Abstract
Cerebral hypoxia and acidosis can follow traumatic brain injury (TBI) and are associated with increased mortality. This study aimed to evaluate a relationship between reduced pHbt and disturbances of cerebral metabolism. Prospective data from 56 patients with TBI, receiving microdialysis and Neurotrend monitoring, were analyzed. Four tissue states were defined based on pHbt and PbtO2: 1—low PbtO2/pHbt, 2—low pHbt/normal PbtO2, 3—normal pHbt/low PbtO2, and 4—normal pHbt/PbtO2). Microdialysis values were compared between the groups. The relationship between PbtO2 and lactate/pyruvate (LP) ratio was evaluated at different pHbt levels. Proportional contribution of each state was evaluated against mortality. As compared with the state 4, the state 3 was not different, the state 2 exhibited higher levels of lactate, LP, and glucose and the state 1—higher LP and reduced glucose (P<0.001). A significant negative correlation between LP and PbtO2 (rho=−0.159, P<0.001) was stronger at low pHbt (rho=−0.201, P<0.001) and nonsignificant at normal pHbt (P=0.993). The state 2 was a significant discriminator of mortality categories (P=0.031). Decreased pHbt is associated with impaired metabolism. Measuring pHbt with PbtO2 is a more robust way of detecting metabolic derangements.
Keywords: brain ischemia, brain trauma, energy metabolism, microdialysis, neurocritial care, pH
Introduction
Following head trauma, brain injury occurs due to the damage of the primary insult followed by potential secondary injury, which may be a result of both ischemic and nonischemic mechanisms. This may contribute to a further loss of potentially viable cerebral tissue thereby decreasing the chances of a good functional outcome. Multimodality neuromonitoring is aimed at early detection of unfavorable physiological conditions, leading to decreased cerebral perfusion or oxygenation, as well as biochemical derangements associated with disturbed cellular metabolism. While low cerebral oxygen tension (PbtO2) is a common pathological finding following severe traumatic brain injury (TBI), the presence of cerebral hypoxia does not universally correlate with deranged cerebral metabolism or neurological outcome. Furthermore, impaired energy metabolism does not invariably improve after induced increases in PbtO2.1 This may be due to the limitations of the monitoring technology, which samples only a small area of extracellular tissue; relatively short duration or tolerable levels of hypoxia, which do not result in metabolic disturbances as well as the presence of nonischemic metabolic perturbations.2, 3
Both ischemic and nonischemic causes of deranged metabolism can lead to cerebral acidosis, which contributes to cerebral tissue injury via a variety of pathophysiological mechanisms. These include (1) increased permeability of the calcium acid-sensing ion channels,4, 5 leading to calcium influx and neuronal death; (2) contribution of acidosis to oxidative6 and excitotoxic injury7; (3) impaired synaptic conduction and long-term potentiation8; (4) reduced glutamate uptake by astrocytes9 and decreased capacity of astrocytes to maintain glucose metabolism during hypoxia10; (5) blood brain–barrier disruption11 and effects on mitochondrial function via an increased opening of the permeability transition pore,12 etc. Although many of the mechanisms above rely on intracellular pH changes, it has been shown that an intracellular pH is strongly dependent on the regulation of extracellular pH.13
Despite differences in the methods of measurement, there is ample evidence in human studies as well as in experimental models of ischemia,14, 15 for the temporary reduction in cerebral extracellular pH (pHbt) after cerebral insults such as TBI16, 17, 18, 19 and subarachnoid hemorrhage.15, 20 It has also been previously demonstrated that following human TBI, acidosis is associated with deranged lactate metabolism and increased mortality.18, 21, 22 However, the relationship between hypoxia and acidosis and their impact on energy metabolism, including other biochemical markers, in our view warrants further exploration. The aim of the present study was, therefore, to test the hypothesis that the presence of cerebral acidosis with or without hypoxia indicates a more profound metabolic derangement, compared with the periods of hypoxia without acidosis or when normal oxygenation and pH are present.
Materials and methods
Neuromonitoring data were prospectively collected from the 56 patients with TBI who met the following criteria:
Inclusion criteria: patients with isolated or combined TBI, who required management in neurocritical care unit and intracranial pressure (ICP) monitoring based on existing guidelines23, 24 and in whom duration of monitoring was expected to exceed 48 hours.
Exclusion criteria: moribund patients, unlikely to survive >24 to 48 hours; refractory blood coagulation disorders that precluded safe insertion of intraparenchymal monitors and patients with penetrating brain injuries, patients younger than 16 years of age (as per general neurocritical care unit admission policy).
Data collection was approved by the Cambridgeshire Local Research Ethics Committee and Research and Development Unit at Addenbrooke's hospital as part of an ongoing ‘Blood flow, swelling and metabolism after head injury' project (protocol 30). An informed assent was obtained from the next of kin or patients' representative in all cases authorizing data collection and Neurotrend sensor (Codman, Raynham, MA, USA) insertion. All consecutive patients, who fulfilled the above criteria and for whom assent could be obtained, were included in the study and no upper age limit was used, other than guided by clinical indications.
All patients were managed according to the standard stepwise management protocol25 with escalation of treatment when ICP (<20 mm Hg) and cerebral perfusion pressure (CPP) (>70 mm Hg) targets were not met. Treatment options included analgesia and sedation, head elevation, muscular relaxants, intravenous colloids or crystalloids, mannitol and hypertonic saline, external ventricular drainage of CSF, moderate hyperventilation with PaCO2≥4.0 kPa, mild-to-moderate hypothermia (35°C to 33°C), inotropes, barbiturates, and decompressive craniectomy.
Monitoring in all patients included mean arterial pressure, ICP, microdialysis, and brain tissue parameters (partial pressure of brain tissue oxygen (PbtO2), carbon dioxide (PbtCO2), and cerebral extracellular pH (pHbt)). In all patients, mean arterial pressure was measured via an arterial line. A parenchymal ICP sensor (Codman), microdialysis catheter (CMA70 and CMA71, CMA Microdialysis, Solna, Sweden) and Neurotrend sensor were inserted via a triple lumen cranial access device (Technicam, Newton Abbot, UK) placed by default in the right frontal region, unless a different position was required for clinical reasons. Microdialysis catheters were perfused with CMA perfusion fluid at a standard rate of 0.3 μL/min and collecting vials were exchanged hourly. Bedside analysis for microdialysis markers was performed by a CMA600 analyzer (CMA Microdialysis) and included extracellular glucose, lactate, pyruvate, glycerol, and glutamate, as well as derived ratios of lactate-to-pyruvate (LP) and lactate-to-glucose (LG). The position of the microdialysis catheter and Neurotrend sensor were confirmed on the next clinical CT (computed tomography) scan and classified according to the proximity of monitor's tip to the traumatic parenchymal lesions as pericontusional (within 5 to 15 mm) or less injured brain (including diffuse injury). No probe was placed directly into the cerebral contusion. All neuromonitoring data were continuously, digitally captured on a bedside computer using ICM+ software.26 At the time of data collection PbtO2 and microdialysis parameters were used as a second-tier and predominantly research monitoring modalities and no direct or protocol-driven clinical treatment interventions (e.g., hyperoxia) were made to correct low PbtO2. However, the observed abnormal values in many instances could influence an aggressiveness of CPP augmentation or ICP lowering maneuvers on an individual patient basis.
Data Processing and Analysis
All monitoring data were combined in a single data set. Artifacts and values outside of the analytical range of the microdialysis analyzer and Neurotrend sensor were excluded. Neurotrend values during the first 2 hours after insertion were not used. The time of microdialysis sample analysis was set back by 17 minutes to allow for transit time from the catheter tip to the vial. ICP, CPP, PbtO2, PbtCO2, and pHbt values, captured at 1-second intervals were averaged to match microdialysis sampling rate, resulting in hourly data points for all included parameters. To avoid potential bias due to the variable length of monitoring in different patients, as well as to prevent possible drift in monitoring values with time and to focus on the most acute stage of pathophysiological response to brain injury, only the first 72 hours of monitoring for each patient were included in the analysis. Four cerebral tissue states were defined, based on PbtO2 and pHbt values:
State 1: low PbtO2 and pHbt
State 2: low pHbt and normal PbtO2
State 3: normal pHbt and low PbtO2
State 4: normal pHbt and PbtO2
Thresholds between ‘normal' and ‘low' PbtO2 and pHbt were defined as follows: PbtO2 <1.5 kPa (≈11 mm Hg) was considered abnormally low and above 1.5 kPa as normal. Previous studies indicated different median human pHbt values as well as thresholds between mortality or functional outcome groups, with the range of discriminating pHbt values from 7.0 to 7.2.18, 21, 22 This values may depend on method of measurement21 as well as timing of assessment.18 In view of lack of universally accepted pHbt thresholds exist, we have based pHbt stratification on mean-1 s.d. pHbt values from the data set calculated separately for less injured (need to define this more) and pericontusional tissue, which also corresponded to the level defining the lowest 25 percentile. Based on this method, pHbt <7.15 and <7.1 were considered low for less injured and pericontusional tissue, respectively.
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). The values of microdialysis glucose, lactate, pyruvate, LP ratio, and LG ratio were compared between four tissue state categories, using one-way ANOVA (analysis of variance). Biochemical values during all three ‘abnormal' tissue states were also compared to periods with normal PbtO2 and pHbt, using Tamhane's T2 method for post hoc multiple comparisons correction. To further reduce individual patient's bias as well as to account for an influence of CPP, ICP, and PbtCO2, a mixed linear model approach was used to compare groups in a similar manner.
Spearman's rank correlations between PbtO2 and LP ratio were evaluated for the whole data set and separately at low and normal pHbt levels.
In addition, the percentage of monitoring time spent in each tissue state was calculated for all patients, and assessed against mortality and Glasgow Outcome Scale Score (GOS)-based functional outcome categories, using Mann–Whitney U test. The relationship between patients clinical and demographic characteristics and percentage of monitoring time in each state was also evaluated. P value <0.05 was considered significant for all statistical tests.
Results
Out of 56 recruited patients, 43 (77%) were males and 13 (23%) were females. Median (interquartile range) age of patients was 38.5 (25; 52) years. Based on postresuscitation preintubation Glasgow Coma Scale (GCS) score, 46 (82%) patients sustained severe (GCS 3 to 8), 8 (14%) moderate (GCS 9 to 12), and 2 (4%) mild (GCS 13 to 15) injury. Out of the two patients with initially mild TBI, one subsequently deteriorated neurologically and the other had significant extracranial injury and abnormal CT brain scan fulfilling criteria of neurocritical care management and invasive neuromonitoring. Major extracranial injury was present in 21 (28%) patients. According to the Marshall CT classification at the time of recruitment, 30 (54%) patients had diffuse brain injury, 16 (29%)—evacuated mass lesion, and 10 (17%)—nonevacuated mass lesion. A microdialysis catheter was placed in less injured brain in 35 (63%) patients and in a pericontusional cerebral tissue in the remaining 21 (37%) patients. According to the GOS at 6 months, 14 (25%) patients died, 1 (2%) patient was in a vegetative state, 15 (27%) patients had severe disability, 17 (31%) patients moderate disability, and 8 (15%) patients had a good recovery. All of the 14 deceased patients died during their stay in the neurocritical care unit—6 due to refractory ICP and 8 due to severe structural brain injury with failure to improve neurologically, despite prolonged weaning of sedation; in four patients systemic injuries or sepsis, contributed to their mortality.
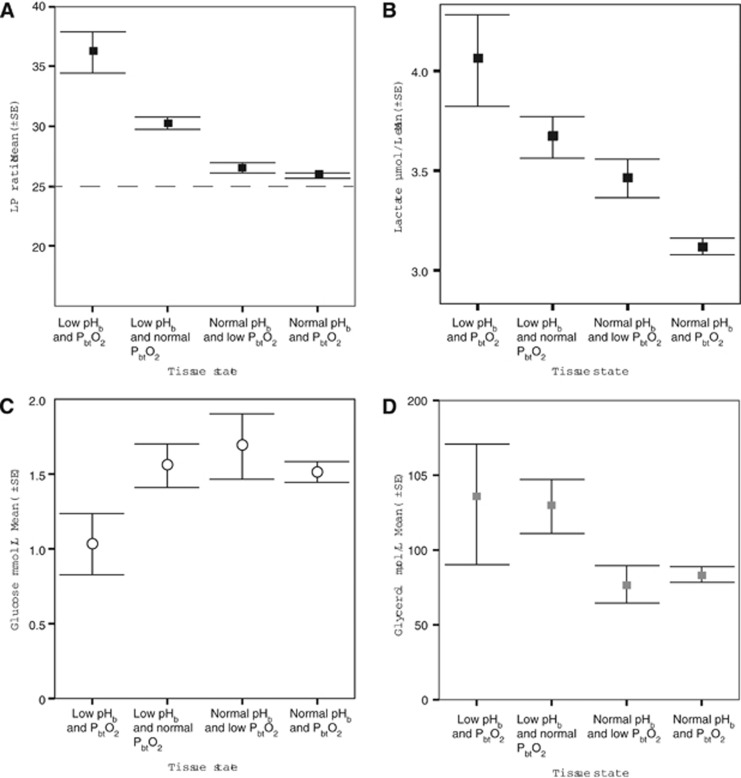
Following removal of the artifacts and the missing data a total of 2,665 hours of monitoring were available for analysis. Proportions of monitoring time belonging to each tissue state, as described above, are presented in Table 1. Mean (±s.d.) values of pHbt and PbtO2 for each tissue state were as follows (respectively): state 1 to 7.05 (±0.1) and 0.9 (±0.5) kPa (6.8 (±3.8) mm Hg), state 2 to 7.08 (±0.06) and 3.0 (±1.0) kPa (22.5 (±7.5) mm Hg), state 3 to 7.24 (±0.06) and 0.99 (±0.4) kPa (7.42 (±3.0) mm Hg) and state 4 to 7.24 (±0.07) and 3.4 (±1.4) kPa (25.5 (±10.5) mm Hg).
Table 1. Proportions of monitoring time belonging to each tissue state category.
| Tissue state | Tissue state 1 low pHbt and low O2 | Tissue state 2 low pHbt and normal PbtO2 | Tissue state 3 normal pHbt and low PbtO2 | Tissue state 4 normal pHbt and normal PbtO2 |
|---|---|---|---|---|
| Overall | 2.6% (68) | 16% (426) | 13.4% (356) | 68% (1,815) |
| Pericontusional brain tissue | 3.8% (40) | 15% (157) | 16.6% (173) | 64.6% (675) |
| Less injured brain tissue | 1.7% (28) | 16.6% (269) | 11.3% (183) | 70.4% (1,140) |
Initial comparison of microdialysis values between the tissue groups demonstrated significant overall difference between them for glucose, lactate, glycerol, and LP ratio (ANOVA) Figures 1A–1D, and no difference in pyruvate, glutamate or LG ratio. Post hoc testing, performed as comparison against the normal tissue state 4 as a control, suggested that only tissue state 1 (low pHbt and PbtO2) and tissue state 2 (low pHbt and normal PbtO2) were biochemically different in terms of all three microdialysis markers, with the exception of lactate, which was significantly higher in all tissue state 1 to 3 groups, when compared with the normal tissue state 4. Analysis of key monitoring parameters, which could have had an impact on cerebral biochemistry and pHbt—CPP, ICP, PbtCO2—also showed that they differed between the four tissue state groups, although the CPP and ICP remained well within the clinical target range. However, mixed model analysis, controlling for ICP, CPP and PbtCO2 as well as individual patient variability suggested that LP ratio was still significantly higher in tissue state 1 and 2 groups, as compared with the state 4. In addition, lactate and glucose were both higher in the tissue state 2 (low pHbt, but normal PbtO2), when compared with the normal tissue state 4. The observed and predicted values of microdialysis markers and other monitoring parameters as well as significance values of statistical tests are presented in Table 2.
Figure 1.
Mean (±s.e.) values of (A) lactate/pyruvate (LP) ratio, (B) cerebral extracellular lactate, (C) extracellular glucose, and (D) extracellular glycerol by the tissue state categories.
Table 2. The values of microdialysis and other monitoring parameters by pHbt/PbtO2 tissue state category.
| Monitoring parameter | Tissue state 1 low pHbtand low PbtO2 | Tissue state 2 low pHbtand normal PbtO2 | Tissue state 3 normal pHbtand low PbtO2 | Tissue state 4 normal pHbt and normal PbtO2 | ANOVA or mixed linear model significance |
|---|---|---|---|---|---|
| Glucose (mmol/L) n=2,586 | |||||
| Observed | 1.0 (±0.8) | 1.6 (±1.5) | 1.7 (±2.0) | 1.5 (±1.5) | P=0.021 |
| Predicted | 1.1 (±0.2) | 1.5 (±0.2) | 1.2 (±0.2) | 1.3 (±0.2) | P<0.001 |
| Lactate (μmol/L) n=2,665 | |||||
| Observed | 4.1 (±1.9) | 3.7 (±2.1) | 3.5 (±1.8) | 3.1 (±1.8) | P<0.001 |
| Predicted | 3.7 (±0.2) | 3.8 (±0.2) | 3.7 (±0.2) | 3.6 (±0.2) | P=0.015 |
| Pyruvate (mmol/L) n=2,641 | |||||
| Observed | 110.1 (±39) | 124.9 (±79) | 127.8 (±57) | 123.4 (±65) | P=0.221 |
| Predicted | 140 (±9.3) | 149.1 (±8.8) | 143 (±8.3) | 145.5 (±8.2) | P=0.104 |
| L/P ratio n=2,624 | |||||
| Observed | 36.2 (±13.9) | 30.3 (±10.4) | 26.5 (±7.9) | 25.9 (±8.6) | P<0.001 |
| Predicted | 29.1 (±2.0) | 27.1 (±1.9) | 26.3 (±1.9) | 25.5 (±1.8) | P<0.001 |
| Glycerol (μmol/L) n=2,085 | |||||
| Observed | 130.4 (±154) | 129.3 (±165.1) | 76.9 (±105.9) | 83.5 (±103.4) | P<0.001 |
| Predicted | 206.9 (±71.9) | 222.5 (±71.5) | 198.0 (±71.1) | 210.4 (±71.0) | P=0.041 |
| CPP (mm Hg) | |||||
| Observed | 72.4 (±8.6 ) | 75.1 (±8.0) | 80.6 (±10.8) | 80.6 (±10.8) | P<0.001 |
| ICP (mm Hg) | |||||
| Observed | 18.5 (±8.3) | 18.5 (±8.3) | 18.6 (±8.4) | 16.9 (±5.5) | P<0.001 |
| PbtCO2 (mm Hg) | |||||
| Observed | 17.2 (±6.7) | 6.9 (±1.4) | 6.9 (±1.2) | 6.2 (±0.8) | P<0.001 |
ANOVA, analysis of variance; L/P, lactate/pyruvate; ICP, intracranial pressure; CPP, cerebral perfusion pressure.
Observed values represent mean (±s.d.), ANOVA is used for significance testing, numbers in bold correspond to values remaining significant after post hoc multiple comparison between groups (Tamhane's T2 method); predicted values represent estimated marginal means (±s.e.) produced by the mixed linear model, with values in bold remaining significant after Bonferroni correction for multiple testing.
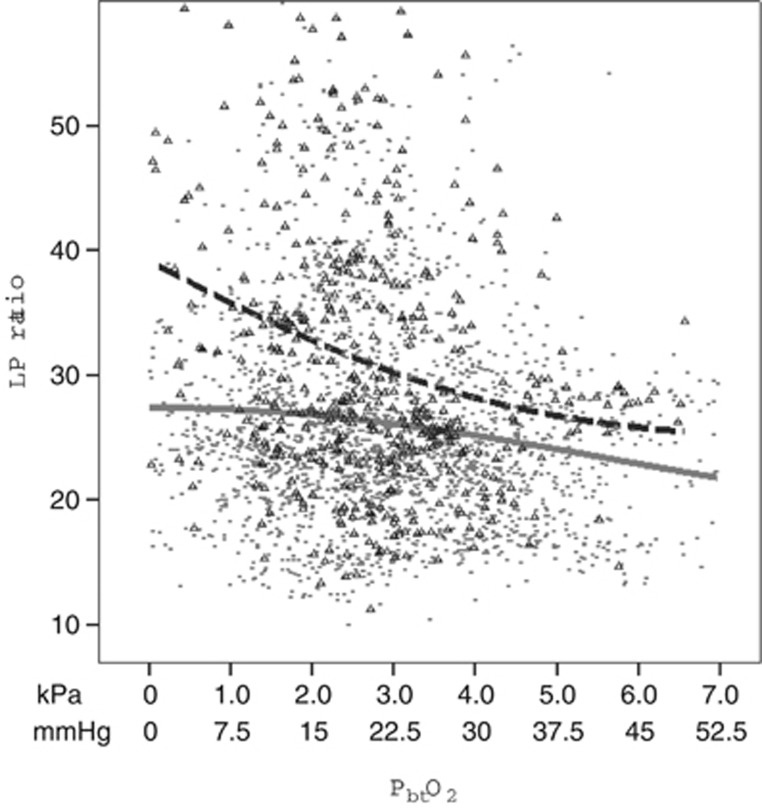
The relationship between LP ratio and PbtO2 was evaluated at two pHbt levels (Figure 2). Spearman rank test suggested significant negative correlation between LP and PbtO2 (rho=−0.159, P<0.001). This relationship was stronger at low pHbt (rho=−0.201, P<0.001) and lost significance at normal pHbt levels (P=0.993). The pHbt was negatively correlated with LP ratio (rho=−0.270, P<0.001) and this relationship remained significant at both low and normal PbtO2 levels.
Figure 2.
Scatterplot depicting relationship between lactate/pyruvate (LP) ratio and PbtO2 depending on the level of pHbt. The dotted fit line represents the subgroup of data with low pHbt (<7.1 in pericontusional brain tissue and <7.15 in less injured brain) and the solid gray line corresponds to the subgroup with normal pHbt (SPSS LOWESS (locally weighted scatterplot smoothing) method of fitting regression lines to the scatterplot was used).
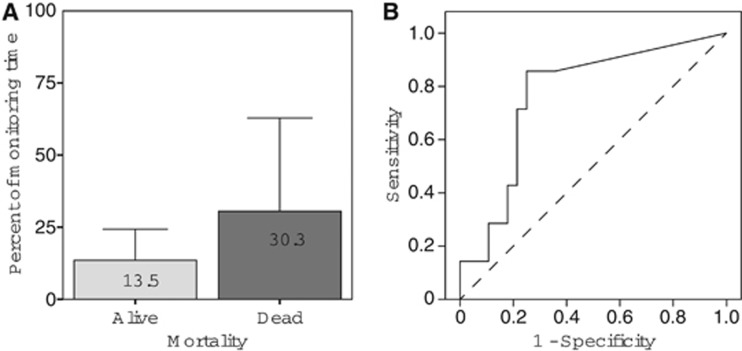
When percentage of time spent in each tissue state (one value per each state per patient) was tested against mortality categories, only the tissue state 2 was found to be a statistically significant discriminator (P=0.031, Mann–Whitney U test), Figure 3. No significant difference or discriminator was found when dichotomy was placed between favorable or unfavorable outcome groups, based on conventional GOS scale. No difference was found in the percentage of time in each tissue state and baseline patients' clinical characteristics including the severity of extracranial injury.
Figure 3.
(A) Mean (±s.d.) percentages of monitoring time belonging to the tissue state 2 (low pHbt and normal PbtO2) by mortality categories, (B) ROC curve representing a duration of time spent in tissue state 2 as a predictor of mortality (P=0.031, AUC: 0.765). AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
The presented results suggest that the four tissue states divided by pHbt and PbtO2 levels exhibit very different metabolic profiles. We did not find any significant difference in proportions of specific baseline patient characteristics, which could explain the differences between the tissue states. Although division into cohorts provides somewhat artificial boundaries to possibly linear continuous data and can be affected by an incorrect selection of thresholds, it allows evaluating and comparing special and often small subgroups within data, which can be missed or diluted by other types of analysis. While the values of PbtO2 below the threshold used in this study are within an accepted hypoxic range, the pHbt thresholds were based on the initial analysis of the data set as described above, since much less conclusive information on significant thresholds of cerebral extracellular pH exists. This approach produced four distinctive groups both in terms of duration of monitoring and associated metabolism. The tissue state 4 represents normal conditions, observed in a majority of monitoring time, and therefore was used as a control group. At the opposite end of the spectrum is the tissue state 1, which is consistent with classic ischemia associated with tissue hypoxia, acidosis, decreased glucose, increased lactate and LP ratio, and PbtCO2 retention. These ischemic conditions were observed very rarely (overall <2% of monitoring time) in line with recent reports3 and expected standards of neurocritical care. The tissue states 2 and 3 together in equal proportions account for one-third of monitoring time. However, tissue state 3, despite low PbtO2 is not metabolically different from normal conditions, apart from significantly higher lactate levels. The latter difference disappears when adjustments for individual patients' variability, ICP, CPP, and PbtCO2 are made. Therefore, despite overt hypoxia, no significant metabolic changes suggesting impaired energy metabolism are present. One possible explanation is that the hypoxia is short-lived and is reversed before causing metabolic perturbations. However, the very nature of the data with values representing hourly averages goes against this suggestion and in many patients hypoxic values of PbtO2 were observed over a prolonged period of time without associated metabolic changes and acidosis. Leaving aside technical reasons (probe failure, focal measurement), it is possible that at least in some situations these perceived hypoxic levels of PbtO2 may be tolerated for a prolonged period of time as the oxygen delivery is matching consumption, although possibly at the very limit of supply/demand capacity. In the majority of patients in this study, metabolic demand was suppressed by pharmacological and other treatment measures. Therefore, even lower levels of PbtO2 may be sufficient, especially if oxygen delivery (optimum perfusion≈CPP) is maintained. Should this balance deteriorate the situation would be likely to shift towards ischemic conditions (tissue state 1) with the development of energy failure and acidosis. It is also possible that acidosis, apart from being a marker of metabolic deterioration (lactate and PbtCO2 accumulation), also actively contributes to energy failure and the deleterious effects of hypoxia.6, 10, 12 This argument is further strengthened by a relationship between PbtO2 and LP ratio at different pHbt levels depicted in Figure 2. However, the absence of any additional data (arterial oxygen and glucose levels, autoregulation, and local perfusion, etc.) makes further elaborations difficult. It should be noted that our treatment protocol placed a strong emphasis on avoidance of aggressive hyperventilation, which can lead to hypoxia and even ischemia in the presence of a relatively alkalotic pHbt due to a reduction in PbtCO2, and therefore this metabolic profile is unlikely to be presented, limiting generalization of the findings to similar management protocols.
Acidosis without hypoxia (tissue state 2 group) represents conditions distinctively different from other tissue states described above. It accounts for a considerable proportion (16%) of monitoring time and is characterized by profound disturbances of energy metabolism with high lactate and LP ratio as well as by the signs of increased cellular membranes breakdown (raised glycerol). At the same time, this tissue state is associated with normal PbtO2, probably normal cerebral perfusion (normal CPP as a surrogate) and normal or relatively high cerebral extracellular glucose. This is the only tissue state associated with increased mortality (although an absence of a relationship between ischemia and mortality is likely to be due to the limited number of observations in that group), which further strengthens the argument that observed metabolic changes are likely to be a genuine representation of unfavorable conditions. We found no statistically significant association with GOS-based dichotomized functional outcome, which may be due to a relatively small number of patients and sample size with the lack of statistical power to detect such relationship.
Several explanations for the observed and evidently nonischemic metabolic disturbances can be proposed. First, it may represent a recovery phase following transient ischemia, after reoxygenation or reperfusion has taken place. Although detailed analysis of temporal profiles was beyond the scope of this study it was clear, following review of the data, that in the majority of time the described changes took place without preceding hypoxia or ischemia. In addition, many patients never exhibited hypoxic levels of PbtO2 before developing nonhypoxic reduction in pH and associated metabolic changes. A second possibility is that this represents an acquired mitochondrial failure,2 with associated reduction of pyruvate utilization in the TCA cycle and accumulation of lactate, which can be further exacerbated by evolving acidosis. Such failure can be sustained or reversible, for instance by supranormal increases in oxygenation to overcome possible tissue diffusion barriers27 or by correction of acidosis.28 Vespa et al3 described a 25% incidence of metabolic crisis without ischemia, which is further supported by the presented data, although with a lower incidence. Third, peripheral hyperglycemia, especially in the presence of increased glucose metabolism or even pathological hyperglycolysis due to nonischemic failure of the TCA cycle, can contribute to acidosis with accumulation of lactate and PbtCO2, a finding well described previously.29, 30, 31 Relatively high extracellular glucose levels, considering that metabolism is likely to be increased, may support this explanation, although without data on arterial glucose values it remains only a theoretical possibility.
It remains unclear which therapeutic methods are most likely to improve the nonischemic metabolic disturbances described and whether they can be reversed at all. Hyperoxia and titration of arterial glucose may play an important role. Furthermore, via the mechanisms described above, acidosis can also contribute to metabolic derangements and cellular injury in its own right and the presence of a significant negative correlation between pHbt and LP ratio, irrespective of PbtO2 levels further supports this notion. However, the cause and effect are difficult to establish in an observational data set and it is not possible to prove that acidosis does indeed exacerbate mitochondrial failure or whether it simply reflects increasing lactate concentration.
In view of this, it would be useful to determine whether the correction of acidosis, with buffering agents for example, will produce metabolic improvement. Several previous encouraging reports32, 33, 34, 35, 36 indicated clinical and monitoring improvements after administration of trometamol (THAM or tris-buffer) and a combination of pHbt monitoring with pH-guided treatment may open new therapeutic avenues; however, further contemporary interventional studies would be required to further evaluate the feasibility and effects of cerebral pH manipulation.
At the present moment, the Neurotrend sensor has been discontinued by the manufacturers for commercial reasons as well as issues related to ease of use and concerns about the monitoring stability and reliability of PbtO2 recording, when compared with other available clinical brain tissue oxygen monitors.37, 38 The lack of comparable clinical monitor further clinical studies are impossible, until a new reliable and clinically approved method of continuous in vivo pHbt monitoring in humans is available. Several groups are working on development of such tool39, 40, 41 and it may be possible in future to resume clinical pHbt monitoring.
Conclusions
Following TBI reduced cerebral extracellular pH (pHbt) with or without concomitant hypoxia is a marker of a significant metabolic derangement and is associated with increased mortality. Continuous monitoring of pHbt in addition to PbtO2 alone may provide an added value in detecting unfavorable physiological conditions, especially when direct biochemical monitoring of brain tissue is not feasible and overt tissue hypoxia is not present.
It is still worthwhile to evaluate whether cerebral acidosis can be reversed by therapeutic maneuvers and if this will lead to improvements in cerebral metabolism. Unfortunately, further continuous clinical monitoring of cerebral extracellular pH is currently impossible due to discontinuation of clinical sensor by the manufacturers until new comparable technology emerges.
The authors declare no conflict of interest.
References
- Nortje J, Coles JP, Timofeev I, Fryer TD, Aigbirhio FI, Smielewski P, et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit Care Med. 2008;36 (1:273–81. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93 (5:815–20. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25 (6:763–74. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101 (17:6752–7. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J Neurosurg. 1992;77 (2:169–84. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73 (4:1549–56. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Bhattacharyya T, Sensi SL, Lobner D, Ying HS, Canzoniero LM, et al. Extracellular acidity potentiates AMPA receptor-mediated cortical neuronal death. J Neurosci. 1998;18 (16:6290–9. doi: 10.1523/JNEUROSCI.18-16-06290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisek L. Extracellular acidosis and high levels of carbon dioxide suppress synaptic transmission and prevent the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Hippocampus. 1998;8 (1:24–32. doi: 10.1002/(SICI)1098-1063(1998)8:1<24::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Farrell K, Simon RP. Acidosis causes failure of astrocyte glutamate uptake during hypoxia. J Cereb Blood Flow Metab. 1995;15 (3:417–24. doi: 10.1038/jcbfm.1995.52. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Benington JH. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18 (5-6:515–21. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Szabo M, Huttner I. Blood-brain barrier impairment by low pH buffer perfusion via the internal carotid artery in rat. Acta Neuropathol (Berl) 1985;68 (2:160–3. doi: 10.1007/BF00688639. [DOI] [PubMed] [Google Scholar]
- Kristian T, Bernardi P, Siesjo BK. Acidosis promotes the permeability transition in energized mitochondria: implications for reperfusion injury. J Neurotrauma. 2001;18 (10:1059–1074. doi: 10.1089/08977150152693755. [DOI] [PubMed] [Google Scholar]
- Mellergard P, Ouyang YB, Siesjo BK. The regulation of intracellular pH is strongly dependent on extracellular pH in cultured rat astrocytes and neurons. Acta Neurochir Suppl (Wien) 1994;60:34–7. doi: 10.1007/978-3-7091-9334-1_9. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Edelman G, Ausman JI.Brain tissue acid-base changes during ischemia Neurosurg Focus 19972(5E2discussion 1 p following E2. [DOI] [PubMed] [Google Scholar]
- Huang Y, McNamara JO. Ischemic stroke: ‘acidotoxicity' is a perpetrator. Cell. 2004;118 (6:665–6. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Shiogai T, Nara I, Saruta K, Hara M, Saito I. Continuous monitoring of cerebrospinal fluid acid-base balance and oxygen metabolism in patients with severe head injury: pathophysiology and treatments for cerebral acidosis and ischemia. Acta Neurochir Suppl. 1999;75:49–55. doi: 10.1007/978-3-7091-6415-0_11. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Faden AI, Bendall MR, Vink R. Traumatic brain injury in the rat: alterations in brain lactate and pH as characterized by 1H and 31P nuclear magnetic resonance. J Neurochem. 1987;49 (5:1530–40. doi: 10.1111/j.1471-4159.1987.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Clausen T, Khaldi A, Zauner A, Reinert M, Doppenberg E, Menzel M, et al. Cerebral acid-base homeostasis after severe traumatic brain injury. J Neurosurg. 2005;103 (4:597–607. doi: 10.3171/jns.2005.103.4.0597. [DOI] [PubMed] [Google Scholar]
- Zauner A, Clausen T, Alves OL, Rice A, Levasseur J, Young HF, et al. Cerebral metabolism after fluid-percussion injury and hypoxia in a feline model. J Neurosurg. 2002;97 (3:643–9. doi: 10.3171/jns.2002.97.3.0643. [DOI] [PubMed] [Google Scholar]
- Brooke NS, Ouwerkerk R, Adams CB, Radda GK, Ledingham JG, Rajagopalan B. Phosphorus-31 magnetic resonance spectra reveal prolonged intracellular acidosis in the brain following subarachnoid hemorrhage. Proc Natl Acad Sci USA. 1994;91 (5:1903–7. doi: 10.1073/pnas.91.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota H, Yamamoto Y, Naoe Y, Fuse A, Sato H, Unemoto K, et al. Measurements of cortical cellular pH by intracranial tonometer in severe head injury. Crit Care Med. 2000;28 (9:3275–80. doi: 10.1097/00003246-200009000-00025. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Zygun DA, Johnston AJ, Steiner LA, Al-Rawi PG, Chatfield D, et al. Extracellular brain pH and outcome following severe traumatic brain injury. J Neurotrauma. 2004;21 (6:678–84. doi: 10.1089/0897715041269722. [DOI] [PubMed] [Google Scholar]
- Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury, 3rd edition. J Neurotrauma. 2007;24 (Suppl 1:S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien) 1997;139 (4:286–94. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- Menon DK. Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br Med Bull. 1999;55 (1:226–58. doi: 10.1258/0007142991902231. [DOI] [PubMed] [Google Scholar]
- Smielewski P, Lavinio A, Timofeev I, Radolovich D, Perkes I, Pickard JD, et al. ICM+, a flexible platform for investigations of cerebrospinal dynamics in clinical practice. Acta Neurochir Suppl. 2009;102:145–151. doi: 10.1007/978-3-211-85578-2_30. [DOI] [PubMed] [Google Scholar]
- Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32 (6:1384–90. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- Hoffman TL, LaManna JC, Pundik S, Selman WR, Whittingham TS, Ratcheson RA, et al. Early reversal of acidosis and metabolic recovery following ischemia. J Neurosurg. 1994;81 (4:567–73. doi: 10.3171/jns.1994.81.4.0567. [DOI] [PubMed] [Google Scholar]
- Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, Al-Rawi PG, Chatfield D, et al. Hyperglycemia and brain tissue pH after traumatic brain injury Neurosurgery 200455(4877–81.discussion 882. [DOI] [PubMed] [Google Scholar]
- Holbein M, Bechir M, Ludwig S, Sommerfeld J, Cottini SR, Keel M, et al. Differential influence of arterial blood glucose on cerebral metabolism following severe traumatic brain injury. Crit Care. 2009;13 (1:6. doi: 10.1186/cc7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PA, Siesjo BK. Role of hyperglycaemia-related acidosis in ischaemic brain damage. Acta Physiol Scand. 1997;161 (4:567–80. doi: 10.1046/j.1365-201X.1997.00264.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Marmarou A. Effects of tromethamine and hyperventilation on brain injury in the cat. J Neurosurg. 1991;74 (1:87–96. doi: 10.3171/jns.1991.74.1.0087. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Corwin F, Marmarou A. Effect of THAM on brain oedema in experimental brain injury. Acta Neurochir Suppl (Wien) 1990;51:317–9. doi: 10.1007/978-3-7091-9115-6_107. [DOI] [PubMed] [Google Scholar]
- Wolf AL, Levi L, Marmarou A, Ward JD, Muizelaar PJ, Choi S, et al. Effect of THAM upon outcome in severe head injury: a randomized prospective clinical trial. J Neurosurg. 1993;78 (1:54–9. doi: 10.3171/jns.1993.78.1.0054. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Holdaway R, Ward JD, Yoshida K, Choi SC, Muizelaar JP, et al. Traumatic brain tissue acidosis: experimental and clinical studies. Acta Neurochir Suppl (Wien) 1993;57:160–4. doi: 10.1007/978-3-7091-9266-5_23. [DOI] [PubMed] [Google Scholar]
- Rosner MJ, Becker DP. Experimental brain injury: successful therapy with the weak base, tromethamine. With an overview of CNS acidosis. J Neurosurg. 1984;60 (5:961–71. doi: 10.3171/jns.1984.60.5.0961. [DOI] [PubMed] [Google Scholar]
- Hoelper BM, Alessandri B, Heimann A, Behr R, Kempski O. Brain oxygen monitoring: in-vitro accuracy, long-term drift and response-time of Licox- and Neurotrend sensors. Acta Neurochir (Wien) 2005;147 (7:767–74. doi: 10.1007/s00701-005-0512-8. [DOI] [PubMed] [Google Scholar]
- Jaeger M, Soehle M, Meixensberger J. Brain tissue oxygen (PtiO2): a clinical comparison of two monitoring devices. Acta Neurochir Suppl. 2005;95:79–81. doi: 10.1007/3-211-32318-x_17. [DOI] [PubMed] [Google Scholar]
- Grant SA, Bettencourt K, Krulevitch P, Hamilton J, Glass R. In vitro and in vivo measurements of fiber optic and electrochemical sensors to monitor brain tissue pH. Sensors and Actuators B: Chemical. 2001;72 (2:174–179. [Google Scholar]
- Wu S, Cheng W, Qiu Y, Li Z, Shuang S, Dong C. Fiber optic pH sensor based on mode-filtered light detection. Sensors Actuators B: Chem. 2010;144 (1:255–259. [Google Scholar]
- Korostynska O, Arshak K, Gill E, Arshak A. Review paper: materials and techniques for in vivo pH monitoring. IEEE Sensors J. 2008;8 (1:20. [Google Scholar]