an increase in neovascular formation by angiogenesis in the periinfarct region.3, 4, 5 Vascular endothelial growth factor (VEGF), the main angiogenic growth factor,6, 7 is a key regulator of this ischemia-induced vascular restructuration. Indeed, in the acute phase of stroke, VEGF is involved in vasogenic edema formation due to its permeabilizing effect.8, 9 Vascular endothelial growth factor also acts on the brain recovering from stroke by stimulating the process of neovascularization.8, 9 This latter effect depends on, and is amplified by another angiogenic factor, angiopoietin-1 (Ang1), which is endowed with antipermeability properties and so counteracts the acute deleterious effects of VEGF.8
Angiopoietin-1 is one of the angiopoietin superfamily, which comprises four members, Ang1 to Ang4; Ang1 and Ang2 are the most studied.10, 11 Angiopoietins bind to the receptor tyrosine kinase Tie2 on endothelial cells and are synergistic with VEGF during developmental and pathological angiogenesis.12 Initially, while Ang1 has been characterized as an agonist for Tie2, Ang2 has been mainly described as an antagonist for this receptor. Over the years, direct effects of Ang2 on endothelial cells have been reported and Ang2 is now known as a context-dependent agonist for Tie2.13, 14, 15 As an example, both pro- and antipermeability properties have been reported for Ang2 in peripheral tissues as a function of the specific microenvironments.16, 17, 18 Furthermore, Ang2 has been described in the brain as an angioneurin, a neologism coined by Zacchigna et al19 to encompass a particular class of molecules that affects both the vascular and the nervous systems. Paradoxically, although Ang2 is commonly described as an angiogenic factor, data reporting a role for this cytokine in the brain mostly relate to the nervous tissue.20, 21, 22, 23, 24 Although some studies indicate that Ang2 might contribute to regeneration within the central nervous system, no one has delineated nor characterized a role for Ang2 in cerebral ischemia, a cytokine characterized by its versatility and pleiotropism.
Accordingly, the aim of this study was to evaluate how this angioneurin affects the neurovascular unit in response to cerebral ischemia.
Materials and methods
In Vivo Studies
Animal studies
The animal investigations were performed under the European directive (86/609/EEC) as enacted in national legislation. The license to investigate was given to SV (14–55) in authorized housing: the Biological Resources Centre (CURB) of the University of Caen (approval D14118015) immediately adjacent to the laboratories of experimental and imaging complex (GIP Cyceron-approval D14118001). The mice were maintained in specific pathogen free housing and were fed laboratory chow and water ad libitum. All experiments were performed on male Swiss mice (25 to 30 g, CERJ, Le Genest-St-Isle, France) under inhalational anesthesia: isoflurane at 5% for induction and 2% for maintenance in 70% N2O/30% O2. Physiological parameters remained in the normal range (body temperature (°C): 37±0.3; PaCO2 (mm Hg): 41±4; PaO2 (mm Hg): 132±4; pH: 7.08±0.08.
Mouse model of permanent middle cerebral artery occlusion
Permanent focal cerebral ischemia was induced by electrocoagulation (occlusion) of the left middle cerebral artery (MCAO) as previously reported.25 In this model, the area of ischemia is restricted to the neocortex. The lesion volume was evaluated 24 hours or 5 days after MCAO by MRI (magnetic resonance imaging) and vascular permeability between 2 and 3 hours after MCAO by MRI and near infrared fluorescence (NIRF). All experiments were randomized and the measurements were made by scientists blinded to the treatment group.
Excitotoxic stress in vivo
Excitotoxic stress was induced in Swiss mice by the intrastriatal injection of NMDA (N-methyl-𝒟-aspartic acid; 10 mmol/L) at coordinates: bregma; 0 mm, parasagittal; 2 mm, and dorsoventral; 4.3 mm. The lesion was evaluated 24 hours after the injection by MRI.
Intracerebroventricular injections
Anesthetized mice were subjected to the intracerebroventricular injection of 3 μL of vehicle (PBS (phosphate-buffered saline) with 1 mg/mL of bovine serum albumin; Sigma-Aldrich, St Quentin-Fallavier, France) or rHuAng2 (8, 40, or 133 μg/mL, R&D Systems, Lille, France) or rHuVEGF (3 μg/mL, Sigma-Aldrich) or both rHuAng2 (40 μg/mL) and rHuVEGF, (n=7 per group) just before either MCAO or the intrastriatal injection of NMDA. The mice were placed on a stereotactic frame and intracerebroventricular injections were made at the following coordinates: bregma; 0 mm, parasagittal; 0.8 mm, and dorsoventral; 3.5 mm.
Magnetic resonance imaging
Experiments were performed on a 7-T Pharmascan horizontal-bore magnet (16 cm bore diameter; Bruker, Ettlingen, Germany). A crosscoil (volume/surface) configuration was used. The mouse was in pronation, its head secured via ear and tooth bars. Respiration was monitored using a pressure-sensitive balloon around the abdomen of the mouse. Pilot imaging was performed for subsequent slice positioning.
Lesion-associated edema was visualized on T2-weighted images (RARE (rapid acquisition with relaxation enhancement), acceleration factor of 8; TR/TEeff=5,000/65 milliseconds; three experiments, 10 slices; resolution=0.078 × 0.078 × 0.5 mm3; acquisition time, 6 minutes).
Changes in vascular permeability were evaluated from signal enhancement between T1-weighted images (RARE, acceleration factor of 4; TR/TE=1,300/7.3 milliseconds; 10 slices; resolution=0.078 × 0.078 × 1.0 mm3; acquisition time, 81 seconds) acquired before and at different times after an injection of the contrast agent Gd-DOTA (Dotarem, Guerbet SA, Villepinte, France, 0.2 mmol/kg). Three precontrast images were acquired before the injection of the contrast agent and seven postcontrast T1-weighted images were acquired (2, 5, 10, 20, 30, 40, and 50 minutes after injection).
Magnetic resonance imaging postprocessing
Image analysis was performed through the use of in-house macros under the ImageJ software (Bethesda, MD, USA). Ischemic lesion volume: The ischemic lesion volume was delineated on T2-weighted images. Vascular permeability: The regions of interest corresponding to the ischemic lesion were delineated on T1 enhanced images {[(postcontrast−precontrast)/precontrast] × 100} at t=50 minutes postcontrast and signal intensity was measured at the different times studied.
Evans blue extravasation
At 2 hours after MCAO, mice were injected in the caudal vein with 100 μL of a solution of 2% Evans blue as a marker of a frank increase in permeability to serum albumin. The mice were intracardially perfused 90 minutes later with NaCl and PBS/4%-paraformaldehyde; the brains were removed. Evans blue diffusion in the brain parenchyma was evaluated both macroscopically and by NIRF (Biospace Lab Photon Imager, Biospace Lab, Paris, France).
Reverse transcription–polymerase chain reaction
Total RNAs were extracted from the ipsilateral and contralateral cortices at 3 or 10 hours after MCAO with the TRIzol Reagent (Fisher Scientific, Illkirch, France) according to the manufacturer's protocol. Reverse transcription–polymerase chain reaction was performed as already described.24 The amount of target gene was given by the formula: 2−(Ct gene of interest−Ct housekeeping), where Ct is the threshold cycle value. Results were expressed relative to the housekeeping gene, cyclophilin. Experiments were repeated at least three times. The primers used are listed in Table 1.
Table 1. Primers used for real-time RT–PCR.
| Ang2 | |
|---|---|
| Forward | 5′-TTAGCACAAAGGATTCGGACAAT-3′ |
| Reverse | 5′-GGACCACATGCGTCAAACC-3′ |
| Claudin5 | |
| Forward | 5′-TAAGGCACGGGTAGCACTCA-3′ |
| Reverse | 5′-GCCCAGCTCGTACTTCTGTG-3′ |
| Cyclophilin | |
| Forward | 5′-TGTCTTTGGAACTTTGTCTGCAA-3′ |
| Reverse | 5′-CAGACGCCACTGTCGCTTT-3′ |
| VE-cadherin | |
| Forward | 5′-TCCTCTGCATCCTCACTATCACA-3′ |
| Reverse | 5′-GTAAGTGACCAACTGCTCGTGAAT-3′ |
| VEGF | |
| Forward | 5′-AAATCACTGTGAGCCTTGTTCAG-3′ |
| Reverse | 5′-GCTGCCTCGCCTTGCA-3′ |
Ang2, angiopoietin-2; RT–PCR, reverse transcription–polymerase chain reaction; VEGF, vascular endothelial growth factor.
In Vitro Studies
Primary neuronal cultures
Primary neuronal cultures were prepared as previously described.26 After 3 days in vitro (DIV), nonneuronal cell division was halted by an exposure to 10 μM cytosine arabinoside. The cultures so treated contained <5% of astrocytes. Cultures were used after 7 DIV for SD (serum deprivation) studies and after 12 DIV for excitotoxic stress studies.
N-methyl-𝒟-aspartic acid excitotoxicity
N-methyl-𝒟-aspartic acid (25 μmol/L) was applied to cultures for 24 hours at 37°C in the presence of glycine (10 μmol/L). rHuAng2 (100 to 800 ng/mL) was coapplied with the excitotoxin and left in the media for 24 hours. Control cultures were submitted to the same procedure but either the addition of rHuAng2 or that of NMDA was omitted. MK-801 (1 μmol/L, R&D Systems), a noncompetitive NMDA antagonist, was used as neuroprotective control. Neuronal death was quantified by the measurement of lactate dehydrogenase released by damaged cells into the culture media.
Statistical analyses
Results are shown as mean±s.d. and analyzed statistically by the Fisher protected least significant difference (PLSD) post hoc test following a significant analysis of variance (P<0.05). All statistics were performed on Statview (SAS, Cary, NC, USA).
Results
Effects of Angiopoietin-2 on Cerebral Ischemia
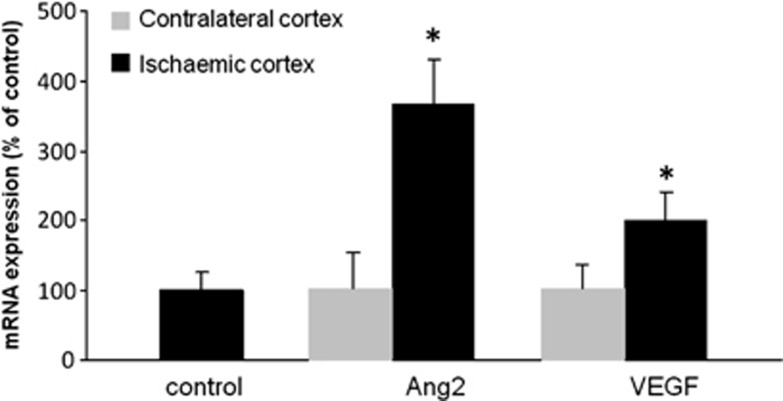
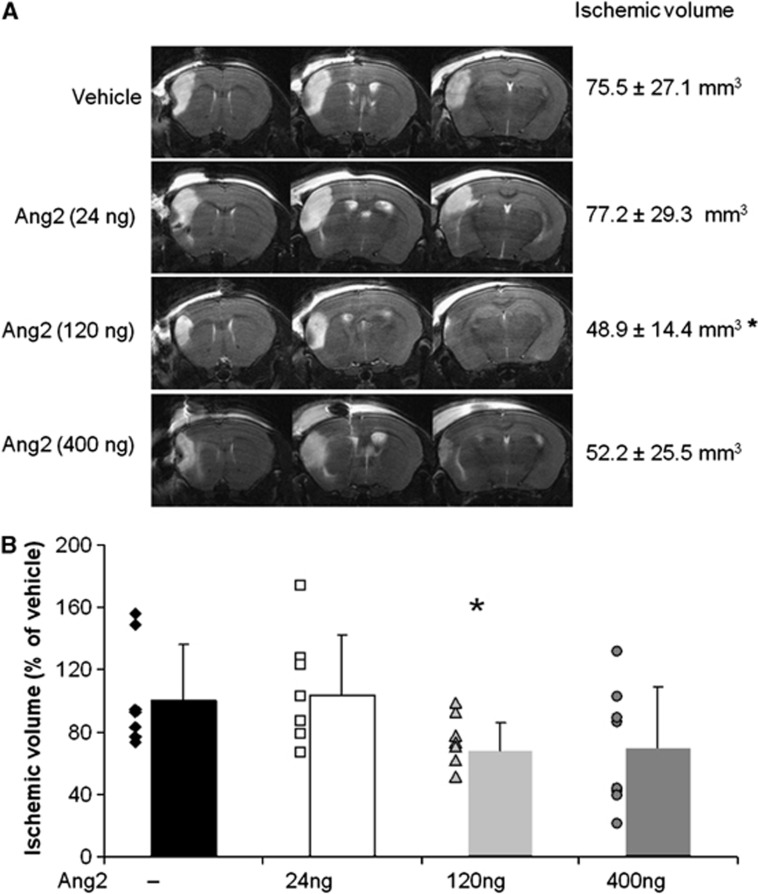
Following the permanent occlusion of the middle cerebral artery, we observed a fourfold increase, in the acute phase of ischemia, in the expression of Ang2 mRNA (Figure 1) in the ischemic cortex in comparison to the contralateral healthy cortex. This overexpression was concomitant with a twofold increase in VEGF mRNA expression (Figure 1), which would suggest that, like VEGF,8 Ang2 contributes to the pathological evolution of the lesion. To verify this hypothesis, we exacerbated this endogenous progression towards infarction by exogenous Ang2 administered intracerebroventricularly22 just before the MCAO. Mice were treated with different doses of Ang2 and brain damage was estimated by measuring, on the T2-weighted MRI images the cortical infarct volume 24 hours after the occlusion (Figures 2A and 2B). Angiopoietin-2 exhibited the common dose-dependent bell-shaped effect of cytokines with the doses tested, from 24 to 400 ng, only the 120 ng dose of Ang2 effected a significant decrease in the lesion volume when compared with the vehicle-treated group (Ang2 group: 48.9±14.4 mm3 versus vehicle-treated group: 75.5±27.1 mm3). This protective effect, although not significant, was also evidenced 5 days after the single injection of Ang2 (−35.1% P=0.09; data not shown).
Figure 1.
Angiopoietin-2 (Ang2) and vascular endothelial growth factor (VEGF) mRNA expression are induced in the injured cortex 10 hours after cerebral ischemia. mRNA were extracted from the ischemic or contralateral cortices 10 hours after middle cerebral artery occlusion (MCAO) and Ang2 and VEGF expression were evaluated. Mean±s.d., n=3 mice/group, *P<0.05 versus both control and contralateral cortices, Fisher protected least significant difference (PLSD) following a significant analysis of variance.
Figure 2.
Dose-dependent effect of different doses of angiopoietin-2 (Ang2) on ischemic volume 24 hours after middle cerebral artery occlusion (MCAO) as determined by T2-weighted magnetic resonance imaging (MRI). (A) Three different coronal planes of T2-weighted images representative of each group. (B) Ischemic volume determined on T2-weighted images. Mean±s.d., Vehicle (n=7), Ang2 24 ng (n=8), Ang2 120 ng (n=7), Ang2 400 ng (n=7), *P<0.05 versus control, Fisher protected least significant difference (PLSD) following a significant analysis of variance.
Effect of Angiopoietin-2 on the Neuronal Compartment
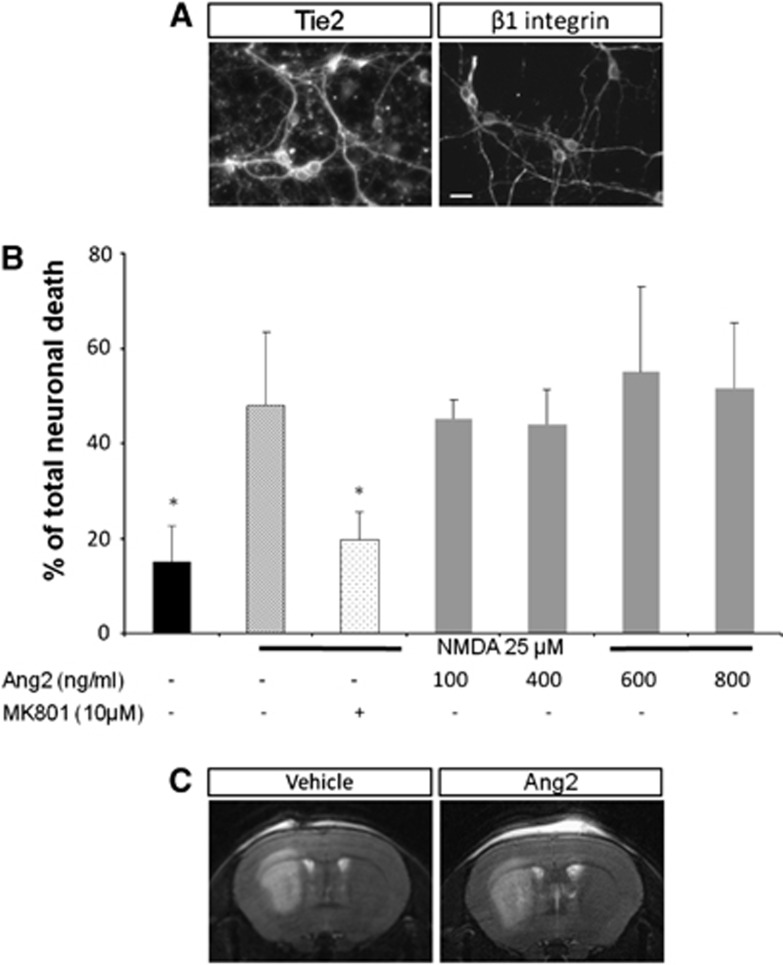
The potential neuroprotective effects of Ang2 were evaluated on both apoptotic and necrotic cell death. In accordance with previously published data,26, 27 we observed in vitro that mature neuronal cells expressed the receptors for Ang2 known as Tie2 and β1 integrin (Figure 3A). Angiopoietin-2 did not protect neurons from apoptosis in vitro (data not shown). In addition, as shown in Figure 3B, whatever the concentrations of Ang2 tested, this cytokine failed to protect neuronal cells from the excitotoxic stress induced by NMDA; an excitotoxicity almost totally antagonized by the coapplication of MK-801. The in vitro lack of neuroprotection with Ang2 was also paralleled by our in vivo results in which Ang2 (120 ng) was without any significant effect on the volume of necrosis induced by the intrastriatal injection of NMDA (Figure 3C).
Figure 3.
Angiopoietin-2 (Ang2) receptors are expressed in primary cultures of neurons but Ang2 does not protect neurons against excitotoxic death. (A) Immunodetection of Tie2 receptor and β1 integrin on neuronal cultures. Scale bar: 20 μm. (B) Lack of effect of Ang2 on excitotoxic cell death induced by a treatment with N-methyl-𝒟-aspartic acid (NMDA) in neuronal cell cultures (12 DIV) assessed by the concentration of lactate dehydrogenase (LDH) released. Mean±s.d., n=3. *P<0.05 versus NMDA, Fisher protected protected least significant difference (PLSD) following a significant analysis of variance. (C) Representative T2-weighted magnetic resonance imaging (MRI) images showing that Ang2 does not protect against excitotoxic death in vivo.
Effect of Angiopoietin-2 on the Vasculature
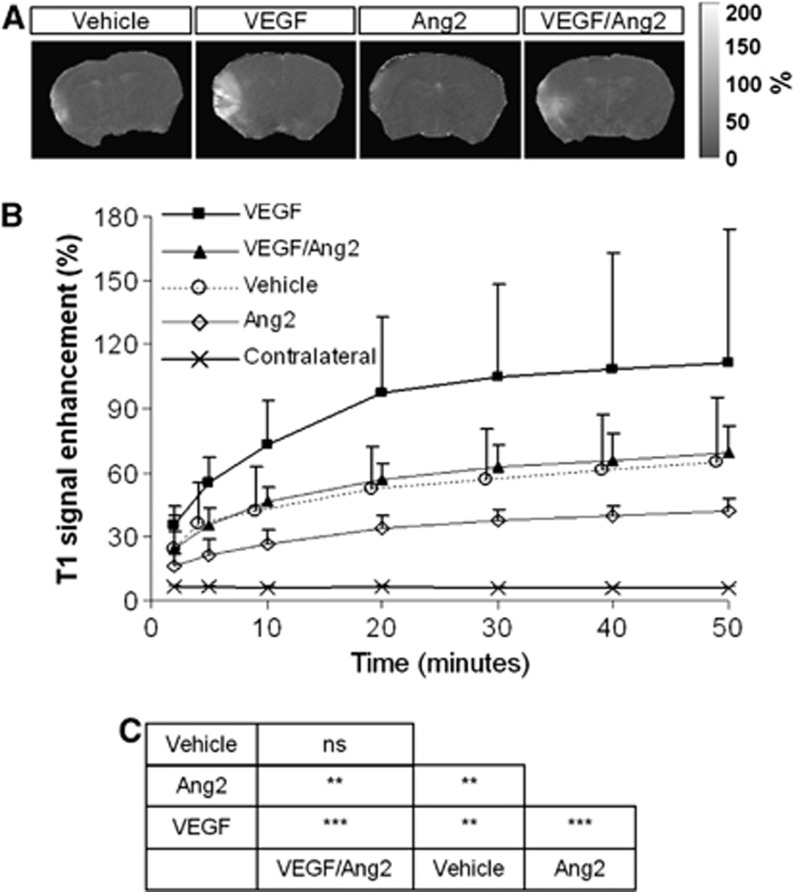
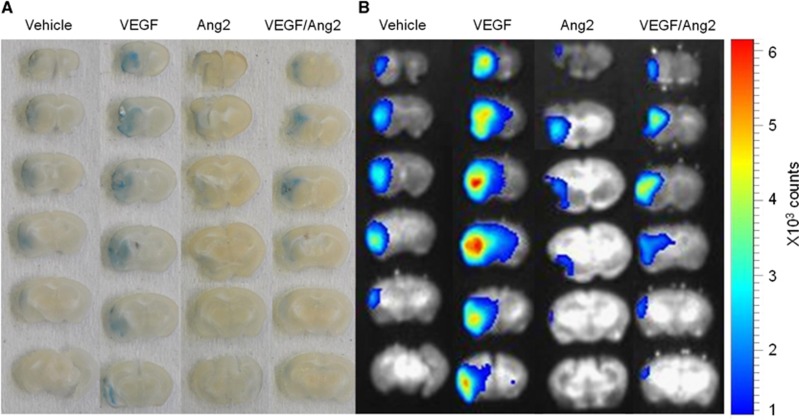
We investigated whether Ang2 or VEGF might modulate vascular permeability, as appreciated by MRI, induced by cerebral ischemia. Mice received an intracerebroventricular injection of either vehicle, Ang2 (120 ng), VEGF or VEGF/Ang2 just before the induction of ischemia. Two hours after MCAO, the contrast agent (Gd-DOTA) was administered and its leakage from the vascular to the parenchymal compartment across the BBB was assessed at 2, 5 minutes, and then at every 10 minutes for 50 minutes. As shown in Figures 4A and 4B, in control occluded mice, BBB permeability was increased over time in the ischemic cortex compared with the contralateral cortex, with, at 50 minutes post-MCAO, a value of T1 enhancement of 6.1%±0.5% in the contralateral cortex compared with 60.9%±30.0% in the ischemic cortex. The administration of VEGF led to a two-fold increase in T1 enhancement in the ischemic cortex (Figures 4A and 4B) since the value measured at 50 minutes post-MCAO was 111.5%±62.3%. In contrast, Ang2 administration led to a significant decrease in vascular leakage (T1 enhancement value: 42.0%±6.0%) compared with vehicle and VEGF groups. Importantly, the presence of Ang2 totally reversed VEGF-induced BBB permeability (T1 enhancement: 69.0%±12.8% for the VEGF/Ang2 group) (Figures 4A and 4B). By analysis of variance, all treatment groups displayed signal enhancement with respect to the contralateral hemisphere and all treatment groups differed between themselves with the notable exception of the VEGF/Ang2 versus the vehicle treatments (Figure 4C). Similar results were obtained when BBB permeability was evaluated on the basis of the extravasation of Evans blue, which binds to serum albumin and thus reflects the leakage of high-molecular weight molecules. Indeed, while VEGF seriously increased Evans blue extravasation into the brain parenchyma compared with vehicle-treated mice, Ang2 administration had the contrary effect and reversed the nefarious impact of VEGF on the BBB (Figures 5A and 5B).
Figure 4.
Angiopoietin-2 (Ang2) decreases the passage of molecules of low-molecular weight across the blood–brain barrier (BBB) after ischemia in the presence or not of vascular endothelial growth factor (VEGF). (A) Representative images of the T1 signal enhancement obtained from T1-weighted magnetic resonance imaging (MRI) images. (B) Quantification of the signal evolution over time. Mean±s.d., n=3 mice/group. (C) Statistical analysis, **P<0.01, ***P<0.001; Contralateral was significantly different from all other conditions tested with P<0.001; Fisher protected least significant difference (PLSD) following a significant analysis of variance.
Figure 5.
Angiopoietin-2 (Ang2) decreases the passage of molecules of high-molecular weight across the blood–brain barrier (BBB) after ischemia in the presence or not of vascular endothelial growth factor (VEGF). Visualization of Evans blue extravasation in coronal sections of a representative mouse brain from each treatment group at 3 hours after middle cerebral artery occlusion (MCAO) at macroscopical level (A) and by near infrared fluorescence (NIRF) (B).
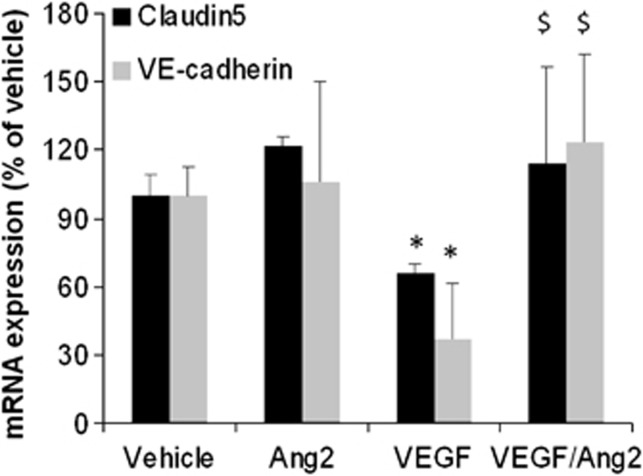
To determine how Ang2 modulates vascular permeability, we assessed whether Ang2 influences the expression of proteins involved in the junctions of the BBB on ischemic cortices. We focused on the tight junction proteins occluding, claudin5 and zonula occludens 1 and the adherens junction protein VE-cadherin. Vascular endothelial growth factor induced a decrease in mRNA expression of claudin5 and VE-cadherin (Figure 6) and Ang2 alone had no effect on the expression of these junctional proteins. However, when associated with VEGF, Ang2 was able to totally attenuate the VEGF-induced decrease in their expression (Figure 6). In contrast, neither VEGF nor Ang2 modulated mRNA expression of occludin and zonula occludens 1 (data not shown).
Figure 6.
Angiopoietin-2 (Ang2) fails to influence mRNA expression of blood–brain barrier (BBB) proteins but counteracts vascular endothelial growth factor (VEGF)-induced decrease in their expression. mRNA were extracted from the ischemic cortices 3 hours after middle cerebral artery occlusion (MCAO) and the expression of claudin5 and VE-cadherin mRNA was evaluated. Mean±s.d., n=3 mice/group, *P<0.05 versus control, $P<0.05 versus VEGF, Fisher protected least significant difference (PLSD) following a significant analysis of variance.
Modulation of the Ischemic Volume (Assessed by T2w Imaging) by Angiopoietin-2 and Vascular Endothelial Growth Factor
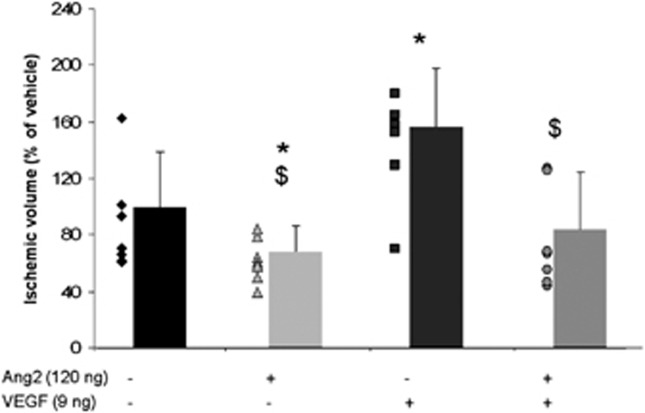
Finally, the effect of a coadministration of Ang2 and VEGF on the MCAO-induced lesion was evaluated and compared with the effect obtained in response to an administration of each factor alone. Interestingly, the effects of VEGF and/or Ang2 on the ischemic volume parallel those observed on vascular permeability. In accordance with our previous data,8 the measure of lesion volume, 24 hours after occlusion, showed that VEGF exacerbated the lesion since an increase of 57% was measured compared with the vehicle-treated group (Figure 7). Conversely, a beneficial effect of Ang2 was observed when the cytokine was administered alone (Figure 2) or in presence of VEGF, which again would suggest that Ang2 significantly reverses the deleterious effects of VEGF (Figure 7).
Figure 7.
Deleterious effect of vascular endothelial growth factor (VEGF) on ischemic volume is counteracted by angiopoietin-2 (Ang2). Ischemic volume determined on T2-weighted images. Mean±s.d., Vehicle (n=7), VEGF (n=7), Ang2 (n=7), VEGF/Ang2 (n=8), *P<0.05 versus control, $P<0.05 versus VEGF, Fisher protected least significant difference (PLSD) following a significant analysis of variance.
Discussion
The loss of BBB integrity (both functional and structural) is a hallmark of a cerebrovascular attack; this loss contributes to the evolution of the stroke from the initial ischemia to the consolidated infarction. Free radicals, proteases, and cytokines may mediate the increase in capillary permeability.28 Among the cytokines, several authors including ourselves have shown that a precocious increase in VEGF expression in the injured brain may be related to BBB disruption after focal cerebral ischemia. When we studied the expression pattern of angiogenic factors in response to cerebral ischemia, we showed that, in accordance with the previous data,29, 30 as well as VEGF, Ang2 was induced early but not in a sustained manner by the ischemic insult. The transient expression of VEGF is now clearly known to contribute to cerebral damage. In contrast, the impact of Ang2 in the acute phase of ischemia is poorly documented. Indeed, although Liu et al21 suggest that endogenous Ang2, like VEGF,31 might be involved in poststroke neurogenesis, to date, there has been no report on a direct effect of Ang2 in the processes that govern the transition of ischemic towards necrotic tissues.
The present study highlights the fact that exogenous Ang2 is a protective factor during the acute phase of cerebral ischemia. This hitherto unknown effect of Ang2 is dose-dependent and observed early after the induction of cerebral ischemia. The decrease in the ischemic volume observed in the presence of Ang2 could—in principle—be due to a protective effect on the vascular compartment as the cytokine obtunds the increase in ischemia-induced vascular permeability. We demonstrated that Ang2 is also able to abolish effects of VEGF on both lesion volume and vascular permeability. The latter effect may be related to a modulation in the expression of junction proteins that are the capital anatomical features of the BBB. Following severe cerebral ischemia, neuronal cell death is accompanied by an increase in vascular permeability. Previous studies in our laboratory, on the same murine model of permanent focal cerebral ischemia, have shown significant damage to the BBB 24 hours after the occlusion but this phenomenon is initiated as early as 2 hours after ischemia.8 Within this time window, BBB leakiness could be a consequence of the increase in VEGF expression, an increase concomitant to that of Ang2. The permeabilizing effect of VEGF has been described in several models of cerebral ischemia and is counteracted by Ang1.8, 32, 33 An unexpected and interesting finding in our study is that our data resemble those obtained for Ang1, despite the fact that Ang1 and Ang2 are usually described as agonists and antagonists in angiogenesis. Indeed, if Ang1 is known to stabilize blood vessels in the brain as well as in peripheral tissues, Ang2 is normally thought to be a factor that favors vascular permeability. However, the effect of Ang2 remains controversial since both pro- and antipermeabilizing effects have been reported for this cytokine.14, 16, 17, 18 Antipermabilizing effects were observed in physiological and in inflammatory contexts or during tumor growth. With respect to models of glioma, we have demonstrated that an overexpression of Ang2 by brain tumor cells participates in vascular normalization and, in particular, reduces their permeability,18 whereas other authors have observed the opposite effect,34 a dichotomy that could be explained by the context-dependent behavior of Ang2. These changes in the behavior of Ang2 could be explained by a differential expression of its receptor Tie2 and integrins,35 as well as by the oligomerization state of Ang2.36 Indeed, multimeric structures of Ang2 can induce responses similar to Ang1 in contrast to lower states of oligomerization.
At the molecular level, an early event involved in the increase of BBB permeability is the modulation of the endothelial expression of junction proteins. Vascular endothelial growth factor is known to limit the expression of such junction proteins.17, 37, 38 In parallel to its permeability effect, we observed that VEGF decreases claudin5 and VE-cadherin expression in the ischemic cortices and this effect was abolished in the presence of Ang2. These effects are similar with those reported by Lee et al,32 who showed that Ang1 counteracts the VEGF-induced increase in vascular permeability by upregulating tight junction protein zonula occludens-2. Interestingly and in contradistinction to Ang2, Ang1 displayed no protective effect when administered alone.8 A therapeutic effect during cerebral ischemia could be the result of both vasoprotection and neuroprotection. Along with others, we have recently reported that Ang2, like Ang1, could be considered as an angioneurin, a word that has been coined to designate molecules that possess both vaso- and neuroactivities.19, 21, 22, 23, 24 We and others have previously shown that neurons express a functional Tie2 receptor and Ang1 protects these cells from apoptosis,26, 39 a similar protection is seen in endothelial cells.40 If this antiapoptotic role was first described specifically for Ang1 on endothelial cells, a similar activity has been ascribed to Ang2 in these cells.10, 14, 40 However, in contrast to Ang1, the neuronal effects of Ang2 do not include neuroprotection and the beneficial effect of Ang2 in cerebral ischemia is restricted to its vasoprotective capacity.
To conclude, our data highlight an original biological role for Ang2 in cerebral ischemia due to a protection of the vascular unit and to a mitigation of VEGF-induced vascular permeability. These results illustrate and reinforce studies that show the particular behavior of Ang2 that acts in a dichotomous manner as a function of the context studied.14, 15, 40 Based on our results, we propose that Ang2 might represent a worthwhile molecular target for cerebral ischemia with, as a therapeutic strategy, a limitation of the brain damage by the protection of vascular compartment.
The authors declare no conflict of interest.
Footnotes
This study was partly supported by the French Centre National de la Recherche Scientifique (CNRS), by the University of Caen Basse-Normandie and by the European Union-Fonds Européen de Développement Régional (FEDER). LM was supported by the French Ministère de l'Education, de la Recherche et de la Technologie. This work was realized as part of an inter-regional research program: TC2N (Trans Channel Neuroscience Network).
References
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanwela N, Koroshetz WJ. Acute ischemic stroke: overview of recent therapeutic developments. Annu Rev Med. 2007;58:89–106. doi: 10.1146/annurev.med.58.070605.115306. [DOI] [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008;39:1563–1568. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KL, Shin I, Kim J, Choi J, Byun J, Jeon E, et al. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc Res. 2006;72:394–402. doi: 10.1016/j.cardiores.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83:852–859. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA. 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lee C, Shen F, Du R, Young WL, Yang G. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- Valable S, Eddi D, Constans J, Guillamo J, Bernaudin M, Roussel S, et al. MRI assessment of hemodynamic effects of angiopoietin-2 overexpression in a brain tumor model. Neuro Oncol. 2009;11:488–502. doi: 10.1215/15228517-2008-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Ward NL, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, et al. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009;284:22680–22689. doi: 10.1074/jbc.M109.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, et al. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci USA. 2009;106:13570–13575. doi: 10.1073/pnas.0905125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rueger MA, Park DM, Boyd JD, Padmanabhan R, Campanati L, et al. Angiogenic factors stimulate growth of adult neural stem cells. PLoS One. 2010;5:e9414. doi: 10.1371/journal.pone.0009414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau L, Pacary E, Valable S, Bernaudin M, Guillemot F, Petit E. Angiopoietin-2 regulates cortical neurogenesis in the developing telencephalon. Cereb Cortex. 2011;21:1695–1702. doi: 10.1093/cercor/bhq243. [DOI] [PubMed] [Google Scholar]
- Welsh FA, Sakamoto T, McKee AE, Sims RE. Effect of lactacidosis on pyridine nucleotide stability during ischemia in mouse brain. J Neurochem. 1987;49:846–851. doi: 10.1111/j.1471-4159.1987.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Valable S, Bellail A, Lesné S, Liot G, Mackenzie ET, Vivien D, et al. Angiopoietin-1-induced phosphatidyl-inositol 3-kinase activation prevents neuronal apoptosis. FASEB J. 2003;17:443–445. doi: 10.1096/fj.02-0372fje. [DOI] [PubMed] [Google Scholar]
- Rosa AI, Gonçalves J, Cortes L, Bernardino L, Malva JO, Agasse F. The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. J Neurosci. 2010;30:4573–4584. doi: 10.1523/JNEUROSCI.5597-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and timps are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR. Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim WJ, Jun H, Choi YK, Kim K. Angiopoietin-1 reduces vascular endothelial growth factor-induced brain endothelial permeability via upregulation of ZO-2. Int J Mol Med. 2009;23:279–284. [PubMed] [Google Scholar]
- Zhao Y, Li Z, Wang R, Wei J, Li G, Zhao H. Angiopoietin 1 counteracts vascular endothelial growth factor-induced blood-brain barrier permeability and alleviates ischemic injury in the early stages of transient focal cerebral ischemia in rats. Neurol Res. 2010;32:748–755. doi: 10.1179/016164109X12445616596562. [DOI] [PubMed] [Google Scholar]
- Chae S, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AMA, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16:3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilä R, Nätynki M, Tammela T, Kangas J, Pulkki KH, Limaye N, et al. Ligand oligomerization state controlsTie2 receptor trafficking and angiopoietin-2-specific responses. J Cell Sci. 2012;125:2212–2223. doi: 10.1242/jcs.098020. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Wu L, Qi X, Shaw L, Li Calzi S, Caballero S, et al. Placenta growth factor-1 exerts time-dependent stabilization of adherens junctions following VEGF-induced vascular permeability. PLoS One. 2011;6:e18076. doi: 10.1371/journal.pone.0018076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bai Y, Meng Z, Cui M, Zhang X, Chen F, Xiao J, et al. An Ang1-Tie2-PI3K axis in neural progenitor cells initiates survival responses against oxygen and glucose deprivation. Neuroscience. 2009;160:371–381. doi: 10.1016/j.neuroscience.2009.01.076. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]