Abstract
Despite its limited regenerative capacity, the central nervous system (CNS) shares more repair mechanisms with peripheral tissues than previously recognized. Scar formation is a ubiquitous healing mechanism aimed at patching tissue defects via the generation of fibrous extracellular matrix (ECM). This process, orchestrated by stromal cells, can unfavorably affect the capacity of tissues to restore function. Vascular mural cells have been found to contribute to scarring after spinal cord injury. In the case of stroke, little is known about the responses of pericytes (PCs) and stromal cells. Here, we show that capillary PCs are rapidly lost after cerebral ischemia in both experimental and human stroke. Coincident with this loss is a massive proliferation of resident platelet-derived growth factor receptor beta (PDGFRβ)+ and CD105+ stromal cells, which originate from the neurovascular unit and deposit ECM in the ischemic mouse brain. The presence of PDGFRβ+ stromal cells demarcates a fibrotic, contracted, and macrophage-laden lesion core from the rim of hypertrophic astroglia in both experimental and human stroke. We suggest that a previously unrecognized population of CNS-resident stromal cells drives a dynamic process of scarring after cerebral ischemia, which appears distinct from the glial scar and represents a novel target for regenerative stroke therapies.
Keywords: cerebral ischemia, extracellular matrix, fibrosis, neurovascular unit, pericyte, platelet-derived growth factor receptor beta
Introduction
The neurovascular unit, comprising the structural and functional links between the central nervous system (CNS) vascular compartment and the neural parenchyma, is a key element in the development, physiology, and pathology of the CNS.1 Vascular mural cells, including the very abundant capillary pericytes (PCs), are positioned at the interface between endothelial cells, neurons, and astrocytes. Experimental evidence supports a variety of functions of these cells: PCs influence the proliferation of endothelial cells during development and adult angiogenesis.2 They contribute to the modulation of blood flow,3, 4 and determine the formation, maintenance, and regulation of the blood–brain barrier.5, 6, 7 Primary cultured CNS pericytes also have immune functions as suggested by phagocytic activity and the expression of macrophage markers like CD11b.8 Moreover, PCs of different organs, including the brain, have been reported to possess mesenchymal stem cell characteristics with the potential to differentiate in vitro into bone, cartilage, or adipose tissue.9, 10, 11 The plasticity of PCs is underscored by reports, which suggested multipotent stem cell characteristics of CNS microvascular PCs.12
With the increasing interest in the complex functions of PCs and mural cells, new questions arise as to how PCs may be affected by pathologic conditions of the CNS. Vice versa, it is also unclear whether PCs can influence neurologic disease progression. During diabetic retinopathy, hyperglycemia leads to the depletion of PCs, causing increased microvascular permeability and microvessel occlusion.13 After experimental traumatic brain injury, ultrastructural evidence suggests that some PCs acutely migrate into the parenchyma while others degenerate.14 The few studies that have addressed the response of PCs to cerebral ischemia have been limited to the acute phase. After ischemia, PC contraction might influence capillary patency,15 while ultrastructural examinations suggest that PCs migrate to the injured parenchyma.16 Recently, a novel subpopulation of mural cells has been described that shares with the PC the perivascular location and a common set of markers, including platelet-derived growth factor receptor beta (PDGFRβ). These cells proliferate extensively into fibroblast-like cells that generate fibrous extracellular matrix (ECM) after spinal cord injury.17
We hypothesized that cerebral ischemia results in the degeneration of brain microvascular PCs, and induces the proliferation of vascular stromal cells which contribute to tissue remodeling. Using (1) a model of experimental stroke in wild-type and transgenic mice expressing GFP under the control of the PC/mural cell-specific rgs5 promoter,18 (2) bone marrow (BM) chimeric mice, and (3) a comprehensive collection of human stroke samples, we show that cerebral ischemia decimates the population of capillary PCs in the brain while inducing the proliferation of a novel population of vessel wall-derived PDGFRβ+ stromal cells. The fibrotic scar with PDGFRβ+ cells constitutes a previously unrecognized element of tissue remodeling after stroke.
Materials and methods
Mice
Wild-type C57BL/6 or mutant rgs5GFP/GFP or rgs5GFP/WT (rgs5GFP) mice were used for this study. Animal experiments were performed in accordance with national and international guidelines for the care and use of laboratory animals (Tierschutzgesetz der Bundesrepublik Deutschland, European directive 2010/63/EU, as well as GV-SOLAS and FELASA guidelines and recommendations for laboratory animal welfare) and subjected to review by an ethics committee (Landesamt für Gesundheit und Soziales, Berlin, Germany). Experiments were reported according to the ARRIVE guidelines (see http://www.nc3rs.org/ARRIVE).
Experimental Stroke Model
Transient focal cerebral ischemia was induced by occlusion of the middle cerebral artery (MCAO). During surgery, anesthesia was maintained with 1.0% to 1.5% halothane in 70% N2O/30% O2, and body temperature was kept constant at 37°C to 37.5°C using a heating pad. A silicone-coated filament was advanced through the incised left internal carotid artery until it occluded the middle cerebral artery. Occlusion time was 60 minutes for the experiments performed in C57BL/6 and GFP BM chimeras, or 45 minutes in rgs5GFP BM chimeras. After successful MCAO, the filament was withdrawn during a second anesthesia to allow reperfusion. Animals survived for 1, 3, 5, 7, 14, or 28 days before histologic analysis of the brain.
Bone Marrow Chimeras
For retroviral transduction of hematopoietic cells, BM was harvested from femurs and tibias 48 hours after treatment with 150 mg/kg 5-fluoruracil. Transduction of BM cells with a murine stem cell virus-based retroviral vector containing the enhanced GFP cDNA was performed as described.19 BM cells were cultured in DMEM supplemented with 15% fetal calf serum, 50 ng/mL rat stem cell factor, 20 ng/mL interleukin-3, and 50 ng/mL interleukin-6. After 2 days, BM cells were transferred onto irradiated (13 Gy) viral producer cells and cultured for another 48 hours. Nonadherent BM cells were finally rinsed off the producer cell monolayer. BM from rgs5GFPmice was harvested and transplanted directly without prior cultivation. For BM transplantation, animals received a total body irradiation of 11 Gy and were reconstituted on the same day via tail vein injection with ∼5 × 106 BM cells. After transplantation, animals were kept under aseptic conditions. In animals receiving GFP-transduced BM cells, levels of chimerism were determined by flow cytometric analysis of GFP expression in peripheral blood cells. Focal brain ischemia and reperfusion were induced at 12 weeks (GFP BM chimeras) or 8 weeks (rgs5GFP BM chimeras) after BM transplantation.
Mouse Brain Histology
For immunohistochemistry, animals were anesthetized with 4% isoflurane in O2, and transcardially perfused with ice-cold phosphate-buffered saline, followed by ice-cold 4% paraformaldehyde in phosphate-buffered saline. After overnight fixation in 4% paraformaldehyde in phosphate-buffered saline and cryoprotection in 30% sucrose-containing phosphate-buffered saline, 30 μm thick sections were cut on a cryostat. Free-floating sections were then incubated in 20% normal goat or donkey serum, 0.3% Triton X-containing Tris-buffered saline for blocking unspecific binding. All further steps were performed in 1% normal goat serum or normal donkey serum, 0.3% Triton X-containing Tris-buffered saline. Sections were then incubated for 12 to 72 hours at 4°C with a combination of two of the following primary antibodies; mouse anti α-smooth muscle (SM)-actin (Merck Millipore, Billerica, MA, USA), rabbit anti-CD105 (Acris Antibodies, Herford, Germany), rat anti-CD13 (Acris Antibodies), rabbit anti-CD31 (BD Pharmingen, Franklin Lakes, NJ, USA), rabbit anti-cleaved caspase-3 (CC3; BD Pharmingen), rabbit anti-fibronectin (Dako, Glostrup, Denmark), rabbit anti-GFAP (Dako), rabbit anti-GFP (Invitrogen, Carlsbad, CA, USA), rabbit anti-Iba-1 (Wako, Osaka, Japan), rabbit anti-Ki67 (Abcam, Cambridge, UK), rabbit anti-laminin (Sigma-Aldrich, St Louis, MO, USA), rabbit anti-NG2 (Merck Millipore), rat anti-PDGFRβ (eBioscience, San Diego, CA, USA), rabbit anti-PDGFRβ (Novus Biologicals, Oakville, ON, USA), or rabbit anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After thorough washing, sections were incubated overnight at 4°C with appropriate secondary antibodies conjugated to Alexa Fluor dyes (Invitrogen), or stained with diaminobenzene (DAB) using an appropriate kit (Vector Labs, Burlingame, CA, USA). For DAB staining, endogenous peroxidase was inactivated with 3% H2O2 during 15 minutes before secondary antibody incubations. When mouse antibodies were used on mouse tissue, unspecific staining was blocked with the M.O.M. kit (Vector Labs). Nuclei were counterstained with DAPI. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stains were performed with the ApopTag fluorescein in situ apoptosis detection kit (Merck Millipore). To increase sensitivity of the stain, we performed a secondary staining with an Alexa Fluor 488-conjugated anti-sheep antibody (Invitrogen).
Human Stroke Histology
Human postmortem stroke samples were obtained from subjects who underwent autopsy for diagnostic purposes in the Department of Neuropathology of the Charité-Universitätsmedizin Berlin. The use of human samples was approved by the appropriate ethics committee (Ethikkommission der Charité-Universitätsmedizin Berlin), and all procedures were performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Tissue blocks of different brain regions were fixed in 4% paraformaldehyde and embedded in paraffin wax. In all, 3-μm-thick sections were obtained from the paraffin wax block with a microtome. After deparaffinization and antigen retrieval (cooking in citrate buffer, pH 6.0 or in borate buffer, pH 8.0), immunofluorescence double staining was performed as described for the mouse tissue. In addition, hematoxylin-eosin staining and DAB immunostaining for single epitopes were performed in an automaton (VENTANA BenchMark XT, Tucson, AZ, USA). The following antibodies were used: rabbit anti-PDGFRβ (clone Y92; Novus Biologicals), rabbit anti-GFAP (Dako), rabbit anti-fibronectin (Dako), and mouse anti-collagen VI (Merck Millipore).
Image Acquisition
Images were obtained with a Leica epifluorescence microscope for quantification. To obtain high-resolution confocal images of the stained sections, a Leica TCS SPE microscope (Leica Microsystems, Wetzlar, Germany) was used. In the figures, either single confocal planes or maximal projections of confocal stacks encompassing the structure of interest are shown. Image processing was performed with ImageJ (http://rsbweb.nih.gov/ij/).
Morphometrical Analysis and Cell Counting
To count mouse PDGFRβ+ cells or CD13+ PCs, and to determine the colocalization with cellular markers (CD13, CD105, α-SM-actin, fibronectin, NG2, Iba-1), markers of apoptosis (CC3) or proliferation (Ki67), we acquired fluorescence images from either ischemic or contralateral nonischemic tissue (or from both hemispheres in controls, 5 to 10 optical fields of 0.15 mm2 for each mouse and condition). Only immunoreactive cells with visible nuclei were counted using custom written software. For CD13+ PCs, only cells were scored with a typical PC morphology (oval or fusiform body of 10 to 15 μm in length, and longitudinal processes along the capillary axis). Lengths of CD31+ vessels were measured by manually delineating vascular contours in an area of 0.15 mm2 per animal and hemisphere. Measurements were then normalized to the contralateral values. The density of PDGFRβ+ cells in human stroke or control tissue was determined off-line by counting PDGFRβ+ cells in 10 to 15 images (each 0.15 mm2) per subject and condition. The densities of laminin fibrous matrix (for mouse brain) and collagen VI fibrous matrix (for human brain) were assessed by counting the number of crossings that immunoreactive structures made with a 50 μm grid overlaid on fluorescent or DAB-stained images (10 to 15 images per subject and condition).
To assess the evolution in size of the stroke-induced lesion, the area of the lesion was measured in one coronal section stained with DAPI (∼0.5 mm anterior to bregma) from each animal 7, 14, or 28 days after ischemia. The area originally occupied by the infarcted tissue (defect area) was determined by subtracting the area of healthy parenchyma ipsilateral to the lesion from the total area of the contralateral hemisphere. The ratios of lesion to defect areas were then calculated for each animal to assess the contraction of the ischemic tissue. To examine the presence of PCs in BM chimeras that received GFP-expressing wild-type BM cells, we analyzed confocal stacks to unequivocally detect two hallmarks of PCs: (1) the expression of the CD13 marker and (2) the inclusion within laminin+ vascular basement membrane.
To determine the nuclear translocation of the p65 subunit of the NF-κB transcription factor, we compared the intensity of p65 staining in the nucleus (stained with DAPI) with the intensity in the cytoplasm (contained between nucleus and membrane CD13 immunoreactivity) using intensity plots traversing PC bodies in single confocal images. The nonischemic contralateral tissue was used as control. Background fluorescence was subtracted from the intracellular signal and, for each animal and condition, the nucleus-to-cytoplasm ratio of p65 signal intensity was averaged from >30 single PCs.
Statistical Analysis
For the assessment of statistically significant differences, we performed one-way ANOVA followed by Bonferroni correction for multiple comparisons, or paired Student's t-test for single within-subject comparisons. In the text and graphs, data are presented as mean values±standard deviation (s.d.). Statistical analysis was performed using MATLAB (Mathworks, Natick, MA, USA).
Results
Loss of CD13+ Capillary Pericytes and CD31+ Endothelium after Cerebral Ischemia
To label PCs, we have stained for PDGFRβ or CD13, two well-established PC markers.20 In healthy brain cortical tissue, staining for PDGFRβ or CD13 revealed the presence of a PC mesh covering the capillaries (Figures 1A and 3A). In rgs5GFP mice, GFP is specifically expressed in PCs and mural cells,18 providing identification independent of surface markers. Coexpression of PDGFRβ and CD13 with GFP showed the high sensitivity of the above-mentioned markers for PCs and mural cells. In normal brain tissue from rgs5GFP mice, 99.3±0.2% and 98.7±1.2% of GFP+ cells expressed PDGFRβ and CD13, respectively (n=3; data not shown). We exposed wild-type mice to 60 minutes MCAO and reperfusion for 1, 3, 5, 7, 14, or 28 days (n=4, 4, 5, 3, 5, and 5, respectively), and compared the ischemic brain tissue with nonischemic control animals (n=5). All animals exposed to MCAO showed ischemic lesions in the cortex and striatum; the analysis focused on the core of the cortical lesion showing loss of glial fibrillary acidic protein (GFAP) immunoreactivity (Figures 2 and 3). One to three days after ischemia, vessel-associated CD13 immunoreactivity appeared disrupted, patchy and was missing at some vascular segments (Figure 1A). Consistent with PC membrane disruption, leakage of CD13 antigen into the surrounding parenchyma was observed along with fragmentation of CD13-immunoreactive PC processes at 24 hours after MCAO (Figures 1D and 1E). Vascular CD13 immunoreactivity was hugely diminished by day 7 after MCAO (Figure 1A). At 14 to 28 days after injury, microglia/macrophages were immunoreactive for CD13 as indicated by colocalization of CD13 with Iba-1 (Figure 1B). We therefore quantified the density of capillary CD13+ PC bodies with typical PC morphology (small cell soma, sleek longitudinal processes) only during the first week after ischemia in the core of the ischemic tissue. Starting from day 1, the number of CD13+ capillary PCs progressively declined to as low as ∼20% of the contralateral density on day 7 after MCAO (Figures 1A and 1C). To confirm that cells with normal PC phenotype are actually lost, excluding the failure to detect PCs due to proteolysis of CD13 after MCAO, we performed MCAO followed by 7 days reperfusion in rgs5GFP mutant mice. Since these mutants have a lower arterial pressure18 and may potentially suffer increased ischemia during MCAO, we reduced the duration of MCAO to 45 minutes; however, this model did not produce cortical lesions in all animals, and we thus limited the analysis to the ischemic striatum. The number of GFP-expressing cells with PC morphology was significantly reduced in the ischemic tissue (43±10/mm2) compared with the contralateral hemispheres (100±15/mm2; n=3, P=0.05; Figures 6A and 6B), corroborating our findings of loss of cells with PC phenotype after MCAO in wild-type mice.
Figure 1.
Loss of CD13+ pericytes after middle cerebral artery occlusion (MCAO). (A) Series of confocal images showing the loss of CD13+ capillary pericytes (PCs) after cerebral ischemia. The CD13-immunoreactive PC cell bodies can be detected in control and ischemic tissues (arrowheads). One day after MCAO, capillary PC CD13 immunoreactivity begins to appear patchy. At days 3 to 7 after MCAO, CD13+ capillary PC cell bodies become increasingly rare. (B) At day 14 (and 28, not shown) after MCAO, the ischemic tissue is filled with CD13/Iba-1 double-immunoreactive macrophages, rendering the analysis of CD13+ PCs impossible. (C) Quantification of CD13+ capillary PC density shows a significant decrease at days 1 to 7 after MCAO. (D, E) CD13+ PCs show structural alterations at day 1 after MCAO, such as spilling of CD13 immunoreactivity outside the vascular basement membrane (arrowheads, D, single stainings and orthogonal reconstruction of the confocal stack at dashed line in the insets) or fragmentation of CD13-immunoreactive PC processes (arrows, E). (F) Example of a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ CD13+ PC in the ischemic tissue at day 1 after MCAO (arrowhead). TUNEL immunoreactivity colocalizes with the nucleus of the PC, although penetration of the labeling into the tissue not complete (arrow). The insets show single confocal planes or an orthogonal reconstruction (orth.) at the plane indicated by the dashed line. (G) Example of a cleaved caspase-3 (CC3)-immunoreactive CD13+ PC in the ischemic tissue at day 3 after MCAO (arrowhead). The DAPI signal reveals condensed and fragmented nucleus (arrows, inset). (H) CC3-immunoreactive CD13+ capillary PCs reach maximum frequency at day 3 after MCAO. (I) Confocal images reveal increase in p65 immunoreactivity in the nucleus of a CD13+ capillary PC at day 1 after ischemia, but not of a contralateral PC (nuclei are marked with stars). The plots show fluorescence intensities for each of the stains at the dashed lines. DAPI and CD13 immunofluorescence was used for determining background, cytoplasm (cyt.) or nucleus of the PC. (J) Quantification of nuclear-to-cytoplasm ratio (N:C) of p65 immunoreactivity in CD13+ capillary PCs. High N:C ratio indicates nuclear translocation of p65. Bars represent means±s.d. *P<0.05; **P<0.01; #P<0.0001 versus control mice (C, H) or the contralateral hemisphere (J). n=5, 4, 4, 5, 3, 5, and 5 for control, 1, 3, 5, 7, 14, and 28 days, respectively. NA, not available; ND, not detected. Scale bars: 20 μm (A–G); 10 μm (insets in D), 5 μm (I).
Figure 2.
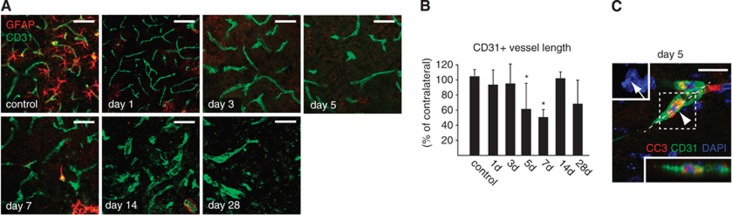
Rarefaction of CD31-immunoreactive endothelium after middle cerebral artery occlusion (MCAO). (A) Series of representative confocal microscopic images showing loss of CD31-immunoreactive endothelium after ischemia. (B) Quantification of vessel lengths based on CD31 immunofluorescence in the ischemic versus nonischemic hemisphere. A significant reduction of vessel length is observed at days 5 and 7 after MCAO. (C) Cleaved caspase-3 (CC3) immunohistochemistry and nuclear morphology reveal apoptosis of CD31+ endothelial cells at day 5 after ischemia (arrowhead). Upper left inset shows nuclear fragmentation of the CD31+ endothelial cell (arrow), lower right inset shows the orthogonal reconstruction obtained from the plane indicated (dashed line), showing CC3 immunoreactivity within the CD31+ endothelial cell. Bars represent means±s.d. *P<0.05 versus control mice. n=5, 4, 4, 5, 3, 5, and 5 for control, 1, 3, 5, 7, 14, and 28 days, respectively. Scale bars: 20 μm.
Figure 3.
Generation of platelet-derived growth factor receptor beta (PDGFRβ+) stromal cells after middle cerebral artery occlusion (MCAO) and contraction of the ischemic tissue. (A) Series of confocal images showing the rise of PDGFRβ+ cell density in the ischemic tissue. PDGFRβ+ pericyte (PC) cell bodies (arrowhead) are frequent in control tissue. At day 1 after MCAO, PDGFRβ immunoreactivity appears patchy, although PCs are still detectable (arrowhead). At day 3, PDGFRβ immunoreactivity increases around larger vessels (arrowhead). GFAP-immunoreactive astrocytes appear to disintegrate from days 1 to 3 (arrows) and disappear from the lesion core at later time points, when a dense mesh of PDGFRβ+ ameboid cells with multiple, irregular cell projections occupies the ischemic parenchyma. (B) Quantification of PDGFRβ+ cell density shows a significant increase in the ischemic area at days 5 to 28 after MCAO versus control animals. (C) Quantification of the area occupied by the lesion at days 7 to 28 after MCAO. The lesion area is defined as the region contained by GFAP immunoreactivity. The ischemic defect area is calculated by subtracting the nonlesioned area of the ipsilateral hemisphere from the total area of the contralateral hemisphere. At day 7, the lesion area roughly equals the original defect area. At days 14 to 28, the lesion area is significantly reduced by ∼2/3 of the ischemic defect. (D) PDGFRβ immunoreactivity in the ischemic tissue at days 5 to 28 after MCAO (arrowheads) is delimited by the progressive formation of a GFAP-immunoreactive astroglial reaction (arrows). At days 14 to 28, occasional cavitation occurs (double arrows). Note the progressive contraction of the ischemic area at days 14 to 28. Bars represent means±s.d. *P<0.05, **P<0.0005. n=5, 4, 4, 5, 3, 5, and 5 for control, 1, 3, 5, 7, 14, and 28 days, respectively. Scale bars: 20 μm (A); 2 mm (D).
We explored whether apoptotic cell death could be involved in the loss of capillary PCs. Indeed, frequent TUNEL-positive CD13+ PCs were observed starting at day 1 after ischemia (Figure 1F). We also stained for CC3, a protein present in early apoptotic cells. CC3-immunoreactive CD13+ PCs were absent in normal brain tissue, but significantly increased by day 3 after ischemia (Figures 1G and 1H). Since CC3 immunoreactivity is not exclusive to programmed cell death, we used condensation and fragmentation of nuclei, consistent with apoptotic bodies, as additional indicators of apoptosis (Figure 1G). Moreover, we examined the nuclear translocation of the p65 subunit of NF-κB, a known stress response potentially leading to apoptosis of PCs.21 At day 1 after ischemia/reperfusion, a significantly increased nuclear-to-cytoplasm ratio of p65 immunoreactivity was observed in PCs (Figures 1I and 1J), suggesting early activation of the NF-κB pathway in CD13+ PCs after ischemia.
To examine the responses of the vascular endothelium to ischemia, we quantified the presence of CD31+ vessel segments in the core of the ischemic tissue. During the first week after MCAO, a rarefaction of CD31+ endothelium was observed (Figure 2A), which reached minimal levels (∼50% of contralateral numbers) on day 7 after MCAO (Figure 2B). CC3-immunoreactive CD31+ endothelial cells containing fragmented nuclei were detected starting on day 5 after MCAO (Figure 2C). It should be noted that our analysis was restricted to the infarct core, whereas increased vascularization has been observed in the penumbra after stroke.22
In conclusion, our results suggest that an early loss of capillary PCs occurs after MCAO (within 24 hours), which precedes the degeneration of vascular endothelium (after day 5) in our MCAO model.
Appearance of a Novel Platelet-Derived Growth Factor Receptor Beta+ Cell Population in the Ischemic Brain
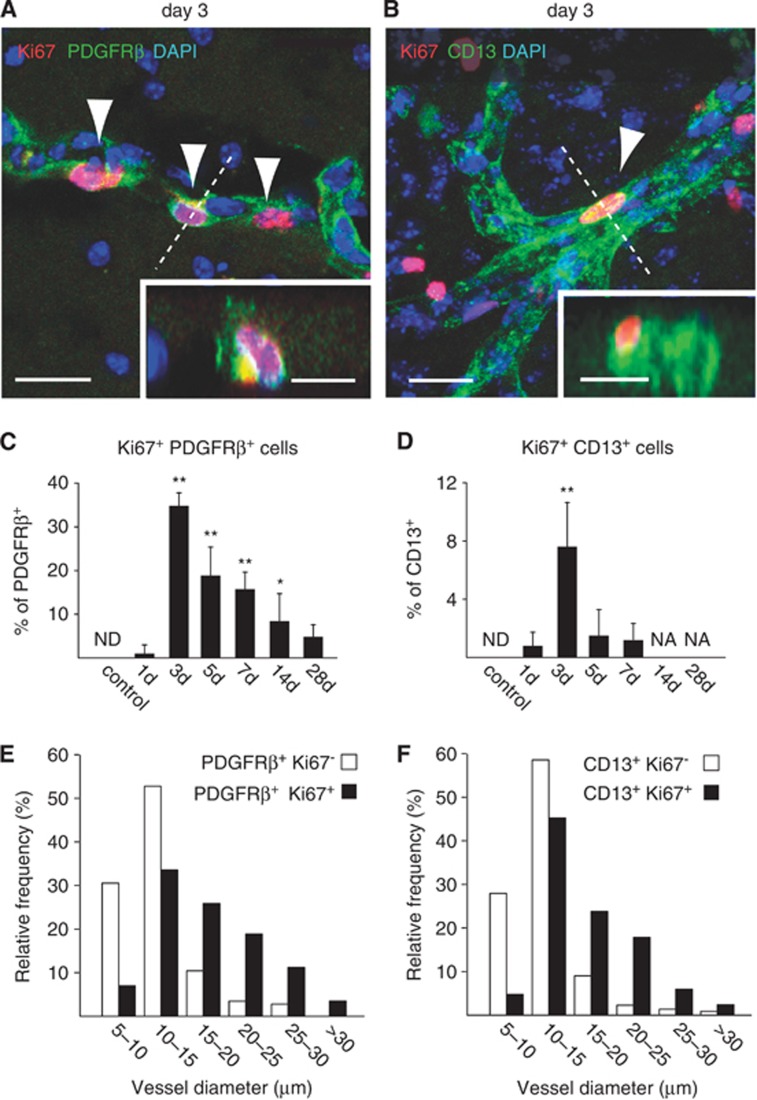
Contrasting with the loss of CD13+ capillary PCs, we detected an increase in PDGFRβ immunoreactivity in the core of the ischemic lesion. On day 1 after MCAO, patchy PDGFRβ expression was observed (Figure 3A). However, this staining pattern changed after 3 days with strong PDGFRβ immunoreactivity in larger vascular structures (Figure 3A). Subsequently, PDGFRβ+ cells with an ameboid shape and multiple irregular cell projections (suggestive of cell migration) generated a densely packed mesh, which was anchored at vessels and persisted for up to 28 days, the last time point examined (Figure 3A). Counting of PDGFRβ+ cells substantiated the increase in PDGFRβ+ cell density at the lesion site starting on day 5 after MCAO (Figure 3B). The increased PDGFRβ immunoreactivity in the lesion core was neatly demarcated by a GFAP-immunoreactive astrogliotic scar (Figure 3D); GFAP+ astrocytes were almost completely absent from the lesion core (Figures 3A and 3D). The area occupied by PDGFRβ+ cells decreased markedly during the second week after ischemia, reflecting the extensive contraction of the damaged tissue (Figure 3C). At these late time points, occasional cavitation was found (Figure 3D). Staining for Ki67, a marker of mitotic cells, showed substantial proliferation of PDGFRβ+ and CD13+ cells, reaching maximal levels by day 3 after MCAO and declining thereafter (Figures 4A to 4D). At this time, proliferative Ki67-immunoreactive PDGFRβ+ or CD13+ cells were predominantly found around vessels larger than capillaries (>10 μm, Figures 4E and 4F). Neither Ki67-immunoreactive PDGFRβ+ cells nor Ki67-immunoreactive CD13+ cells were detected in healthy CNS tissue (Figures 4C and 4H). The results suggest that PDGFRβ+ and CD13+ mural cells that reside in large microvessels (rather than capillaries) proliferate and contribute to the vast parenchymal PDGFRβ+ cell population in the core of the ischemic lesion.
Figure 4.
Proliferation of platelet-derived growth factor receptor beta (PDGFRβ+) and CD13+ cells after middle cerebral artery occlusion (MCAO). (A, B) At day 3 after MCAO, Ki67 staining reveals proliferation of PDGFRβ+ cells (A) and CD13+ cells (B) at vessel walls (insets, orthogonal reconstructions from the planes indicated by dashed lines). (C, D) Quantification of Ki67-immunoreactive PDGFRβ+ cells (C) and Ki67-immunoreactive CD13+ cells (D) reveals significant bursts of proliferation at day 3 after MCAO. Whereas PDGFRβ+ cells still maintain robust proliferation at days 5 to 7, the proliferation of CD13+ cells subsides after day 3. (E, F) At day 3 after MCAO, frequency histograms of vessel size show that vessel-associated Ki67-immunoreactive PDGFRβ+ and CD13+ cells are predominantly found at microvessels with large diameters (>10 μm), rather than capillaries. Bars represent means±s.d. *P<0.05, **P<0.0005 versus control animals. n=5, 4, 4, 5, 3, 5, and 5 for control, 1, 3, 5, 7, 14, and 28 days, respectively. NA, not available; ND, not detected. Scale bars: 20 μm; 10 μm (insets).
Platelet-Derived Growth Factor Receptor Beta+ Cells Coexpress Stromal Cell Markers and Parallel the Generation of Fibrotic Tissue
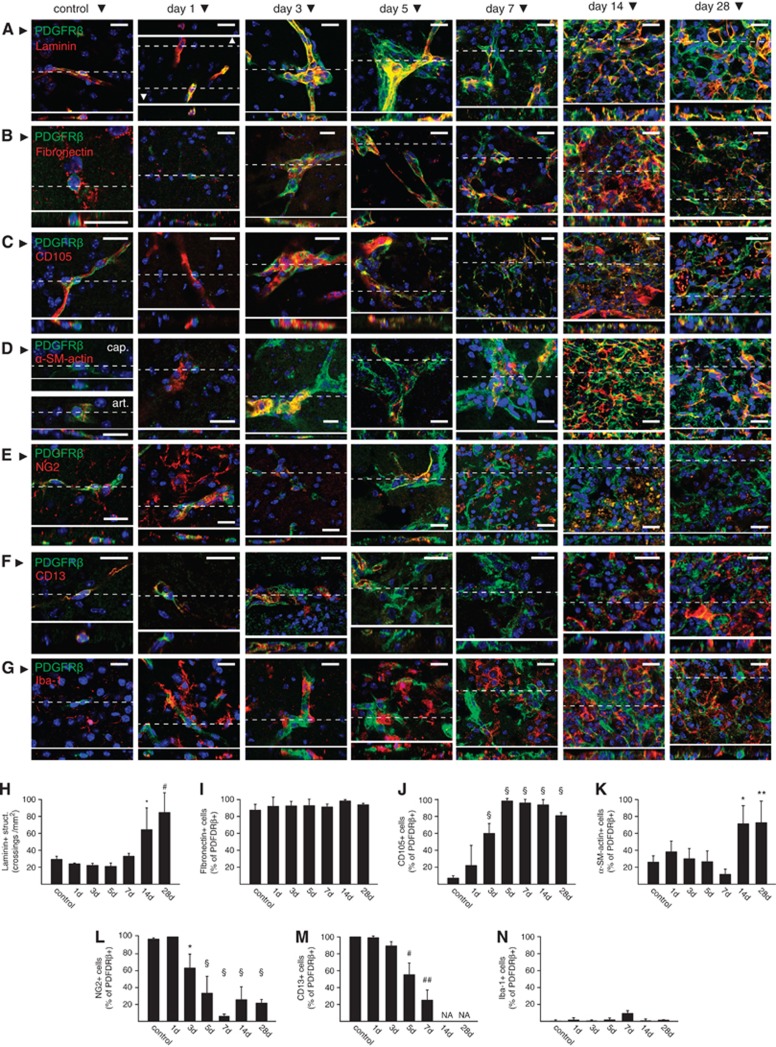
In the ischemic lesion, a marked upregulation of laminin immunoreactivity was observed starting on day 14 after MCAO (Figures 5A and 5H), which apposed or colocalized with PDGFRβ+ cells (Figure 5A). Very low expression of the fibroblast marker fibronectin was detected in the majority of PDGFRβ+ PCs and mural cells in control tissue. After ischemia, a marked upregulation of fibronectin expression was observed in the PDGFRβ+ cell population (Figures 5B and 5I). CD105 (endoglin) is a transforming growth factor-β receptor-associated protein expressed by mesenchymal stem cells and fibroblasts, and is upregulated during fibrosis.23 Induction of CD105 expression was observed in PDGFRβ+ cells starting on day 3 after MCAO (Figures 5C and 5J). Only a quarter of PDGFRβ+ cells (including smooth muscle cells and PCs) in control tissue were immunoreactive for α-SM-actin (Figures 5D and 5K). The fraction of PDGFRβ+ cells expressing α-SM-actin rose to ∼80% starting on day 14 after MCAO, coinciding with tissue contraction. Collectively, our results show that PDGFRβ+ cells in the ischemic mouse brain acquire a set of markers which are normally present in stromal cells or scar tissue myofibroblasts. Importantly, the population of PDGFRβ+ cells in the ischemic core progressively lost PC/mural cells markers, such as NG2 or CD13 (Figures 5E, 5F, 5L, and 5M). The majority of PDGFRβ+ cells were negative for Iba-1 immunoreactivity, a marker of microglia/macrophages that also proliferate in the ischemic tissue (Figures 5G and 5N).
Figure 5.
Characterization of platelet-derived growth factor receptor beta (PDGFRβ+) cells in the ischemic brain tissue. (A–G) Representative examples showing single confocal planes of immunofluorescence stains for PDGFRβ and laminin (A), fibronectin (B), CD105 (C), α-smooth muscle (SM)-actin (D), NG2 (E), CD13 (F), and Iba-1 (G) in control tissue or at the core of ischemia at different time points after middle cerebral artery occlusion (MCAO). Orthogonal reconstructions of the confocal stacks (insets) obtained from the planes marked by dashed lines are shown for each figure. (H–N) Quantification of laminin immunoreactive extracellular matrix (ECM) structures (H) and the fraction of PDGFRβ+ cells immunoreactive for fibronectin (I), CD105 (J), α-SM-actin (K), NG2 (L), CD13 (M), and Iba-1 (N) in control tissue or in the core of ischemia at different time points after MCAO. Collectively, the data show the proliferation of laminin+ ECM in the core of ischemic tissue, gain of a stromal or myofibroblast phenotype by PDGFRβ+ cells (colocalization with fibronectin, CD105, or α-SM-actin), and loss of pericyte (PC)/mural cell markers (loss CD13 or NG2 immunoreactivity). Bars represent means±s.d. *P<0.005, **P<0.001, #P<0.0001, ##P<0.00001, §P<0.000001 versus control animals. n=5, 4, 4, 5, 3, 5, and 5 for control, 1, 3, 5, 7, 14, and 28 days, respectively. NA, not available. Scale bars: 20 μm.
Mural Cell Origin of Platelet-Derived Growth Factor Receptor Beta+ Stromal Cells
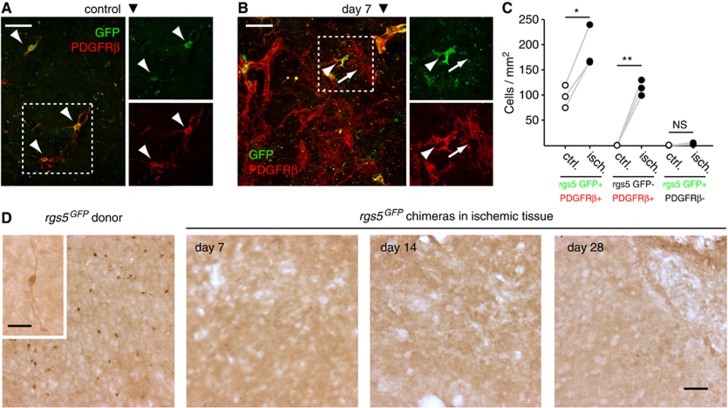
To ascertain the vascular mural origin of the novel PDGFRβ+ cell population in the ischemic tissue, we performed MCAO in rgs5GFP mutant mice (n=3). In these mice, PCs and mural cells express GFP under the control of the mural cell-specific rgs5 promoter, obviating the reliance on cell surface markers for their detection. We exposed mutant mice to 45 minutes of ischemia followed by 7 days of reperfusion. Similar to our findings in wild-type animals after 60 minutes of MCAO, a remarkable increase in PDGFRβ+ cells was observed in the ischemic striatum at 7 days after 45 minutes MCAO (Figures 6A to 6C). GFP immunoreactivity was exclusively detected in PDGFRβ+ cells, and the number of GFP-expressing PDGFRβ+ cells roughly doubled (Figures 6A to 6C). In all, 44.5±3.5% of PDGFRβ+ parenchymal ameboid cells were immunoreactive for GFP (Figures 6A to 6C).
Figure 6.
Local mural cell origin of platelet-derived growth factor receptor beta (PDGFRβ+) cells in the ischemic brain tissue. (A) In control rgs5GFP mice, brain pericytes (PCs) and mural cells coexpressed GFP and PDGFRβ (arrowheads). (B) Seven days after middle cerebral artery occlusion (MCAO), rgs5 promoter-driven expression of GFP increased in the ischemic tissue, colocalizing with PDGFRβ+ cells (arrowhead). Not all PDGFRβ+ cells expressed GFP (arrow). (C) Plot of the paired counts of PDGFRβ+ GFP-stained cells, showing a significant increase in both PDGFRβ+ GFP+ and single positive PDGFRβ+ cells after ischemia. (D) In rgs5GFP BM chimeras exposed to 45 minutes MCAO, GFP immunoreactivity (indicative of rgs5 promoter-driven expression of GFP in BM-derived cells) is absent from the ischemic brain parenchyma. As a positive control, GFP immunoreactivity is detected in mural cells and PCs of naive rgs5GFP donor mice (left panel). Maximal projections of confocal images are shown. In (A–C), n=3. In (D), n=3, 3, or 4 for days 7, 14, or 28, respectively. *P<0.05, **P<0.01 versus control animals. NS, not significant. Scale bars: 100 μm (A, B, D); 10 μm (inset in D).
It has been proposed that BM-derived cells differentiate into PCs in a number of neuropathological conditions, including cerebral ischemia.24, 25 We therefore wanted to determine the potential contribution of BM-derived cells to the populations of PCs and stromal cells in the ischemic brain tissue. To this end, we generated BM chimeric mice by transplanting GFP-expressing wild-type BM cells into lethally irradiated recipient mice as described.19 After 60 minutes MCAO and 7 days of reperfusion (n=3), <2% of >3,600 GFP-positive donor-derived cells expressed CD13 and localized within the laminin-immunoreactive vascular basement membrane in the ischemic core. None of these GFP-positive cells showed characteristic PC morphology (data not shown), suggesting that BM cells do not significantly contribute to the PC population after MCAO. In a second set of BM chimera experiments, we wanted to provide additional evidence against a BM origin of PCs and the PDGFRβ+ stromal cell population in the ischemic brain. We transplanted BM from rgs5GFPmice into lethally irradiated recipients, and subjected the chimeras to 45 minutes MCAO and 7 days of reperfusion. Using double immunofluorescence stainings for GFP and CD13, or single DAB stainings for GFP, we screened every third section of the ischemic tissue for GFP-expressing cells. No GFP-immunoreactive cells were observed at 7, 14, or 28 days after MCAO (n=3, 3, and 4, respectively; Figure 6D). As a control, we also stained brain tissue from rgs5GFP donor mice for GFP expression, which revealed GFP-immunoreactive PCs and mural cells as expected (Figure 6D). Overall, our results suggest that BM cells do not significantly contribute to the populations of capillary PCs and PDGFRβ+ stromal cells in the ischemic mouse brain.
Pericyte Loss and Proliferation of Platelet-Derived Growth Factor Receptor Beta+ Cells in Human Stroke
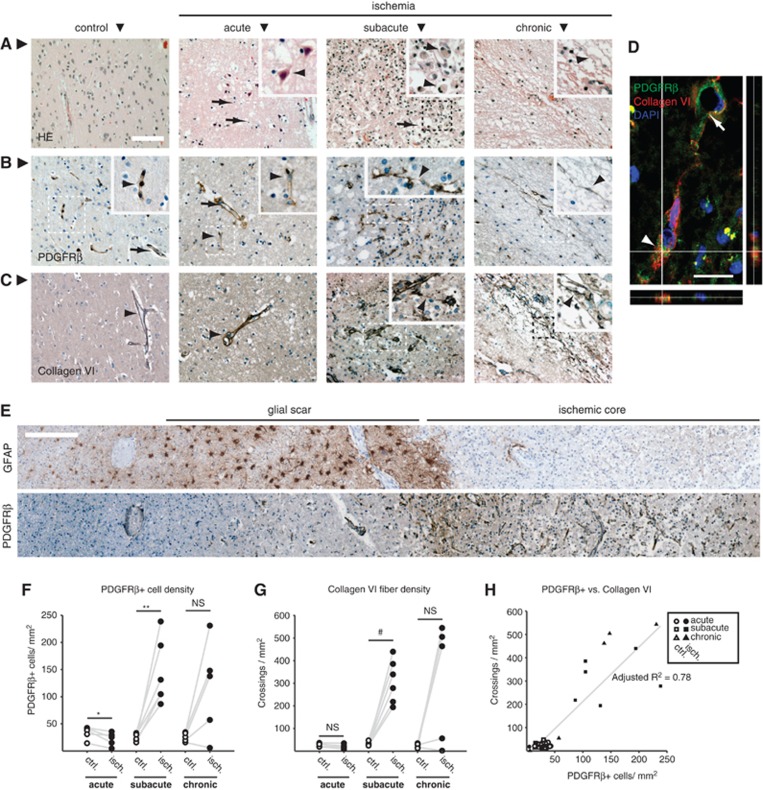
Given the substantial differences in plasticity and tissue remodeling after cerebral ischemia between humans and lissencephalic rodents, we were intrigued to find out whether PCs are also lost and PDGFRβ+ stromal cells also proliferate in human stroke. Postmortem histological samples from 16 individuals with a neuropathological diagnosis of stroke were obtained. These had been classified into acute, subacute, or chronic lesions according to neuropathological findings obtained by routine hematoxylin-eosin stains. Five individuals with acute, six with subacute, and five with chronic stroke lesions were included in the study (Supplementary Table 1). The presence of edema, pyknotic nuclei, shrunken or eosinophilic neurons and a minor inflammatory infiltrate defined the acute lesions (Figure 7A). Subacute lesions were characterized by a major inflammatory infiltrate, mainly composed of macrophages, and incipient pseudocystic reorganization of the ischemic tissue (Figure 7A). Finally, chronic lesions showed a complete pseudocystic rearrangement of the tissue with few inflammatory cells (Figure 7A). In control (nonischemic) tissue from the same brain regions and subjects, PDGFRβ immunohistochemistry revealed PCs and mural cells, whereas staining for collagen VI revealed ECM surrounding larger vessels (Figures 7B and 7C). Acute stroke lesions showed a significant reduction in the density of PDGFRβ+ cells (Figures 7B and 7F). In contrast, the density of PDGFRβ+ cells increased sharply in subacute lesions, and remained elevated in the majority of chronic lesions, which showed a more heterogeneous density of PDGFRβ+ cells (Figures 7B and 7F). The morphology of PDGFRβ+ cells in subacute or chronic stroke lesions differed from mural cells in controls, often showing a ramified morphology and bridging between different tissue segments (Figure 7B). Similar to the findings in murine experimental stroke (Figures 3A and 3D), increased PDGFRβ immunoreactivity in human subacute stroke lesions was neatly demarcated by a GFAP-immunoreactive astroglial scar, and GFAP+ astrocytes were almost completely absent from the lesion core (Figure 7E). The density of collagen VI-immunoreactive ECM fibers was unchanged in human acute stroke lesions, but increased dramatically in subacute lesions (Figures 7C and 7G). As for PDGFRβ+ cells, the density of collagen VI fibers remained elevated in the majority of chronic lesions (Figures 7C and 7G). Both parameters were highly correlated among all samples (adjusted R2=0.78; Figure 7H). PDGFRβ+ cells were found to express collagen VI (Figure 7D), suggesting production of collagen VI by PDGFRβ+ cells.
Figure 7.
Pericyte loss and proliferation of platelet-derived growth factor receptor beta (PDGFRβ+) cells in human stroke. (A) Representative examples of hematoxylin-eosin (HE) stains in postmortem samples of control tissue or acute, subacute, and chronic brain stroke lesions. Acute lesions were recognized by the presence of edema, pyknotic cell nuclei (arrows) and shrunken/eosinophilic neurons (arrowhead). Subacute lesions were characterized by incipient reorganization of the tissue into pseudocysts (arrow) and a prominent inflammatory infiltrate (arrowhead points to a monocyte/macrophage). Chronic lesions showed a marked pseudocystic reorganization with multiple cavitations and septa (arrowhead) and few inflammatory cells. (B) In control healthy and acute stroke tissue, pericytes (arrowheads) and mural cells in large vessels (arrows) were immunopositive for PDGFRβ. Subacute lesions presented proliferation of PDGFRβ+ cells, which often ramified in the inflamed parenchyma, bridging vessels (arrowhead). Chronic lesions showed slender PDGFRβ+ cells located in septa (arrowhead). (C) In control healthy and acute stroke tissue, collagen VI immunoreactivity was present around large vessels (arrowhead). In subacute lesions, collagen VI immunoreactive extracellular matrix (ECM) fibers invaded the parenchyma, often bridging adjacent vessels (arrowhead). In chronic lesions, collagen VI immunoreactivity localized to the septa between pseudocysts (arrowhead). (D) Single confocal plane and orthogonal reconstructions of a PDGFRβ+ cell (arrowhead), showing colocalization with collagen VI immunoreactivity. The collagen VI+ fiber reaches for a neighboring vessel (arrow). (E) In subacute lesions, the area occupied by proliferative PDGFRβ+ cells in the lesion core contained few GFAP+ astrocytes and was sharply demarcated by hypertrophic reactive GFAP+ astroglia. Images were obtained from contiguous sections of the same subject. (F) Quantification of PDGFRβ+ cells in the ischemic tissue. Whereas the density of PDGFRβ+ cells diminished significantly in acute stroke lesions, their presence rose markedly in subacute stroke samples. Chronic lesions showed a heterogeneous response. (G) Quantification of collagen VI+ fibers in the ischemic tissue. While no difference in collagen VI density was observed in the acute lesion, it rose markedly in subacute ischemic lesions. In similarity to the PDGFRβ response, chronic lesions showed a heterogeneous change in density of collagen VI+fibers. (H) Scatterplot of the pooled data for PDGFRβ+ cell and collagen VI+fiber density, showing a high degree of correlation between both parameters. *P<0.03, **P<0.005, #P<0.0008 versus control tissue. NS, not significant. n=5, 6, and 5 for acute, subacute, and chronic stroke lesions, respectively. Scale bars: 100 μm (A–C), 20 μm (D), 250 μm (E).
Overall, the study of human stroke pathology specimens revealed a strikingly similar pattern compared with the murine ischemia model. This includes both the loss of PCs in the acute stages and the proliferation of PDGFRβ+ cells in the subacute to chronic stages, and the generation of fibrotic tissue during reorganization of the ischemic lesion core.
Discussion
We examined the behavior of PCs and other vascular mural cells in the acute and chronic phases after cerebral ischemia in rodents and humans. This is the first study to show (1) the loss of capillary PCs, (2) the proliferation of newly formed PDGFRβ+CD105+ stromal cells, and (3) the formation of a fibrotic scar distinct from the well-known astroglial scar after stroke.
Proliferation of Platelet-Derived Growth Factor Receptor Beta+ Cells
Our findings suggest that a previously unrecognized population of PDGFRβ+ stromal cells is a major cellular component in the ischemic brain tissue and participates in the generation of scar tissue within the boundary of the reactive astroglial response in rodents and humans. We propose that PDGFRβ+CD105+ cells originate locally from vessel walls expressing PDGFRβ, CD13 and the mural cell-specific protein, regulator of G protein signaling (RGS)5. No significant contribution of BM-derived cells was detected. The PDGFRβ+CD105+ cells observed in the ischemic brain tissue are likely stromal cells derived from ‘type A' PCs, which have recently been reported to proliferate and seal the tissue defect after surgical lesion of the spinal cord.17 The authors of this important study were able to track the origin of PDGFRβ+ cells to mural cells, which express both PDGFRβ and CD13.
Multipotent mesenchymal stem cells, which are able to differentiate into fibroblasts, are suspected to reside as PCs or mural cells in adult tissues, including the brain.9, 10, 11 In agreement with this notion, we propose that a cell population which shares with the PC a vascular niche and some cellular markers (PDGFRβ, CD13) could be the source of the PDGFRβ+ stromal cells in the ischemic brain. Future lineage tracing experiments will help to clarify this issue.26 The appearance of PDGFRβ+ cells in the ischemic core parallels the generation of abundant fibrotic tissue after stroke. As yet, the impact of fibrosis on the outcome of CNS pathology has received little attention. Our results suggest that, far from being a mere collection of debris, the ischemic CNS tissue exhibits a surprisingly high degree of remodeling in rodents and humans. Although scarring may seal the injured tissue after dorsal funiculus incision or dorsal hemisection of the spinal cord in mice,17 the generation of excessive ECM may also negatively affect the structure and function of organs.27 In the injured CNS, fibroblast-like stromal cells may impede axonal outgrowth by expressing semaphorin3.28 While astroglia can provide a favorable environment for regeneration,29 fibroblast-like cells are known to segregate astrocytes from the lesion via ephrin-B2/EphB2 signaling.30 Conditional deletion of PDGFRβ during stroke resulted in larger infarcts and disruption of astroglial scar formation,31 suggesting a potential crosstalk between PDGFRβ+ cells and astroglia. Inflammatory cells such as macrophages, neutrophils, and lymphocytes modulate fibrosis via the release of interleukins and growth factors, including PDGF and transforming growth factor-β.27 Conversely, stromal cells may modulate the proliferation of inflammatory cells after stroke.32 Recently, a PDGFRβ+ perivascular cell population has been described to give rise to follicular dendritic cells that may appear de novo in chronically inflamed tissue.33 We are currently investigating the role of PDGFRβ+ stromal cells in neuroinflammatory disorders.
Loss of Capillary Pericytes
While some CD13+, PDGFRβ+, RGS5-expressing mural cells residing predominantly at larger vessels may proliferate and differentiate into scar tissue, our results suggest that PCs in capillaries succumb to cerebral ischemia acutely after the insult. Thus, there may be functional differences of PCs depending on their location in the vascular tree.20 Although endothelial cell death and vascular rarefaction occurred in the ischemic brain tissue, PC loss appears to precede and exceed vessel degeneration in our MCAO model. PC loss represents a previously unrecognized feature of brain ischemia and reperfusion injury, whose impact needs to be further characterized. In other experimental models, PCs have been shown to be very sensitive to hypoxia34 and to reactive oxygen species.35 Adenosine triphosphate, which is released by necrotic cells and secreted by astrocytes in the injured brain tissue, may cause PC death via activation of P2X7 purinergic receptors.36 In our model, TUNEL-positive PCs, indicative of cell death, were frequent starting at day 1 after ischemia; expression of CC3 in the ischemic tissue was also detected with a peak 3 days after the insult. Although CC3 expression is not exclusive to apoptosis,37 the presence of nuclear condensation and fragmentation corroborated that apoptosis occurs in capillary PCs. We also observed nuclear translocation of NF-κB in capillary PCs within 24 hours after ischemia, which might drive PCs into apoptosis as described for diabetic retinopathy.13, 21
Contraction of PCs has been observed during hypoxia or adenosine triphosphate stimulation,3, 38 and could contribute to blood flow impairment during the first hours after stroke in vivo.15 PCs are intimately linked to the formation of the highly specialized brain vessels2 and regulate the function of the blood–brain barrier. Notably, CD13 (or aminopeptidase N) in CNS pericytes acts as an enzyme at the blood–brain barrier that limits the passage of signaling peptides into the neural parenchyma.39 Hence, its absence may contribute to blood–brain barrier derangement after ischemia. Interventions aimed at maintaining or restoring the defective PC population after stroke might promote angiogenesis and support the function of newly generated vessels, thereby offering new therapeutic opportunities for stroke.40
In summary, we show that rapid loss of capillary PCs occurs after experimental and human stroke. This is accompanied by the proliferation of a novel population of PDGFRβ+ stromal cells, which likely originates from the neurovascular unit and contributes to the deposition of fibrous ECM. The fibrotic scar with PDGFRβ+ cells constitutes a previously unrecognized element of the tissue remodeling process after stroke. We propose that the fibrotic reaction may have different functions compared with the rim of hypertrophic astrocytes, which classically constitutes the so-called astroglial scar. While the latter may support regeneration, the fibrotic scar could be a conserved tissue response to injury with potential detrimental functions in the brain. Specific inactivation of PDGFRβ+ stromal cells, e.g., using conditional knockout mice, may help to provide a better understanding of the process of scar formation in the ischemic brain, and hopefully uncover novel targets for regenerative stroke therapies.
Acknowledgments
The authors are grateful to Vincent Prinz and Mihovil Mladinov for providing tissue samples and to Dr Andreas Meisel for his critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the Deutsche Forschungsgemeinschaft (FOR1336/B3 and TRR43/A7 to JP).
Supplementary Material
References
- Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, Larue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52:127–142. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen C, Corselli M, Park T, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Kang S-G, Shinojima N, Hossain A, Gumin J, Yong RL, Colman H, et al. Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery. 2010;67:711–720. doi: 10.1227/01.NEU.0000377859.06219.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS ONE. 2012;7:e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Melgar M, Rafols J, Gloss D, Diaz F. Postischemic reperfusion: ultrastructural blood-brain barrier and hemodynamic correlative changes in an awake model of transient forebrain ischemia. Neurosurgery. 2005;56:571–581. doi: 10.1227/01.neu.0000154702.23664.3d. [DOI] [PubMed] [Google Scholar]
- Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, Genové G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- Romeo G, Liu W-H, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- López-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2010;299:H959–H974. doi: 10.1152/ajpheart.01251.2009. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Piquer-Gil M, García-Verdugo JM, Zipancic I, Sánchez MJ, Alvarez-Dolado M. Cell fusion contributes to pericyte formation after stroke. J Cereb Blood Flow Metab. 2009;29:480–485. doi: 10.1038/jcbfm.2008.150. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, De Winter F, Holtmaat AJ, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J Neurosci. 1998;18:9962–9976. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Ishii Y, Xu G, Dang TC, Hamashima T, Matsushima T, et al. PDGFR-β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;32:353–367. doi: 10.1038/jcbfm.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Radulescu A, Chen C-L, James IO, Besner GE. Heparin-binding EGF-like growth factor protects pericytes from injury. J Surg Res. 2010;172:165–176. doi: 10.1016/j.jss.2010.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem. 1999;75:118–129. [PubMed] [Google Scholar]
- Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG, Kobayshi M. Enhancement of P2X(7)-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci. 2004;45:1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- Wagner D-C, Riegelsberger UM, Michalk S, Härtig W, Kranz A, Boltze J. Cleaved caspase-3 expression after experimental stroke exhibits different phenotypes and is predominantly non-apoptotic. Brain Res. 2011;1381:237–242. doi: 10.1016/j.brainres.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, et al. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2009;551:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Rutin J, Leenen P, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58:367–378. [PubMed] [Google Scholar]
- Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.