Abstract
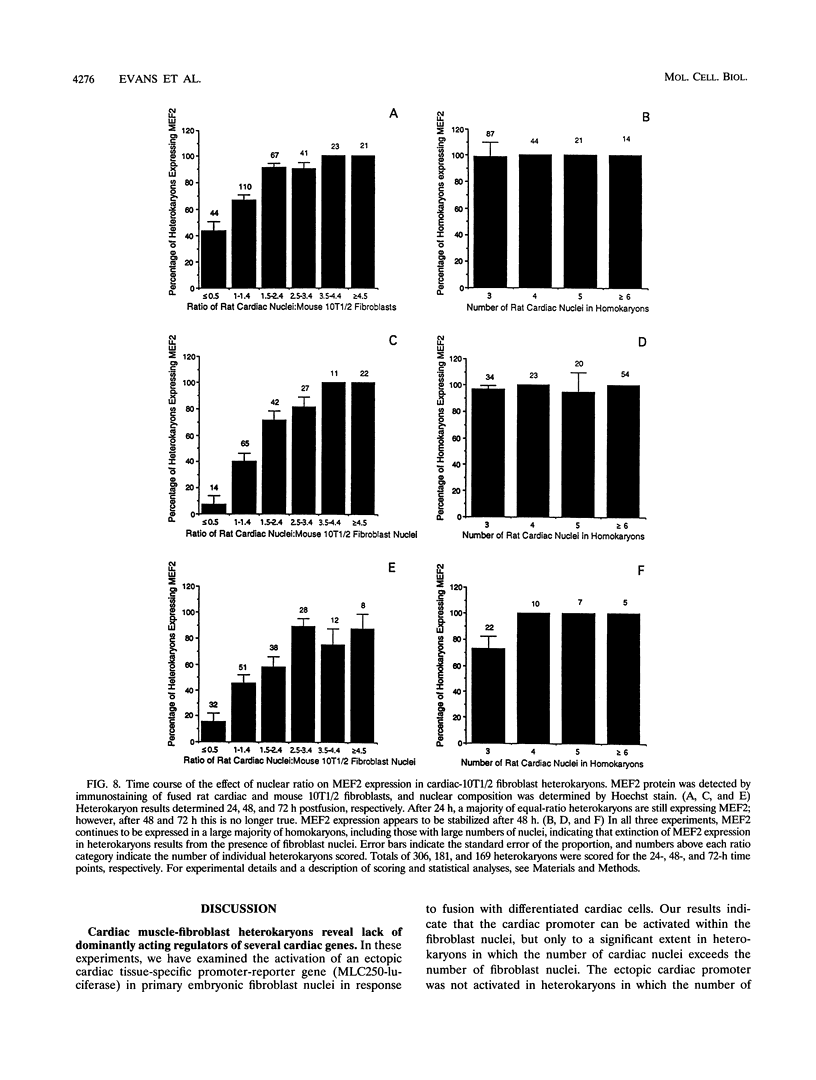
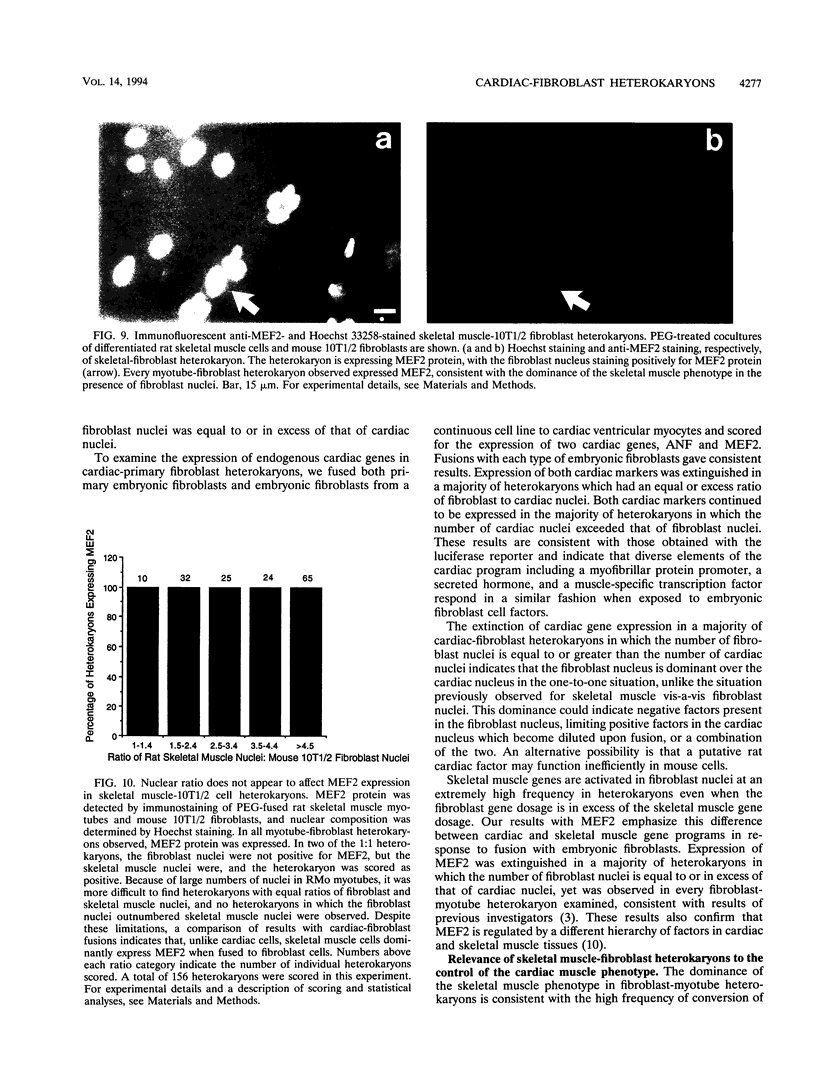
The molecular characterization of a cardiac determination gene has been an elusive goal for the past several years. Prior to cloning of the skeletal muscle determination factor MyoD, the presence of a dominantly acting skeletal muscle determination factor had been inferred from the observation that the skeletal muscle phenotype was dominant in skeletal muscle-fibroblast heterokaryons (H. M. Blau, G. K. Pavlath, E. C. Hardeman, C.-P. Chiu, L. Siberstein, S. G. Webster, S. C. Miller, and D. Webster, Science 230:758-766, 1985). In these experiments, we have examined cardiac-fibroblast heterokaryons to investigate the existence of a dominantly acting cardiac determination factor. We have employed a novel experimental approach using primary embryonic fibroblasts from transgenic mice as a means of assaying for the activation of a cardiac promoter-luciferase reporter transgene within fibroblast nuclei. This approach provides a potential means of genetic selection for a dominantly acting positive factor and can be generalized to other systems. We have examined the expression of three markers of the cardiac lineage: a myofibrillar protein promoter (MLC2), a secreted protein (ANF), and a transcription factor (MEF2). MEF2 is specific to both cardiac and skeletal muscle cells. Our results indicate that in a majority of heterokaryons with an equal ratio of cardiac to fibroblast nuclei, none of these cardiac markers are expressed, indicating that the cardiac phenotype is not dominant over the embryonic fibroblast phenotype. The distinction from previous results with skeletal muscle is emphasized by our results with MEF2, which is dominantly expressed in skeletal muscle-fibroblast but not cardiac-fibroblast heterokaryons, supporting its divergent regulation in the two cell types.
Full text
PDF


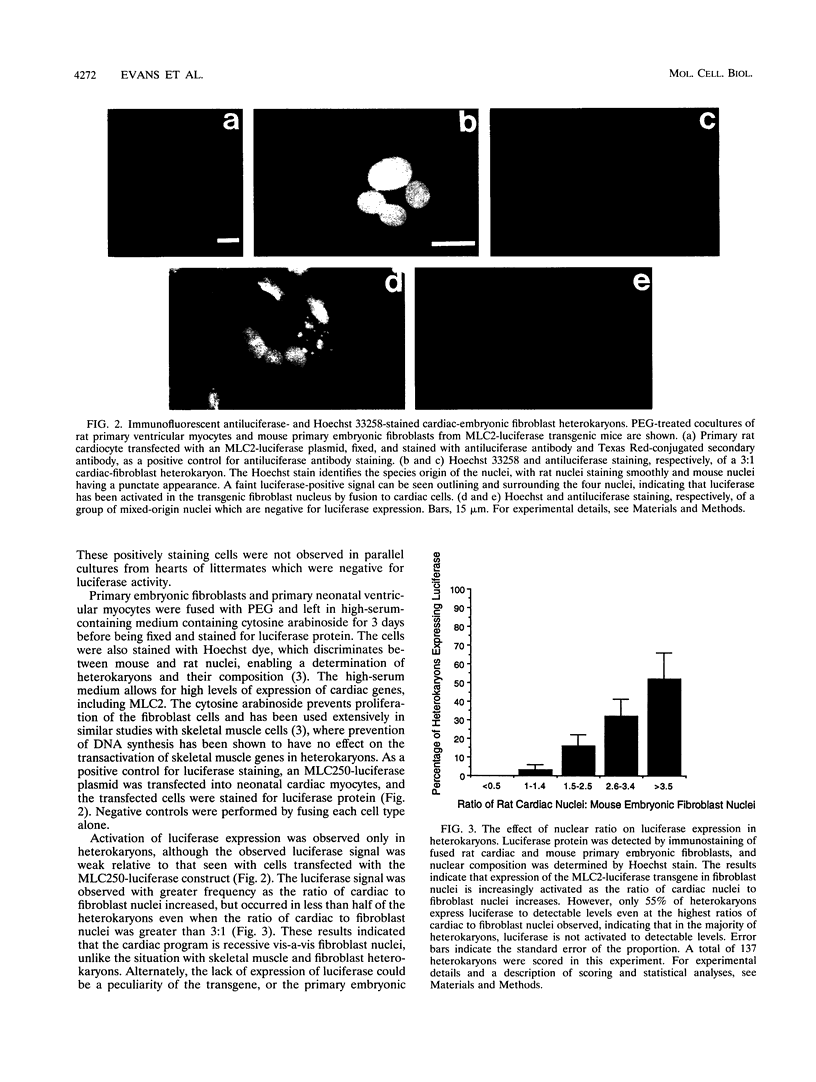
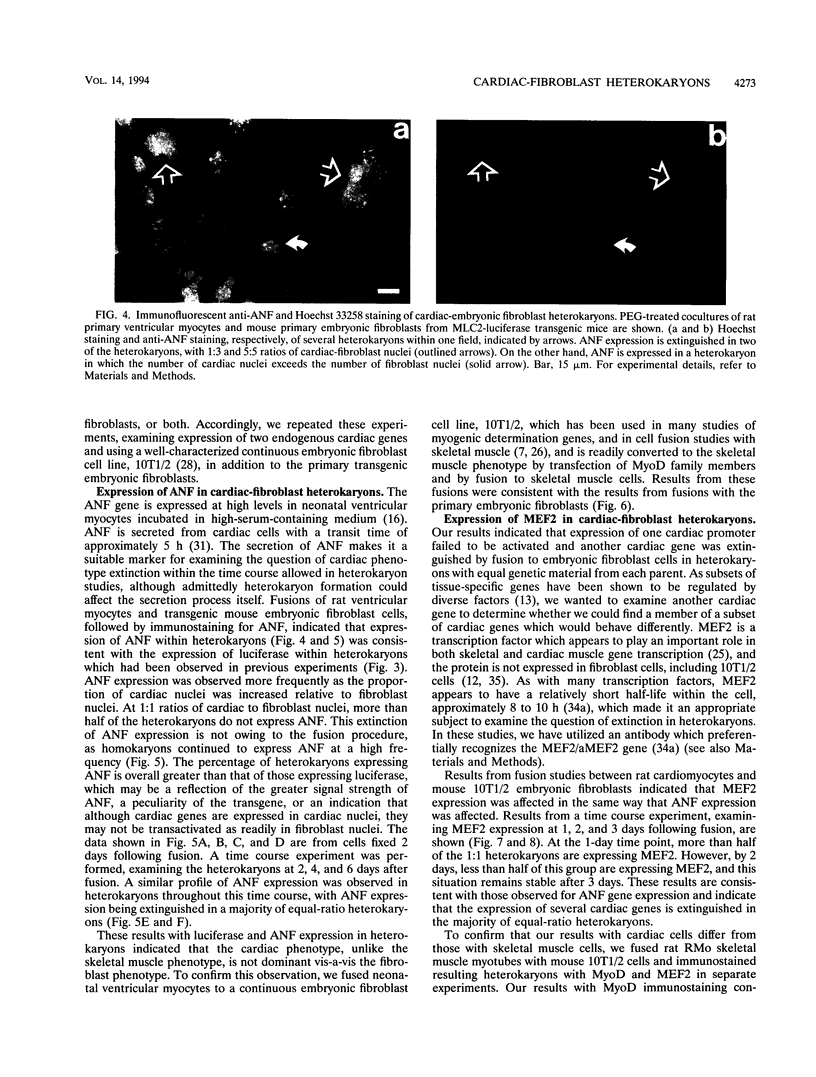
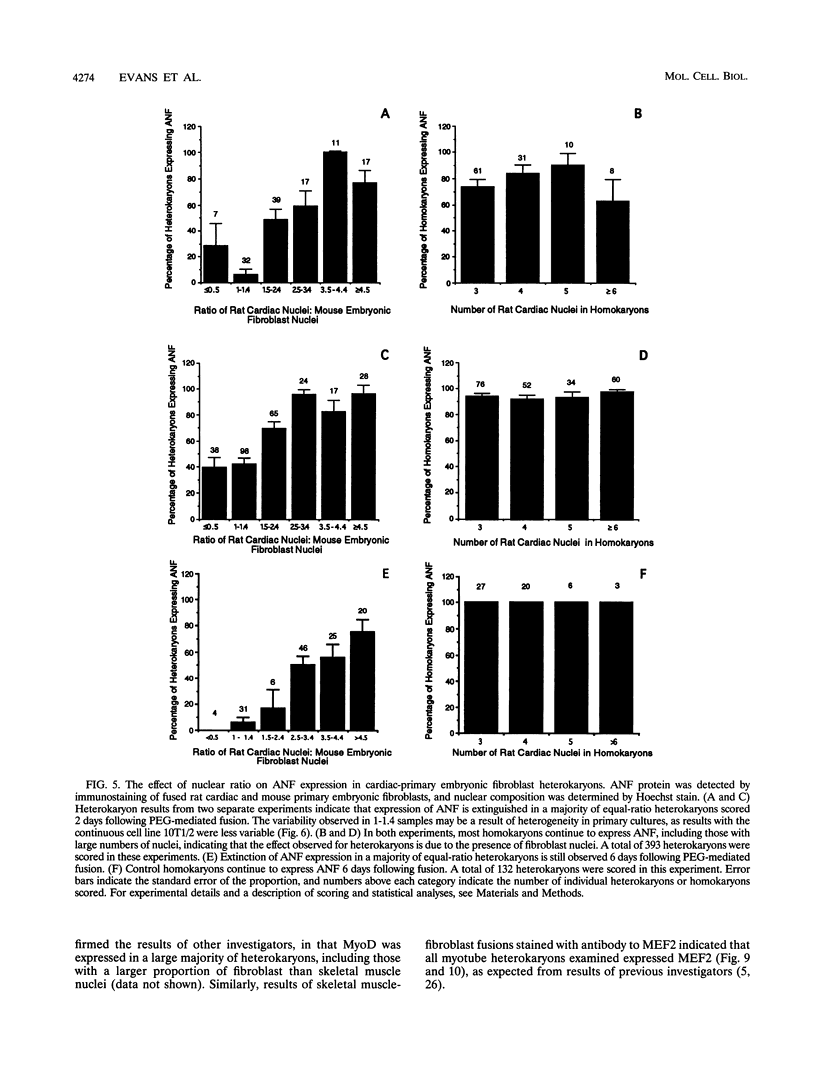
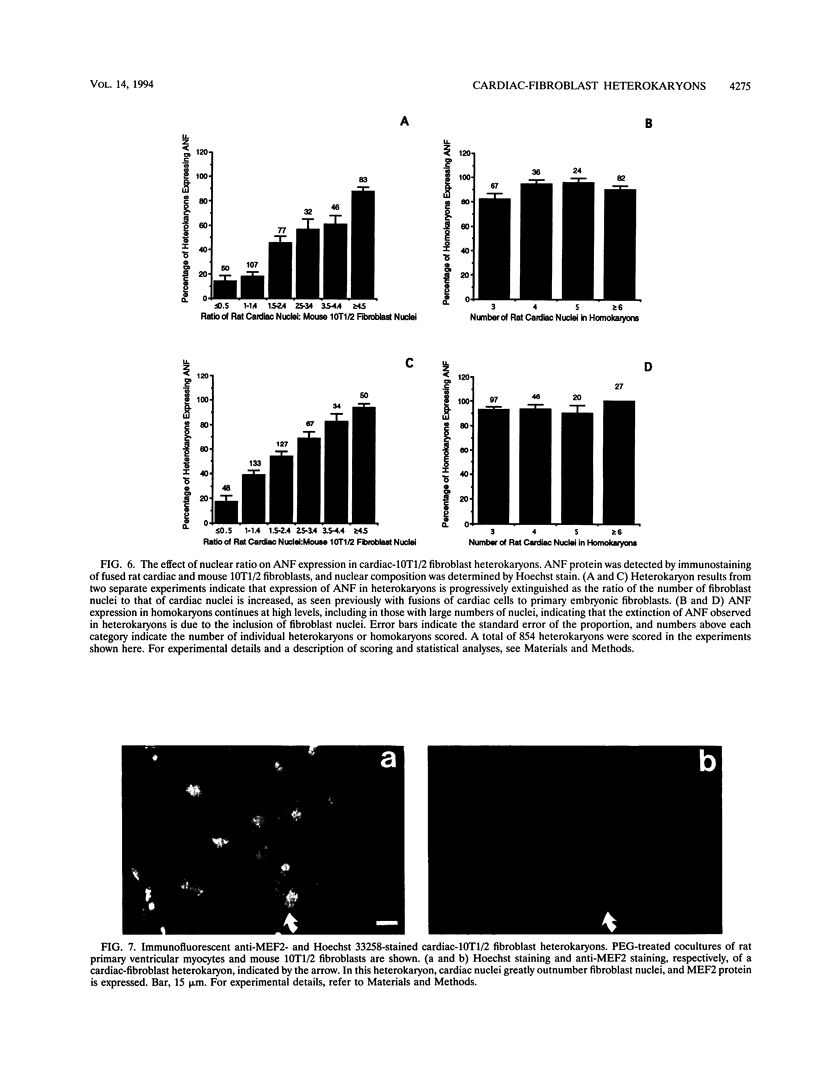


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H. M., Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M. Differentiation requires continuous active control. Annu Rev Biochem. 1992;61:1213–1230. doi: 10.1146/annurev.bi.61.070192.010025. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Zhu H., Knowlton K. U., Miller-Hance W., van-Bilsen M., O'Brien T. X., Evans S. M. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Tapscott S. J., Houghton P. J. Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res. 1992 Dec 1;52(23):6431–6439. [PubMed] [Google Scholar]
- Edmondson D. G., Cheng T. C., Cserjesi P., Chakraborty T., Olson E. N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992 Sep;12(9):3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. Helix-loop-helix proteins as regulators of muscle-specific transcription. J Biol Chem. 1993 Jan 15;268(2):755–758. [PubMed] [Google Scholar]
- Evans S. M., Walsh B. A., Newton C. B., Thorburn J. S., Gardner P. D., van Bilsen M. Potential role of helix-loop-helix proteins in cardiac gene expression. Circ Res. 1993 Sep;73(3):569–578. doi: 10.1161/01.res.73.3.569. [DOI] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdeau H., Fournier R. E. Genetic analysis of mammalian cell differentiation. Annu Rev Cell Biol. 1990;6:69–94. doi: 10.1146/annurev.cb.06.110190.000441. [DOI] [PubMed] [Google Scholar]
- Hardeman E. C., Minty A., Benton-Vosman P., Kedes L., Blau H. M. In vivo system for characterizing clonal variation and tissue-specific gene regulatory factors based on function. J Cell Biol. 1988 Apr;106(4):1027–1034. doi: 10.1083/jcb.106.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton K. U., Baracchini E., Ross R. S., Harris A. N., Henderson S. A., Evans S. M., Glembotski C. C., Chien K. R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991 Apr 25;266(12):7759–7768. [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell. 1984 Oct;38(3):791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Paterson B. M., Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986 Dec 5;47(5):649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- Lee K. J., Hickey R., Zhu H., Chien K. R. Positive regulatory elements (HF-1a and HF-1b) and a novel negative regulatory element (HF-3) mediate ventricular muscle-specific expression of myosin light-chain 2-luciferase fusion genes in transgenic mice. Mol Cell Biol. 1994 Feb;14(2):1220–1229. doi: 10.1128/mcb.14.2.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J., Ross R. S., Rockman H. A., Harris A. N., O'Brien T. X., van Bilsen M., Shubeita H. E., Kandolf R., Brem G., Price J. Myosin light chain-2 luciferase transgenic mice reveal distinct regulatory programs for cardiac and skeletal muscle-specific expression of a single contractile protein gene. J Biol Chem. 1992 Aug 5;267(22):15875–15885. [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Merrill G. F. Clonal derivation of a rat muscle cell strain that forms contraction-competent myotubes. In Vitro Cell Dev Biol. 1989 May;25(5):471–476. doi: 10.1007/BF02624635. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Pavlath G. K., Blakely B. T., Blau H. M. Muscle cell components dictate hepatocyte gene expression and the distribution of the Golgi apparatus in heterokaryons. Genes Dev. 1988 Mar;2(3):330–340. doi: 10.1101/gad.2.3.330. [DOI] [PubMed] [Google Scholar]
- O'Brien T. X., Lee K. J., Chien K. R. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res. 1993 Jan;72(1):1–6. doi: 10.1161/01.res.72.1.1. [DOI] [PubMed] [Google Scholar]
- Peterson C. A., Gordon H., Hall Z. W., Paterson B. M., Blau H. M. Negative control of the helix-loop-helix family of myogenic regulators in the NFB mutant. Cell. 1990 Aug 10;62(3):493–502. doi: 10.1016/0092-8674(90)90014-6. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Schäfer B. W., Blakely B. T., Darlington G. J., Blau H. M. Effect of cell history on response to helix-loop-helix family of myogenic regulators. Nature. 1990 Mar 29;344(6265):454–458. doi: 10.1038/344454a0. [DOI] [PubMed] [Google Scholar]
- Sei C. A., Hand G. L., Murray S. F., Glembotski C. C. The cosecretional maturation of atrial natriuretic factor by primary atrial myocytes. Mol Endocrinol. 1992 Mar;6(3):309–319. doi: 10.1210/mend.6.3.1533896. [DOI] [PubMed] [Google Scholar]
- Spear B. T., Tilghman S. M. Role of alpha-fetoprotein regulatory elements in transcriptional activation in transient heterokaryons. Mol Cell Biol. 1990 Oct;10(10):5047–5054. doi: 10.1128/mcb.10.10.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Breitbart R. E., Smoot L. B., Lee Y., Mahdavi V., Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992 Sep;6(9):1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zhu H., Garcia A. V., Ross R. S., Evans S. M., Chien K. R. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991 Apr;11(4):2273–2281. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]







