Abstract
In vivo imaging of translocator protein 18 kDa (TSPO) has received significant attention as potential biomarker of microglia activation. Several radioligands have been designed with improved properties. Our group recently developed an 18F-labeled TSPO ligand, [18F]-FEPPA, and confirmed its reliability with a 2-tissue compartment model. Here, we extended, in a group of healthy subjects, its suitability for use in voxel-based analysis with the newly proposed graphical analysis approach, Relative-Equilibrium-Gjedde-Patlak (REGP) plot. The REGP plot successfully replicated the total distribution volumes estimated by the 2-tissue compartment model. We also showed its proof-of-concept in a patient with possible meningioma showing increased [18F]-FEPPA total distribution volume.
Keywords: inflammation, kinetic modeling, microglia, neurooncology, positron emission tomography
Introduction
Activated microglia has a pivotal role in neuroinflammation and abundantly expresses a protein in their mitochondria called the translocator protein 18 kDa (TSPO).1, 2 Increased TSPO expression has been shown in different brain diseases such as cerebral ischemia, HIV, encephalitis, Alzheimer's disease, multiple sclerosis, and stroke.3
To date, there have been a few attempts to use positron emission tomography (PET) to assess the level of microglial activation. First, [11C]-PK11195 has been used to quantify TPSO expression.4 However, this radiotracer had several limitations including high nonspecific binding, low brain penetration, high plasma protein binding, and a difficult preparation.4 Newly developed TSPO tracers that overcame the deficiencies of [11C]-PK11195 include [11C]-PBR28 (ref. 5) and [11C]-DPA713 (ref. 6). Recently, our imaging group has developed a new radiotracer, [18F]-FEPPA (ref. 7) which, while it has overcome some of the previous limitations associated with older tracers, being an 18F-labeled radiotracer (cf., [18F]-DPA714) it has the advantage of wider distribution to imaging sites distant from the production location particularly useful for larger clinical application.
Recently, Rusjan et al8 showed that the 2-tissue compartment model-estimated total distribution volume (VT) is the most reliable outcome for [18F]-FEPPA quantification. However, because of the heavy computation and high susceptibility to noise, full kinetic modeling is limited to region-of-interest (ROI) analyses where ROIs must be predefined with specific hypothesis. Thus, it would be important to validate a method to estimate VT in voxel-wise manner which could be useful especially for clinical applications, e.g., where only a small brain region may be affected or no a priori hypothesis exists.
In the absence of a reference region (i.e., brain area that expresses negligible binding9), graphical approaches may be the only solution to estimate voxel-wise VT. The Logan plot10 is the most widely used graphical analysis that estimates VT, but it underestimates VT in the presence of noise,11 and as it requires the tracer kinetics to reach its equilibrium, it may not be suitable for voxel-wise calculation for [18F]-FEPPA. Zhou et al.12 recently proposed the Relative-Equilibrium-Gjedde-Patlak (REGP) method for reliable estimation of voxel-wise VT for radioligands that reach equilibrium slowly, i.e., it does not require the tracer kinetics to reach its equilibrium. Mathematically, the total distribution volumes estimated by the Logan (DVL) and REGP plot (DVREGP) are the same if, and only if, the noise in the tissue tracer kinetics is negligible.12
In the present study, we have validated the use of the REGP method for [18F]-FEPPA PET in a group of healthy subjects and visualized its proof-of-concept in a patient who had a possible meningioma. Various forms of brain tumors, including meningioma, have been reported to be associated with increased TSPO expression.13, 14
Materials and methods
Subjects
Fifteen healthy subjects (age 58.9±2.56, mean±s.e.) and one patient with Parkinson's disease (PD) underwent an [18F]-FEPPA PET and magnetic resonance imaging scan. The PD patient (female, 68-year-old) had taken part in an on-going [18F]-FEPPA PET study but was excluded because of this incidental finding (i.e., possible meningioma). All subjects provided written informed consent after all procedures were fully explained, and were approved by the Center for Addiction and Mental Health Ethics Review Board. The ROI analysis of the healthy subjects was published elsewhere.8
Radiochemistry
Details of the [18F]-FEPPA synthesis have been described elsewhere.7 It is reliably and quickly labeled with [18F] by nucleophilic displacement of a tosylate leaving group in a fast one-step reaction, yielding a sterile, pyrogen-free product after purification and formulation.
Positron Emission Tomography
A dose of 175.5±3.3 MBq (4.74±0.09 mCi) of intravenous [18F]-FEPPA was administered as a bolus for the PET scans (mass 1.03±0.22 μg, range: 0.16 to 3.22). An automatic blood sampling system (ABSS, Model #PBS-101 from Veenstra Instruments, Joure, The Netherlands) was used to measure arterial blood radioactivity levels continuously at a rate of 2.5 mL/min for the first 22.5 minutes. Manual blood samples were obtained at 2.5, 7, 12, 15, 30, 45, 60, 90, and 120 minutes. These samples were used to determine the temporal evolution of the ratio of radioactivity in whole blood to radioactivity in plasma, and the amount of unmetabolized radioligand in plasma needed to create the input function for the kinetic analysis.8 The scan duration was 125 minutes after the injection of [18F]-FEPPA. The images were reconstructed into 34 time frames. Frames were acquired as followed: 1 frame of variable length, 5 × 30, 1 × 45, 2 × 60, 1 × 90, 1 × 120, 1 × 210, and 22 × 300 seconds. The PET images were obtained using 3D HRRT brain tomography (CPS/Siemens, Knoxville, TN, USA), which measures radioactivity in 207 slices with an interslice distance of 1.22 mm. All PET images were corrected for attenuation using a single photon point source, 137Cs (T50=30.2 years, Eg=662 keV) and were reconstructed by filtered back projection algorithm, with a HANN filter at Nyquist cutoff frequency.
Region of Interest- and Voxel-Based Analyses
For the anatomic delineation of ROIs, a brain magnetic resonance image was acquired for each subject. 2D axial proton density magnetic resonance images were acquired with a General Electric (Milwaukee, WI, USA) Signa 1.5T magnetic resonance image scanner (slice thickness=2 mm, repetition time >5,300 milliseconds, echo time=13 milliseconds, flip angle=90 degree, number of excitations=2, acquisition matrix=256 × 256, and field of view=22 cm). Regions of interest were automatically generated using the in-house software, ROMI (ref. 15). Briefly, ROMI (CAMH, Toronto, Ontario, Canada) fits a standard template of 29 ROIs to an individual high-resolution proton density magnetic resonance image scan based on the probability of gray matter, white matter, and cerebrospinal fluid.15 For the detailed description of the ROIs, see ref. 15. The individual magnetic resonance image with ROIs properly superimposed is then coregistered to the summed [18F]-FEPPA PET image using a mutual information algorithm to generate the time activity curves from each ROI. Time activity curves and metabolite-corrected plasma input function were used to estimate the VT, DVL, and DVREGP using a 2-tissue compartment model,8 Logan plot,10 and REGP plot,12 respectively. VT was considered reliable only if its coefficient of variation (COV) was <15% (ref. 8). In addition, DVL and DVREGP were also calculated in voxel-by-voxel basis.10, 12 The voxel-wise DVL and DVREGP were averaged within each ROI for comparisons with VT estimated by full kinetic analysis results.
To examine if the noise was in fact the source of underestimation in the voxel-based analysis using Logan plot,11 the voxel-based Logan analysis and regression analysis were repeated with smoothed dynamic PET images (full width at half maximum=(8, 8, 8 mm) and (16, 16, 16 mm)).
The DVREGP map of the PD patient with meningioma was coregistered with the anatomic magnetic resonance imaging then transformed to Montreal Neurological Institute space for visualization. For this patient, binding potential of [18F]-FEPPA is also calculated in the tumor and contralateral region.
Statistical Analysis
All DVL and DVREGP of both ROI-based and voxel-based analysis were tested for linear regression with VT estimated by the 2-tissue compartment model which is the ‘gold standard' for [18F]-FEPPA PET quantification.8 The r2>0.9 (i.e., >90% of variance is explained by regression model) is considered for reliable estimation of VT. The regression coefficients were also tested as to whether they are significantly different from reference line of y=x (i.e., b0=0 and b1=1) with a one-sample t-test.
Results
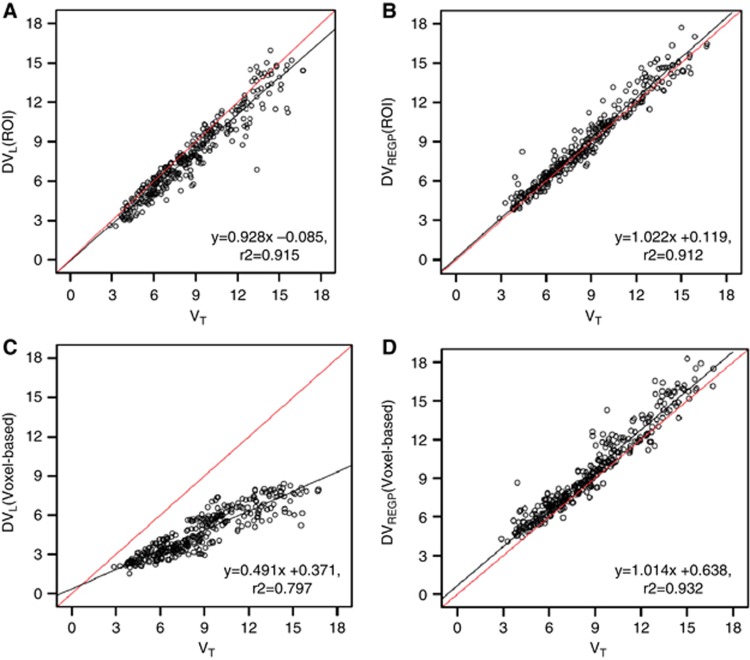
In ROI analysis, both DVL (r2=0.915) and DVREGP (r2=0.912) reliably estimated the VT calculated by the 2-tissue compartment model. DVL slightly underestimated the slope of regression (b0=−0.085, t(326)=−0.586, P=0.558; b1=0.928, t(326)=−4.5, P<0.001; Figure 1A). In contrast, as also showed by Zhou et al.,12 DVREGP reliably estimated both the slope and intercept of the regression (b0=0.119, t(326)=0.737, P=0.462; b1=1.022, t(326)=1.22, P=0.223; Figure 1B). This difference was even greater in voxel-based analysis, i.e., DVL calculated in voxel-by-voxel manner did not reliably estimate VT (r2=0.797, b0=0.371, t(326)=2.945, P=0.003; b1=0.491, t(326)=36.36, P<0.001; Figure 1C), while voxel-wise DVREGP reliably predicted VT (r2=0.932; b0=0.638, t(326)=4.576, P<0.001; b1=1.014, t(326)=0.93, P=0.351; Figure 1D).
Figure 1.
Linear regression of total distribution volume (VT) estimated by 2-tissue compartment model and graphical approaches. In region of interest (ROI) analysis, (A) Logan plot (DVL) slightly underestimated the slope of regression of VT calculated by 2-tissue compartment model (r2=0.915; b0=−0.085, t(326)=−0.586, P=0.558; b1=0.928, t(326)=−4.5, P<0.001). (B) Relative-Equilibrium-Gjedde-Patlak (REGP) plot (DVREGP) reliably estimated VT (r2=0.912; b0=0.119, t(326)=0.737, P=0.462; b1=1.022, t(326)=1.22, P=0.223). In voxel-based analysis, (C) Logan plot (DVL) did not reliably estimate VT (r2=0.797, b0=0.371, t(326)=2.945, P=0.003; b1=0.491, t(326)=36.36, P<0.001), while (D) REGP method (DVREGP) reliably predicted VT (r2=0.932; b0=0.638, t(326)=4.576, P<0.001; b1=1.014, t(326)=0.93, P=0.351). VT and DVL were taken from 29 ROIs in 15 healthy subjects (see Materials and methods).
The underestimation of VT by DVL was improved when the dynamic PET images were smoothed with full width at half maximum=8 mm (r2=0.865, b0=−0.225, t(326)=−1.303, P=0.193, b1=0.860, t(326)=−7.368, P<0.001). However, r2 was slightly decreased as bigger smoothing kernel was used (full width at half maximum=16 mm, r2=0.817, b0=0.606, t(326)=3.010, P=0.003, b1=0.836, t(326)=−7.455, P<0.001).
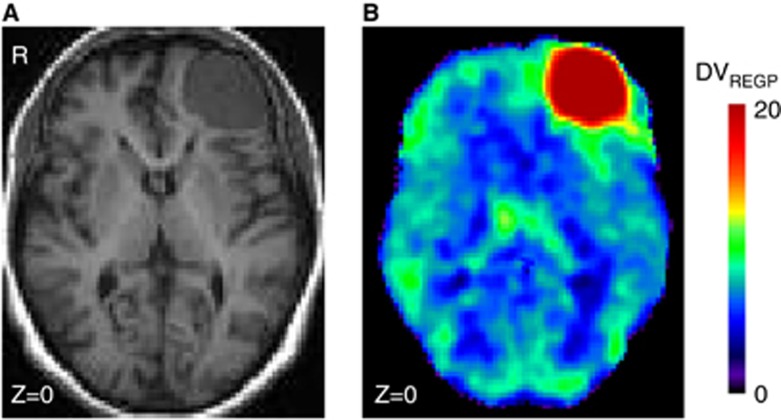
The PD patient (Figure 2) was excluded from our on-going research study in PD patients because of an incidental finding of possible meningioma in the left frontal lobe. A full kinetic analysis with input function of the ROI showed that the tumor (left frontal) presented a VT of 25.1 mL/cm3 (%COV=7%), which was three times higher than the contralateral, right frontal side VT=7.6 mL/cm3 (%COV=3.7).
Figure 2.
Patient with possible meningioma. (A) T1-MRI (B) DVREGP of [18F]-FEPPA PET. DV, distribution volume; MRI, magnetic resonance imaging; PET, positron emission tomography; REGP, Relative-Equilibrium-Gjedde-Patlak.
Discussion
We extended the suitability of [18F]-FEPPA PET for use in voxel-based analysis with the newly proposed graphical analysis approach, i.e., REGP plot.12 The REGP plot successfully replicated the total distribution volumes estimated by the 2-tissue compartment model. The underestimation of slope and r2 of regression in the voxel-based Logan plot analysis was improved when the dynamic PET image was smoothed, which indicates that the noise in fact was an important source of error. Nevertheless, the highest r2 of regression was achieved when voxel-based REGP method was used without smoothing (r2=0.932).
We also showed its proof-of-concept in a patient with possible meningioma showing increased [18F]-FEPPA total distribution volume. Therefore, voxel-based analysis of [18F]-FEPPA PET may be reliably used for explorative research studies that aim to probe microglia activation in vivo.
In the absence of a blocking experiment (as no TSPO drug is approved for human use), it is not possible to know with certainty how much of this binding is specific and how much is free or nonspecific. Moreover, the shape of the TACs is difficult to compare because the delivery in the tumor is different than in regular tissue; K1 in the tumor ROI was double than that in the right frontal (0.27 (COV=5%) versus 0.15 (COV=3%) mL/cm3 per minute), and there is no evidence to assume that the distribution volumes of the free and nonspecific compartment are the same in the tumor and the right frontal side. However, relying in the best fitting solution for the full kinetic analysis, the tumor presents a binding potential of 9.9 (COV=9%) which is 57% higher than the contralateral, right frontal binding potential=6.3 (COV=8%). Based on the previous reports that TSPO expression is increased in meningioma13, 14 and increased binding of [18F]-FEPPA in the brain region with abundant TSPO expression in rats,7 our results support the utility of [18F]-FEPPA PET as a valid probe for TSPO in vivo.
The authors declare no conflict of interest.
Footnotes
This work was supported by Canadian Institutes of Health Research (MOP 110962). APS is also supported through the Canada Research Chair program.
References
- Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Suh HS, Morgan J, Natividad R, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–328. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camsonne R, Crouzel C, Comar D, et al. Synthesis of N-(C-11) methyl, N-(methyl-1 propyl), (chloro-2 phenyl)-1 isoquinoleine carboxamide-3 (Pk-11195) - a new ligand for peripheral benzodiazepine receptors. J Labelled Comp Radiopharm. 1984;21:985–991. [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H, Chauveau F, Thominiaux C, Grégoire MC, James ML, Trebossen R, et al. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S, et al. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:305–314. doi: 10.1016/j.nucmedbio.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, et al. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab. 2011;31:1807–1816. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ye W, Brasic JR, Wong DF. Multi-graphical analysis of dynamic PET. Neuroimage. 2010;49:2947–2957. doi: 10.1016/j.neuroimage.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese C, Appollonio I, Frigo M, Gaini SM, Piolti R, Frattola L. Benzodiazepine receptors and diazepam-binding inhibitor in human cerebral tumors. Ann Neurol. 1989;26:564–568. doi: 10.1002/ana.410260411. [DOI] [PubMed] [Google Scholar]
- Black KL, Mazziotta JC, Becker DP. Brain tumors. West J Med. 1991;154:186–197. [PMC free article] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]